Journal of Animal Health and Production
Research Article
Ascorbic Acid Attenuated the Hepatic Parenchymal Necrosis induced by Azithromycin- Etoricoxib Interaction in Rats
Fatma Omara2, Sayed A. Aziz1, Sawsan M. El-Sheikh1, Mahmoud A. A. Said2*
1Department of Pharmacology, Faculty of Veterinary Medicine, Zagazig University, Egypt; 2Zagazig University Hospital, Zagazig University, Egypt.
Abstract | Adverse drug reactions are still facing difficulties until these days. Azithromycin is commonly prescribed for serious infectious diseases of farm animals caused by gram positive cocci and gram negative bacilli. Some data reported that azithromycin has a potential interaction with etoricoxib. The objective of this study was to investigate the possible hepatoprotective effect of ascorbic acid against hepatotoxicity due to azithromycin- etoricoxib interaction using biochemical and histopathological approaches. One hundred and eight adult male albino rats were divided into six equal groups Viz., control, ascorbic acid, azithromycin, etoricoxib, azithromycin+etoricoxib and azithromycin+etoricoxib+ascorbic acid. The results showed a significant (p < 0.05) decline in aspartate transaminase (AST) and alanine transaminase (ALT) level of rats given ascorbic acid alone and in combination with etoricoxib and azithromycin corresponding to control and etoricoxib/azithromycin groups respectively. The ascorbic acid significantly (p < 0.05) reduced the gamma-glutamyl transferase (GGT) and alkaline phosphatase (ALP) level in rats given combination of etoricoxib and azithromycin. A significant increase (p<0.05) in serum glutathione (GSH), glutathione peroxidase (GPx) and decrease (p<0.05) in malondialdehyde (MDA) was observed in ascorbic acid+etoricoxib+azithromycin group as compared with etoricoxib+azithromycin group. Moreover, ascorbic acid restored the liver histomorphological architecture deteriorated by azithromycin+etoricoxib to its normal state. It was concluded that ascorbic acid could prevent hepatocellular damage caused by azithromycin and etoricoxib.
Keywords | Liver, Ascorbic acid, Azithromycin, Etoricoxib, Histopathology
Received | July 21, 2021; Accepted | August 08, 2021; Published | August 25, 2021
*Correspondence | Mahmoud AA Said, Zagazig University Hospital, Zagazig University, Egypt; Email: [email protected]
Citation | Omara F, Aziz SA, El-Sheikh SM, Said MAA (2021). Ascorbic acid attenuated the hepatic parenchymal necrosis induced by azithromycin- etoricoxib interaction in rats. J. Anim. Health Prod. 9(s1): 42-48.
DOI | http://dx.doi.org/10.17582/journal.jahp/2021/9.s1.42.48
ISSN | 2308-2801
Copyright © 2021 Omara et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Globally, Drug- induced liver disease is considered one of most adverse drug events having high mortality rate and their prevention and treatment guidelines still remain inadequate in spite of advancement of pharmacovigilance field (Ramappa et al., 2013). The main cornerstone for pathogenesis of this type of disease is oxidative stress and inflammation which makes it progress to more serious complications e.g. steatosis, chronic hepatitis, fibrosis, and even cirrhosis. Azithromycin, a macrolide antibiotic, is extensively used for gram positive cocci and gram negative bacilli infections. Despite its effectiveness and clinically improvement outcome, many hepatic disorders have been reported because of the use of this drug (Sultana et al., 2020).
Some controversial data reported that etoricoxib (a selective cox II inhibitor) increases chances of developing hepatic injury induced by azithromycin via inhibiting its metabolism. Nevertheless, some anti-oxidant drugs like sylimarin and N-acetyl cysteine have been assessed for their hepatoprotective effect against different experimentally induced liver toxicities and have shown to be therapeutically useful agents (Camini et al., 2020). Additionally, many diverse plant extracts were extensively established for its role against oxidative stress- induced hepatotoxicity through various mechanisms (Mossa et al., 2015).
