Journal of Animal Health and Production
Case Report
Jowl Edema and Malodorous Diarrhoea in Cattle and its Clinical Management
M.Venkatesan1*, K. Jayalakshmi1, A. Latchumikanthan2, M. Saravanan3, M. Veeraselvam1, N. Premalatha1
1Department of Veterinary Medicine, VCRI, Orathanadu – 614 625; 2Department of Veterinary Parasitology, VCRI, Orathanadu – 614 625; 3Veterinary Clinical Complex, VCRI, Orathanadu – 614 625; Large Animal Medicine Out Patient Unit, Veterinary College and Research Institute, TANUVAS, Orathanadu – 614 625, Thanjavur.
Abstract | Edema of gastrointestinal parasitic infestation is very common in food animals. Cattle (n=4 cases) brought to Large Animal Medicine Outpatient Unit of Veterinary College and Research Institute, Orathanadu with history of inappetence, foul smelling diarrhoea and jowl edema were taken for this study. Clinical examination revealed pale pink mucous membrane, jowel edema and malodorous watery diarrhoea with blood clots. Faecal examination revealed eggs of Amphistome spp. (in 3 cases) and Strongyloides spp. (in one case). Haemato-biochemical examination revealed anaemia and hypoproteinemia. No radiographic abnormalities could be seen. Percutaneous thoraco- ultrasound revealed displaced abomasum, dilated intestinal loops and hepatic vessels in three cases. Malodorous diarrhoea and jowl edema due to helminths infection was confirmed by differentiated from other edema causing diseases of cattle. All the affected animals were treated with oxyclozanide and levamisole at the rate of 5 ml/10 kg bw twice a day along with supportive therapy. Out of four, 03 animals were recovered.
Keywords | Amphistome spp., Strongyloides spp., Cattle, Ultrasonography, Jowl edema.
Received | June 07. 2020; Accepted | June 14, 2020; Published | July 28, 2020
*Correspondence | M Venkatesan, Department of Veterinary Medicine, VCRI, Orathanadu – 614 625; Email: [email protected]
Citation | Venkatesan M, Jayalakshmi K, Latchumikanthan A, Saravanan M, Veeraselvam M, Premalatha N (2020). Jowl edema and malodorous diarrhoea in cattle and its clinical management. J. Anim. Health Prod. 8(3): 145-149.
DOI | http://dx.doi.org/10.17582/journal.jahp/2020/8.3.145.149
ISSN | 2308-2801
Copyright © 2020 Venkatesan et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Gastrointestinal parasitic infestation are consider to be major constrain in the large animal practice and it cause huge economic loss to the poor farming community. Among the gastrointestinal parasites, fluke plays major role to cause production loss in the cattle (which recorded up to 80-90% due to paramphistomes) as well as mortality (Juyal et al., 2003). The major clinical signs of parasitic infestations are mandibular edema, poor weight gain, diarrhoea and loss of productivity. Even though the mandibular edema and diarrhoea are the common clinical signs and it should be differentiated from traumatic reticulo peritonitis, pericarditis, coccidiosis, liver diseases and hypoalbuminemia (Michell, 1996), because these conditions also causes edema and diarrhoea. Identification of various types of faecal parasites, faecal examination could be the reliable method of diagnosis in ruminants (Phiri et al., 2006) but accurate as well as identify the early infection in animals we should rely on serological and molecular techniques. Application of ultrasound in large animals has great importance in many liver diseases like hepatic abscesses, hepatic neoplasia, facioliasis, fatty liver diseases (Braun, 2009a). The supportive diagnostic methods like haemato-biochemical and ultrasonography examination helps to differentiate, confirm diseases and initiate appropriate therapeutic management. Hence, the present study reported the clinical management of paramphistomosis with valuable application of imaging techniques to rule out other conditions like traumatic reticulo pericarditis, peritonitis and liver disorders of cattle.
Case Presentation and Treatment
The present cases were recorded in Large Animal Medicine outpatient unit of Veterinary Clinical Complex, Veterinary College and Research Institute, Orathanadu, dis
Table 1: Haematology and serum biochemistry of animals with jowl edema and malodorous faeces.
| Parameter* | Cow 1 | Cow 2 | Cow 3 | Cow 4 |
| Breed | crossbred Jersey | crossbred Jersey | crossbred Jersey | crossbred Holstein Friesian |
| Calving age | 3 calving | 1 calving | 1 calving | 2 calving |
|
Positive for |
Amphistome spp (++) |
Strongyloides spp (+) |
Amphistome spp (++) |
Amphistome spp (+++) |
| Hb (g/dl) | 3.2 | 4.0 | 3.4 | 2.4 |
| PCV (%) | 19 | 14 | 16 | 14 |
| RBC (millions/cubic millimetre) | 1.89 | 2.3 | 1.2 | 2.6 |
| WBC (per cubic millimetre) | 3750 | 5600 | 6540 | 7600 |
| Neutrophils (%) | 24 | 58 | 22 | 29 |
| Lymphocytes (%) | 72 | 40 | 58 | 61 |
| Monocytes (%) | 2 | 2 | 8 | 4 |
| Eosinophils (%) | 2 | 0 | 12 | 6 |
| Basophils (%) | 0 | 0 | 0 | 0 |
| Total protein (mg/dl) | 4.3 | 5.6 | 5.8 | 6.1 |
| Albumin (mg /dl) | 1.3 | 2.1 | 3.1 | 3.2 |
| AST (U/L) | 121 | 79 | 91 | 93 |
| ALP (U/L) | 136 | 85 | 69 | 41 |
| Status of animal after treatment | Recovered | Recovered | Recovered |
Collapsed |
* Hb: Haemoglobin; PCV: Packed cell volume; RBC: Red blood cell; WBC: White blood cell; AST: Aspartate aminotransferase; ALP: Alkaline phosphatase
trict Thanjavur, Tamil Nadu, India during mid-February to March 2020. Out of four cases three cattle had eggs of Amphistome spp. and one had Strongyloides spp. All the animals were subjected for clinical examination were revealed all animals had jowl edema, anorexia, pale mucous membrane, slight to moderate sunken eyeball and malodorous watery dung with blood clots and other vitals were normal. Faecal samples subjected to diagnose parasitic ova/egg. Blood samples were collected in Ethylenediaminetetraacetic acid (EDTA) coated tube and plain clot activator tube for haematology and serum biochemical examination respectively. Haematology samples were analyzed immediately using haemotology auto analyzer (VetScan HM5, Abbott) as per manufacturer instructions. The samples collected in clot activator vials kept undisturbed at room temperature for 1 hr and centrifuged at 3000 rpm for 15 minutes, and the serum was separated and analysed. Serum biochemical activities of total protein, albumin, aspartate aminotransferase (AST) and alkaline phosphatase (ALP) were determined using autoanalyzer with commercially available biochemical testing kits (SELECTRA PROxs Clinical Chemistry Analyzer, ELITechGroup Clinical System, Netherland). Peripheral blood smear was collected to ruled out blood parasites. Blood smears were stained by Leishman’s staining method and parasitological examination of faeces using both floatation and sedimentation methods (Soulsby, 1982). Samples were examined under the microscope to reveal any ova/ oocysts of parasites. Positive for parasitic ova/egg animals were subjected radiography and ultrasound examination were performed to ruled out traumatic reticulo-pericarditis (TRP), pericarditis, peritonitis and hepatobiliary disorders, because these conditions also having signs of edema and anorexia. Survey radiography was done to rule out foreign body in the reticulum and pericarditis as per standard operating procedure. Percutaneous thoraco-abdominal ultrasonography was done by using 2.5 to 5 MHz Curvilinear transducer (Esaote MyLabVET™ one) according to previous reports (Braun et al., 1997a; Kurt and Cihan, 2013) on standing without any sedation.
Radiography of thorax and reticulum exhibited no evidence of radio opaque foreign bodies, pericarditis, reticulitis and peritonitis. Percutaneous thoraco-adominal ultrasonography examination revealed normalcy of the heart, reticulum and spleen. Unusual finding of abomasum contour and leaves with emptiness were found on left side lateral thorax at 7th to 8th intercostal space in all animals (Figure 1). In the three animals, dilated caudal vena cava, hepatic veins and hyperechoic foci with anechoic cavity was observed in right side approach on 11th and 12th inter costal space (Figure 2). Distended gall bladder (diameter of 7.65 cm) with clear bile was observed. Dilated loops of small intestinal (>4.5cm diameter) were notice at the level of 10th intercostal space to caudal abdomen (Figure 3).