Ascorbic acid is a water soluble vitamin and it’s one of the dominant antioxidants, sustaining the intracellular antioxidant system. It has many different therapeutic uses like immunostimulant and promoting wound healing. And yet, its role in preventing hepatic fibrosis and non-alcoholic steatohepatitis due to its free radicle scavenging effect has been recognized (Naziroglu et al., 2011).
This study was carried out to detect the possible drug interaction between azithromycin and etoricoxib, to assess the hepatocellular damage induced by this interaction and to attempt to counteract this damage by ascorbic acid by performing serum biochemical analysis and histopathological techniques.
MATERIAL AND METHODS
Drugs
Azithromycin powder for oral suspension (100 mg/5ml), etoricoxib and ascorbic acid (500 mg each) were purchased from EGYPHAR, Obour City-Egypt.
Animals and experimental design
One hundred and eight adult male albino rats weighing from 150-180 g were used. They were obtained from Animal Breeding Unit, Faculty of Vet. Med. Zagazig University. The rats were left to acclimatize in wire cage with natural ventilation and were given standard pelleted diet and clean tap water ad libitum 2 weeks prior to the experiment. The rats were allocated into 6 equal groups, each of 18 and were treated according to experimental design shown in Table 1. This study was done according to ethical guidelines of Zagazig University committee (Nr. ZU-IACUC/2/F/69/2020).
Sample collection
At the end of the experiment, six rats from each group were sacrificed on days 1, 7, and 14 post treatments. Blood samples were collected to clot and serum was separated by centrifugation at 503 xg for measuring biochemical liver function parameters and liver tissues were collected in a jar containing 10% neutral formation for histopathological examination.
Histopathological study
According to procedures of Suvarnet et al. (2013) liver was cut into slices and preserved in jar containing 10% neutral formalin. They were processed in an automated tissue processor. Paraffin sections (4–5 um) were stained with hematoxylin and eosin. Stained sections were examined for inflammatory reactions, degenerative, necrotic, apoptotic changes and any other pathological lesions in experimental rats (Mohammed et al., 2019).
Biochemical analysis of liver enzymes
Serum Liver enzymes including AST and ALT were analyzed with IDEXX Catalyst One Chemistry Analyzer (IDEXX Laboratories Inc., Westbrook, USA) while gamma-glutamyl transferase (GGT) and alkaline phosphatase (ALP) in the liver homogenate was analyzed by following specific ELISA kit procedures using manufacturer’s instructions (Elabscience Biotechnology Co., Ltd., Houston, TX, USA) (Akinnuga, 2019).
Oxidant/Antioxidant Assessment
The determination of the superoxide dismutase (SOD) activity was based on the generation of superoxide radicals produced by xanthine and xanthine oxidase, which react with 2-(4-iodophenyl)-3-(4-nitrophenol)-5-phenyltetrazolium chloride to form a red formazon dye. Briefly, 300 μL of mixed substrate was added to 200 μL of diluted hemolysates (Madkour, 2013). The samples were mixed well and 75 μL xanthine oxidase was added to reactions. The absorbance was measured at 505 nm and the SOD activity was then calculated according to the manufacturer’s instruction (Ransod®-Randox Lab, Antrim, UK) and expressed as U/mL.
Glutathione peroxidase (GPx) activity was determined based on the fact that GPx catalyses the oxidation of glutathione by cumene hydroperoxide. In presence of the glutathione reductase and nicotinamide adenine dinucleotide phosphate (NADPH), the oxidized glutathione was immediately converted to the reduced form with concomitant oxidation of NADPH to NADP+. To evaluate GPx activity in hemolysates 10 μL of samples was mixed with 500 μL reagent R1 and 20 μL cumene R2. The absorbance was measured at 340 nm and the GPx activity was then calculated according to the manufacturer’s instruction (Ransel®-Randox Lab, Antrim, UK). The enzymes activities were expressed as U/mL (Fatemeh et al., 2016).
The procedure to estimate the reduced glutathione (GSH) level followed the method described by Ellman, (1959). In this method thiols react with Ellman’s reagent (5,5′-dithiobis-(2-nitrobenzoic acid) or DTNB), cleaving the disulfide bond to give 2-nitro-5-thiobenzoate (TNB−), which ionizes to the TNB2− dianion in water at neutral and alkaline pH. To evaluate GSH level in samples, 15 μL of hemolysates was mixed with 260 μL assay buffer (0.1 M sodium phosphate and 1 mM EDTA, pH: 8) and 5 μL Ellman reagents. Samples were incubated for 15 min at room temperature and the TNB2− formation was quantified in a spectrophotometer by measuring the absorbance of visible light at 412 nm. Absorbance values were compared with a standard curve generated from standard curve from known GSH.
Catalase (CAT) activity was determined spectrophotometrically by the method of (Koroliuk et al., 1988). Briefly, 10 μL of sample was incubated with 100 μmol/mL of H2O2 in 0.05 mmol/L Tris-HCl buffer pH = 7 for 10 min. The reaction was terminated by rapidly adding 50 μL of 4% ammonium molybdate. Yellow complex of ammonium molybdate and H2O2 was measured at 410 nm. One unit of catalase activity was defined as the amount of enzyme required to decompose 1 μmol H2O2 per min.
Malondialdehyde (MDA) levels in samples were measured using the thiobarbituric acid reaction method of Placer et al. (1966). Quantification of the thiobarbituric acid reactive substances was determined at 532 nm by comparing the absorption to the standard curve of MDA equivalents generated by acid-catalyzed hydrolysis of 1,1,3,3-tetramethoxypropane. To measure MDA level, a working solution containing 15% trichloroacetic acid, 0.375% thiobarbituric acid, and 0.25 N hydrochloric acid was prepared. For each sample, 250 μL serum and 500 μL working solution were mixed and placed in boiling water for 10 min. After cooling the samples were centrifuged at 3000 rpm for 10 min. Finally 200 μL of each supernatant was transferred to microplates and the optical density of samples was measured at 535 nm. The values of MDA were expressed as μmol/L.
Statistical analysis
The obtained data were analyzed and graphically represented using the statistical package for social science (SPSS, version 16), for obtaining [Mean value ± standard error]. The results were statistically analyzed by using one-way ANOVA test. Subsequent multiple comparisons between the different groups were analyzed by Duncan’s multiple comparison tests. Values at p < 0.05 were considered significant (Armitage et al., 2008).
RESULTS
Liver function parameters
The data shown in Table (2) illustrated significant (p < 0.05) decline in AST and ALT of rats given ascorbic acid alone and in combination with etoricoxib and azithromycin corresponding to control and etoricoxib/azithromycin respectively along the entire period of the study. Ascorbic acid also reduced (p > 0.05) the serum AST and ALT level of rats when given along with etoricoxib+azithromycin combination.
Changes in ALP and GGT parameters were represented in Table 3. The ascorbic acid significantly (p < 0.05) reduced the GGT level in rats given combination of etoricoxib and azithromycin on 1st and 7th day post administration as compared to rats of group etoricoxib+azithromycin. The ALP level was reduced (p < 0.05) in rats given etoricoxib and azithromycin combination on 7th and 14th day post administration as compared to etoricoxib+azithromycin group.
Antioxidant parameters
The data provided on Table 4 clarified a significant increase (p<0.05) in serum GPx of ascorbic acid+etoricoxib+azithromycin group as compared with etoricoxib+azithromycin group on 1st and 7th day post administration. The obtained results revealed that treatment with all drugs and their combination in their recommended doses showed no significant (p > 0.05) changes in serum CAT. Table (5) revealed that treatment of ascorbic acid in etoricoxib+azithromycin rats exhibited a significant (p < 0.05) increase in GSH on day-7 post oral administration, but no difference (p > 0.05) detected on days 1 and 14 post-treatment. Serum SOD levels of rats given ascorbic acid together with azithromycin and etoricoxib has decreased in a significant manner (p < 0.05) on days 1, 7 and 14 post treatment in contrast to azithromycin+etoricoxib group.