Blood profile investigation of animals showed anaemia and hypoproteinemia (Table 1). Blood smears stained with Leishman’s stain did not reveal any Pasturella organisms or parasites in the animals. Faecal examination on the first day of animals presented to clinic, did not reveal any ova/
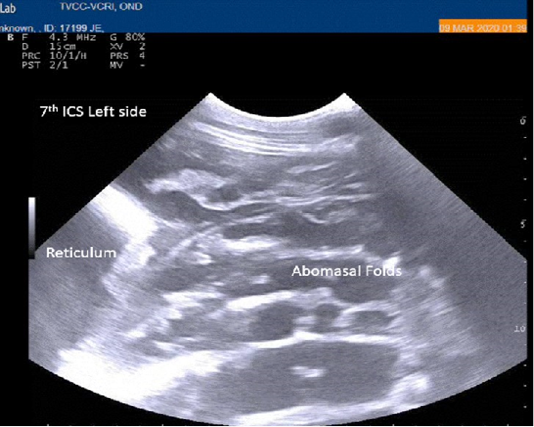
Figure 1: Percutaneous ultrasonography of left lateral thorax at 7th inter coastal space showed inflamed abomasal leaves with emptiness.
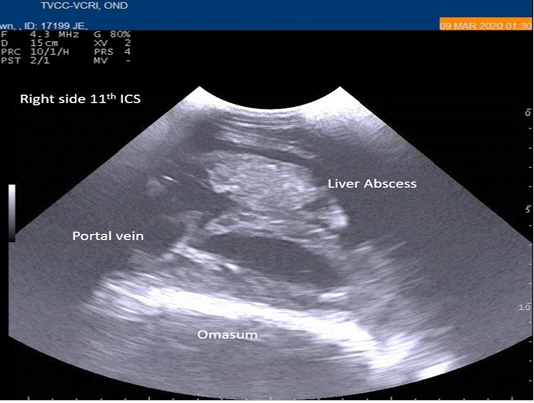
Figure 2: Percutaneous ultrasonography of right-side abdomen at 11th inter coastal space showed liver hyperechoic foci with anechoic cavity.
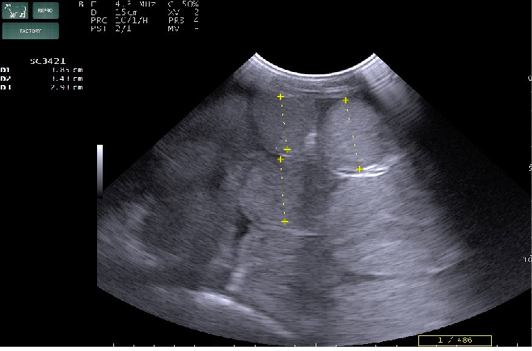
Figure 3: Percutaneous ultrasonography of right side mid abdomen showed dilated (>4.5cm) intestinal loops with mild peritonitis (Arrow).
oocysts of parasites. Since persistence of diarrhoea, again faecal sample was collected for re-examination and found to have Amphistome eggs in 3 animals (Figure 4) and stray Strongyloides eggs (Figure 5) in other animal. Oval shaped eggs, having distinct operculum at anterior pole and a small knob at posterior end with compact yolk cells inside confirmed as Amphistome eggs and Strongloides eggs were having a thin shell and with fully developed larva inside the egg.
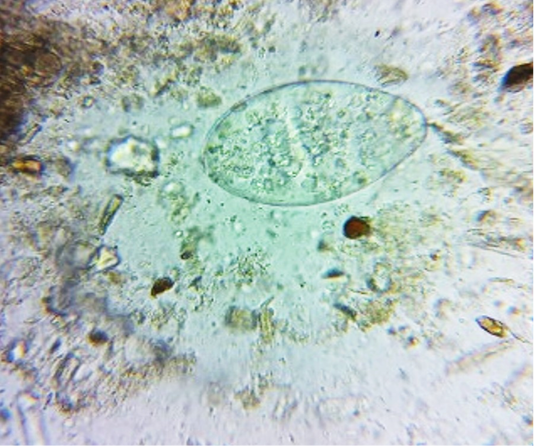
Figure 4: Faeces examination by sedimentation method showed Amphistome eggs (x 400) in three animal.