The treatment of ascorbic acid to etoricoxib+azithromycin rats resulted a significant (p < 0.05) decrease in serum MDA value on days 1 and 7 post-treatments (Table 6).
Histopathological findings
Hepatic parenchyma and hepatocytes were apparently normal (Fig.1A). The dose of etoricoxib induced hydropic degenerative and edematous portal vein with periductular fibrosis containing lymphocyte, which were the main detectable lesions in the hepatic parenchyma on the 14th day post administration (Fig.1B). Degenerative changes, mainly acute cell swelling or coagulative necrosis occurred in the hepatic cells. Moreover, congested blood vessels with proliferation of bile ductules and fibroblasts were seen in portal areas and in interlobular tissue as shown in Fig.1C. On day 7 post administration of etoricoxib and azithromycin combination, an extensive degenerative or necrotic changes of the hepatic cells, congested portal vein, while numerous bile ductules and fibroblasts proliferation were evident (Fig 1D). On the 14th day post administration, these necrotic changes in the hepatic parenchyma were replaced by extravasted erythrocyte with acute cell swelling in the adjacent cells were the main encountered lesions (Fig. 1E). Addition of ascorbic acid with the two drugs caused mild reversible changes varied from cloudy swelling beside dilated portal vein and a few lymphocytes in some portal areas were noticed (Fig. 1F). Fourteen days after the treatment, the majority of the hepatic parenchyma restored its normal morphological character except few cells have a cloudy swelling or vacuolar degeneration beside hyperplastic kupffer cells (Fig. 1G). There were no detectable changes in the portal areas.
Table 1: Experimental animals grouping and treatment strategy.
| Experimental groups* | Dose** |
| I. Control group | Rats were orally given 1 ml distilled water. |
| II. Treated with vitamin C (Ascorbic acid) | Rats were injected with vitamin C 40mg/kg BW intraperitoneally for14 consecutive days. |
| III. Treated with etoricoxib, azithromycin and vitamin C | Rats were given etoricoxib 1 mg/kg BW and azithromycin 45mg/kg BW orally using stomach tube, and vitamin C 40mg/kg BW intraperitoneally for 14 consecutive days. |
| IV. Treated with etoricoxib and azithromycin | Rats were orally given etoricoxib 1 mg/kg BW and azithromycin 45mg/kg BW for 14 consecutive days. |
| V. Treated with azithromycin | Rats were orally given azithromycin 45mg/kg BW for 14 consecutive days. |
| VI. Treated with etoricoxib | Rats were orally given etoricoxib 1 mg/kg BW for 14 consecutive days. |
* n= 18 in each group.
** Doses of vitamin C, etoricoxib and azithromycin were adopted from the studies of Ebuehi et al., (2012), Moraes et al., (2007), and El-Sayed, (2017) respectively.
Table 2: Effect of administration of azithromycin, etoricoxib and ascorbic acid on ALT and AST of male albino rats. (Mean± S.E)
| Parameters | ALT(U/L) | AST (U/L) | ||||
| Groups |
1st day |
7th day |
14th day |
1st day |
7th day |
14th day |
| Control |
262.33 10.19ab |
259.54 10.6ab |
264.33 10.61bc |
50.3 3.38ab |
48.66 3.23d |
49.073.15bc |
| Ascorbic acid |
222 9.53cd |
281.33 3.89ab |
281.33 4.6 b |
49.66 4.17ab |
39.33 3.41de |
45.33 5.19bc |
| Azithromycin+ etoricoxib+ ascorbic acid |
273.66 12.81ab |
279.33 11.79ab |
251 4.01cd |
44.66 3.9b |
73.33 4.43ab |
55.33 6.41b |
| Azithromycin+ etoricoxib |
286.66 12.46a |
304.33 10.68a |
271 10.61bc |
52.33 1.42ab |
77.66 6.08a |
66.66 5.33ab |
| Azithromycin |
226.33 10.94cd |
146.33 10.06d |
245.5 5.05cd |
56 .28a |
65.66 3.89bc |
56.41 1.81b |
| Etoricoxib |
185 4.5e |
200.33 12.6bc |
320.66 2.2a |
49 1.32b |
63 3.53bc |
78.33 .83a |
Mean with different superscript letters in the same column are significantly different at (p ≤ 0.05).
Table 3: Effect of administration of azithromycin, etoricoxib and ascorbic acid on GGT and ALP of male albino rats. (Mean± S.E)
| Parameters | GGT (U/L) | ALP(U/L) | ||||
| Groups |
1st day |
7th day |
14th day |
1st day |
7th day |
14th day |
| Control |
1.13 0.058c |
1.14 0.052d |
1.13 0.05d |
315.83 10.94a |
303 10.68a |
307 10.26e |
| Ascorbic acid |
1.35 0.1d |
1.33 0.22d |
1.03 0.25c |
169 3.96b |
221 4.44c |
374.33 15.83d |
| Azithromycin+ etoricoxib+ ascorbic acid |
4.91 0.63c |
6 0.86c |
3.33 0.44b |
177 9.004b |
198.66 10.58 c |
586 21.09 b |
| Azithromycin+ etoricoxib |
10 1.31a |
29 3.87a |
3.66 0.44b |
180.33 5.18b |
245.66 10.88b |
737 16.74a |
|
Azithromycin |
7.10 0.36b |
14 1.42b |
8.1 0.83a |
197.66 10.93b |
191 10.21c |
448.5 5.34 c |
| Etoricoxib |
1.55 0.36d |
26 1.24a |
4.33 0.6b |
177.66 5.73b |
252 11.85b |
590.66 15.83 b |
Mean with different superscript letters in the same column are significantly different at (p ≤ 0.05).
Table 4: Effect of administration of azithromycin, etoricoxib and ascorbic acid on GPx and CAT of male albino rats. (Mean± S.E)
| Parameters | GPx (U/ ml) | CAT (U/L) | ||||
| Groups |
1st day |
7th day |
14th day |
1st day |
7th day |
14th day |
| Control |
24.85 .12 a |
24.2 .103 a |
25.06 .2a |
5.47 .25 c |
5.48 0.28 ab |
5.26 0.25c |
| Ascorbic acid |
24.2 .17 b |
23.46 .12 b |
24.26 .21b |
6.21 .47bc |
4.93 0.34 b |
6.05 0.4b |
| Azithromycin+ etoricoxib+ ascorbic acid |
22.2 .05 c |
22.8 .25 c |
22.66 .14c |
8.33 .43 a |
6.13 0.38 ab |
9.59 0.72a |
| Azithromycin+ etoricoxib |
21.60 .05 e |
22.18 .102 d |
22.4 .15 c |
8.39 .59 a |
7.69 0.45 a |
9.51 1.1a |
| Azithromycin |
21.80 .05de |
22.16 .09 d |
22.06 .08 c |
6.9 .28 b |
7.71 0.41 a |
7.26 0.58bc |
| Etoricoxib |
22.06 .12cd |
22.4 .057 d |
22.4 .15 c |
7.23 .24 ab |
8.06 0.33 a |
7.02 0.58 c |
Mean with different superscript letters in the same column are significantly different at (p ≤ 0.05).