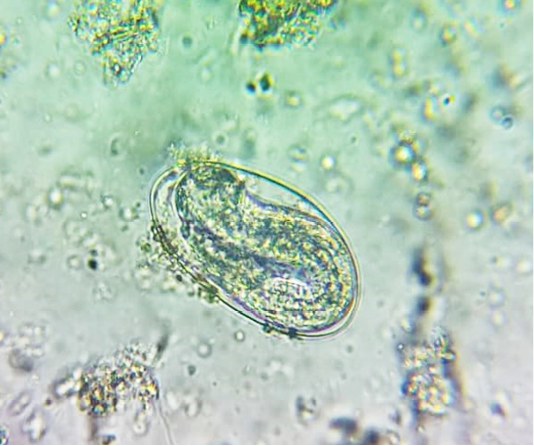
Figure 5: Faeces examination by sedimentation method showed Strongyloides eggs (x 400) in one animal.
Initially animals were treated with Inj. Multiple Electrolyte Solution at the rate of 10ml/kg bw IV to correct electrolyte loss. Inj. Sulphatrimethoprime at the rate of 25 mg/kg bw IM to prevent secondary bacterial infection due to diarrhoea and supportive therapy viz., Inj. Tribivet at the rate of 10ml IV, Inj. Ascorbic acid at the rate of 10 ml IM and 300g of charcoal with 5 egg white mixture were given orally for a week. Suspension Oxyclozanide with Levamisole at the rate of 5 ml/10 kg bw twice a day were given to all the animals. Three animals were recovered uneventfully except one cross breed Holstein Friesian cow which was died 7 days after start of treatment.
Discussion
In current case repport, faecal examination done on the 1st day of animal presentation to clinic did not exhibited parasite eggs. This may be because the parasites may not be fully matured to release eggs during that day. The 2nd day of faecal examination revealed Amphistome eggs in three animals and Strongyloides egg in one animal. The presence of amphistome eggs in the atery faeces was due to acute paramphistomosis. This is in contrary with that of Ballweber (2014) who reported no eggs in the faeces due to acute paramphistomosis. Saravanan et al. (2015) reported higher incidence of Amphistome infection in cattle in the delta region of Thanjavur district.
Chauhan et al. (2015) reported that in severe cases of amphistomosis in buffaloes production loss in terms of milk and meat occurs due to high intensity of anaemia along with hepatic damage caused by Explanatum explanatum. Intermandibular edema was start to subside from 3rd dayof treatment. It was agreement with previous reports of Jayalakshmi et al. (2017) and Suchita et al. (2017). In the present study, anaemia and hypoproteinemia due to severity of the parasitic infestations which leads anaemic crisis, could be the cause of death of animal even with continuous care. Anaemia is also a feature of Amphistomosis and high intensity of anaemia along with hepatic damage could lead to death of the animals (Chauhan et al., 2015).
There are several literatures report regarding normal and abnormal ultrasound findings of abomasum (Braun et al., 1997b; Braun 1997c; Braun, 2009b); but no such reports are available about displacement of abomasum in cows affected by helminth parasites. Authors hypothesized that in the presented cases abomasal displacement was less sever because displaced abomasum was observed from 7th-8th intercostal area only. Hence, the abomasal displacement could be the a) incidental finding, b) due to continuous diarrhoea and emptiness of abomasum causes microbial load (i.e secondary bacterial infection) leads bloat formation and may displaced the abomasum. Radostitis et al. (2000) stated that maturation of immature flukes takes six weeks to four months, in due course it migrates.
Conclusion
Amphistomosis spp. induced jowl edema and malodorous diarrhoea in cattle were managed successfully. Further research on this aspect with a greater number of animals may clear the pathophysiology of organ displacement in gastrointestinal parasite infested cattle. To prevent such type of disorders, regular and rotational deworming schedule could be effective preventive method for parasitic infestation in cattle.
Acknowledgements
The authors are thankful to the Dean, Veterinary College and Research Institute, Orathanadu, TANUVAS, Thanjavur, Tamil Nadu, India for the help.
Conflict of interest
The authors declare, no conflict of interest.
authors contribution
M.Venkatesan: Corresponding author, Clinical examination, Ultrasound examination, Treatment of the cases and preparation of article, K. Jayalakshmi: Contributed for literature collection, handling of case and blood sample processing, A. Latchumikanthan: Contributed in processing of fecal sample and additional reference literature for article preparation, M. Saravanan: Contributed while ultrasound examination of cases, proof reading of article and final shaping. M. Veeraselvam: Contributed for literature collection, final proof reading and guiding in treatment aspect. N. Premalatha: Contributed in guiding of therapeutics for the cases and final proof reading of the article.
References