Table 5: Effect of administration of azithromycin, etoricoxib and ascorbic acid on GSH and SOD of male albino rats. (Mean± S.E)
| Parameters | GSH (µmol/ mL) | SOD (U/L) | ||||
| Groups |
1st day |
7th day |
14th day |
1st day |
7th day |
14th day |
| Control |
68.32 2.93a |
77.95 2.02a |
79.99 .64a |
44.33 0.72 b |
44.62 1.04 ab |
45.2 1.58a |
| Ascorbic acid |
74.06 .98a |
77.77 2.39a |
79.25 2.25a |
49.33 1.3 a |
48.66 2.02 a |
49 1.15a |
| Azithromycin+ etoricoxib+ ascorbic acid |
55.55 1.92 b |
62.73 1.33b |
55.55 2.13c |
32.33 1.01d |
32 1.04 d |
33.66 1.58c |
| Azithromycin+ etoricoxib |
49.81 1.82 b |
40.73 1.61 c |
58.51 3.74 c |
41 0.57 c |
39.66 1.45 bc |
38.33 1.2b |
| Azithromycin |
42.22 .64 c |
31.1 1.28 d |
70.36 2.03ab |
32 1.32 d |
31.33 1.01 d |
30 1.06c |
| etoricoxib |
40.73 .74 c |
62.95 2.96 b |
65.91 1.95b |
29.33 0.88 d |
31.33 1.16 d |
24.66 0.79 d |
Mean with different superscript letters in the same column are significantly different at (p ≤ 0.05).
Table 6: Effect of administration of azithromycin, etoricoxib and ascorbic acid on MDA value of male albino rats. (Mean± S.E)
| Parameter |
|
MDA(mmol/L)after | |
| Groups |
1st day |
7th day |
14th day |
|
49.89 2.86 c |
50.12 2.8d |
49.75 1.91d |
|
| Control |
48.23 2.16 c |
47.25 2.87d |
41.59 1.87e |
| Ascorbic acid |
71.58 3.75 b |
71.59 1.87 c |
84.6 1.87b |
| Azithromycin+ etoricoxib+ ascorbic acid |
97.62 1.87 a |
108.46 4.72a |
73.75 3.9bc |
| Azithromycin+ etoricoxib |
99.79 2.87 a |
95.45 5.59 ab |
112.8 5.73a |
| Azithromycin |
106.29 7.11a |
71.59 1.87 c |
82.43 2.16b |
| Etoricoxib |
|
|
|
Mean with different superscript letters in the same column are significantly different at (p ≤ 0.05).
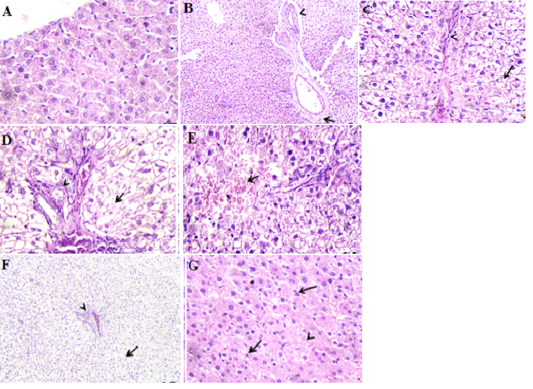
Figure 1: Photomicrographs of rat liver. A. section of control group showing normal hepatic parenchyma. H&E X400. B. etoricoxib group (on day 14 post administration) showing hydropic degeneration, edema of portal vein (arrow) and periductular fibrosis (arrow head). H&E X120. C. azithromycin group (on day 7 post treatment) showing diffuse acute cell swelling or necrosis (arrow) of the hepatic cells beside fibroblasts proliferation in interlobular tissue (arrow head). H&E X 400. D. etoricoxib+azithromycin group (on day 7 post treatment) showing extensive necrosis of the hepatic cells (arrow) and numerous bile ductules (arrow head) in the portal area. H&E X400. E. etoricoxib+azithromycin group (on day 14 post treatment) showing necrotic hepatic cells replaced by extravasted erythrocyte (arrow). H&E X400. F. etoricoxib+azithromycin group received ascorbic acid (on day 7 post treatment) showing mild reversible change (cloudy swelling) of the hepatic cell (arrow) and hyperemic portal vein (arrow head). H&E X120. G. etoricoxib+azithromycin group received ascorbic acid (on day 14 post treatment) showing vacuolar degeneration of the hepatic cells (arrow) and hyperplastic kupffer, s cells (arrow head). H&E X400.
DISCUSSION
In this study, we used rats and divided them as mentioned before. Biochemical analysis including oxidative parameters and liver function tests and histopathological study were performed to evaluate the severity of liver damage induced by etoricoxib-azithromycin interaction and how to avoid this adverse drug reaction by using ascorbic acid.
Further, the combination of etoricoxib and azithromycin caused a significant increase in ALT and AST along the entire course of study as well as major necrotic changes in the hepatic parenchyma on days 7 and 14 post treatment. No previous studies have been reported on the hepatotoxicity caused due to the interaction between azithromycin and etoricoxib. But the same dose of azithromycin alone caused a coagulative necrosis associated with congested blood vessels. These data agreed with Sayed et al. (2020) who stated that the dose of 5 mg/ kg. body weight given orally for 5 successive days caused centrolobular necrosis with a presence of congested central vein. Also, Olayinka and Ore, (2014) supported our findings, because they concluded that the hepatotoxic effects of azithromycin appeared due to the generation of highly reactive free radicals because of oxidative threat caused by the drug which disrupted normal cellular functioning of the liver.
As is known, increased serum GGT is one of the major adverse effects caused by azithromycin. For that reason, it caused a significant rising of serum GGT values of rats given azithriomycin and its combination with etoricoxib. This result was in accordance with Singh et al. (2015) who concluded that azithromycin 200 mg/kg and paracetamol 250 mg/kg given to Wister rats for 7 successive days caused liver toxicity characterized by increased GGT and other liver enzymes and increased oxidative parameters. Notably, increased levels of AST, ALT and ALP indicate cellular leakage and hepatocyte dysfunction, so this leakage makes these enzymes escape into the circulation. In addition, elevated serum GGT levels are indicative of increased biliary pressure caused by azithromycin (Duan et al., 2016).
Many research studies have widely revealed that Vitamin C (Ascorbic acid) has anti-oxidant properties (Hoang et al., 2020). Our results are nearly similar to (Riffel et al., 2020) who reported that that administration of vitamins C prevented changes in pro-oxidant and antioxidant markers in rats exposed to chronic constriction injury. After adding ascorbic acid to the two drugs, it resulted in normalizing serum liver enzymes and returned hepatic histomorphological architecture to its normal state. It was explained by Oularbi et al. (2017) who confirmed that Vit. C attenuated oxidant markers, liver biochemical and histological abnormalities in rats whose liver was exposed to Emamectin. On the other hand, these provided data strengthened the hypothesis that ascorbic acid is potent anti-oxidant works by 1) Inhibiting cytochrome C that’s involved in activation of caspase enzymes 2) Decrease DNA damage 3) Enhancement of cytochrome oxidase, which is important for mitochondrial O2 entry (Agnieszka et al., 2019).
CONCLUSION
It was concluded that the interaction (combination) of azithromycin and etoricoxib have serious hepato-damaging effects. Ascorbic acid clearly abolished liver damage and histomorphological abnormalities induced by both azithromycin and etoricoxib. This would be of interest in the upcoming days in the clinical practice to establish a concept role in management and avoidance of potential hepatotoxicity induced by azithromycin- etoricoxib interaction.
acknowledgements
The authors would like to thank all members of Pharmacology Department, Faculty of Veterinary Medicine, Zagazig University for their assistance.
conflict of interest
The authors declare that they have no competing interests.
AUTHORS CONTRibution
All authors contributed equally.
REFERENCES






