Advances in Animal and Veterinary Sciences
Research Article
The Ameliorative Role of Synbiotic Culture Techniques Application in White Shrimp (Litopenaeus vannamei) During Nursery Stage
Eman M. Moustafa1*, Talaat T. Saad2, Riad H. Khalil2, Mahmoud A.O. Dawood3, Emad E. Lolo4
1Department of Fish Diseases and Management, Faculty of Veterinary Medicine, Kafr El-Sheikh University, Kafr El-Sheikh governorate, Postal code: 33516, Egypt; 2Department of Fish Diseases and Management, Faculty of Veterinary Medicine, Alexandria University, Egypt; 3Department of Animal Production, Faculty of Agriculture, Kafrelsheikh University, Egypt; 4Department of Aquaculture, Faculty of fisheries and aquatic science, Kafr El-Sheikh University, Egypt.
Abstract | The current study was conducted to analyze the potential benefits of dietary supplementation of synbiotics on water quality, microbial population, growth performance, proximate analysis, non-specific immune response, oxidative status, cytokine gene expression and susceptibility to experimental infection by V. harveyi in L. vannamei juveniles. A total number of 9000 white shrimp (L. vannamei) in post-larval stage (PL12) with mean initial weight (0.5 ± 0.02 g/ piece) obtained from Barket-Ghalioun fish farm, Kafr El-Sheikh Governorate were used. After the accommodation period, shrimps were randomly distributed into three experimental groups of 3000 post-larvae / each group allotted into three replicates of 1000 post-larvae / each replicate (experimental tanks). Salinity of all tanks was adjusted from 25‰ - 35‰. The first experimental group (G-I) was kept as control (feed on basal diets with continous change of water and aeration). The second experimental group (G-II) (synbiotic culture treatment) was fed on 50 % basal diets + fermented rice bran with Bacillus subtilis without change of water and high aeration). However, the last experimental group (G-III) was fed on fermented rice bran with Bacillus subtilis (without basal diets and change of water and high aeration). The synbiotic containing probiotic B. subtilis in shrimp diets could significantly improve the growth by enhancing the immune response, antioxidative activity, water quality and biofloc composition. The increase of non-specific responses of the shrimp fed the synbiotic supplemented diets may suggest that the antioxidant defense system and innate immune system could work synergistically to improve the physiological performance of the shrimp leading to higher resistance against bacterial challenge. This system can play a key role in developing a sustainable aquaculture via better water quality, maintenance decrease in feed requirements and higher production to achieve more profit in shrimp farming. Consequently, the synbiotic could be practically used as a viable alternative dietary supplement.
Keywords | Growth, Immunity, Litopenaeus vannamei, Nursery stage, Synbiotic
Received | December 11, 2019; Accepted | February 17, 2020; Published | March 03, 2020
*Correspondence | Eman Moustafa Moustafa, Department of Fish Diseases and Management, Faculty of Veterinary Medicine, Kafr El-Sheikh University, Kafr El-Sheikh Governorate, Postal code: 33516, Egypt; Email: emantarek2002@yahoo.com
Citation | Moustafa EM, Saad TT, Khalil RH, Dawood MAO, Lolo EE (2020). The ameliorative role of synbiotic culture techniques application in white shrimp (Litopenaeus vannamei) during nursery stage. Adv. Anim. Vet. Sci. 8(3): 260-277.
DOI | http://dx.doi.org/10.17582/journal.aavs/2020/8.3.260.277
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2020 Moustafa et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
The aquaculture of shrimp has quickly developed to a significant industry around the world, giving not just financial pay and top notch nourishment item, yet additionally gives work to several thousand gifted and unskilled workers. It has been anticipated that by 2050, the absolute populace on the planet will be 9 billion and aquaculture will have significant job in taking into account the expanded interest for nourishment (Godfray et al., 2010).
Penaeid shrimps are highly valued seafood commodities. Driven by the market demand, intensive and semi-intensive shrimp farming have steadily expanded over the last two decades in many parts of the world. With the rapid expansion and intensification, there is a growing concern for ecological sustainability of shrimp farming (Naylor et al., 2000). Commercial shrimp feed constitutes 50–60 % of the operational cost of the shrimp culture (Tan et al., 2005). However, cultured shrimp retains only 20–30 % of feed nutrients and remaining 70–80 % accumulate in aquatic system which leads to eutrophication and water quality deterioration (Paez–Osuna, 2001).
The white shrimp Litopenaeus vannamei is the most widely cultivated species in the world. Recently, L. vannamei farming is facing some issues and problems in the culture which needs a serious contemplation. Diseases such as black gill disease etc. associated with severe growth retardation have been reported in L. vannamei (Newman, 2015). In addition to the disease problems, other issues like limited quarantine facilities, production cost towards feed, quality seed availability, feed quality and availability and environmental impact and management are also affecting the L. vannamei culture in Egypt (Babu et al., 2013). Pacific white shrimp is one of the most important shrimp species currently being cultured in many countries. Over the past decade, mass production of L. vannamei has been demonstrated in biofloc-based intensive culture systems under high aeration and negligible water exchange (Haslun et al., 2012).
Recently, it was demonstrated that dietary inclusion of biofloc had enhanced growth performance of L. vannamei (Ju et al., 2008; Kuhn et al., 2010; Bauer et al., 2012). There is a lack of information to support the role of synbiotic in diets on growth and immune response in the species. Extensive studies on the biofloc based nursery and grown out phases of L. vannamei in the recent years had shown improvement in the growth performance, feed utilization and disease resistance (Kuhn et al., 2008; Xu et al., 2012, 2013).
Synbiotic refers to nutritional supplements combining a mixture of probiotics and prebiotics in a form of synergism. The concept of synbiotics was proposed to characterize some colonic foods with interesting nutritional properties that make these compounds candidates for classification as health-enhancing functional ingredients’ (Gibson and Roberfroid, 1995). Certainly, synbiotics have become widely used in human and veterinary medicine as they could potentially be useful to treat and/or prevent infection (Cheng et al., 2014). However, after the first finfish study was published by Rodriguez-Estrada et al. (2009), several studies have emerged (Cerezuela et al., 2011; De et al., 2014). The focus of these studies has spanned from growth performance, feed utilization, body composition, immunological responses, disease resistance, survival rate and modulation of the water microbiota of synbiotic fed to larvae of L. vannamei. Synbiotic studies reviewed and discussed by different researcher Ringø et al. (2014). Newaj-Fyzul and Austin (2015) have evaluated the effect of plant products on innate and adaptive immune response and their ability to control finfish and shellfish diseases, and key players in the innate immune response include phagocytic cells, such as neutrophils, monocytes and natural killer cells. However, the combined use of probiotics and plant products as a disease control strategy has received less attention (Peraza-Gomez et al., 2014). In shrimp culture, carbohydrate addition improves the growth of black tiger shrimp, Penaeus monodon (Anand et al., 2013), western white leg shrimp, L. vannamei (Xu and Pan, 2012), kuruma shrimp, Marsupenaeus japonicus (Zhao et al., 2012) and pink shrimp, Farfantepenaeus paulensis (Emerenciano et al., 2011).
Many authors determined that biofloc particles are a potential source of lipids and fatty acids that could contribute to juvenile shrimp nutrition (Shyne Anand et al., 2014). Moreover, several studies have shown that dietary biofloc could enhance the immune cellular response and antioxidant status of cultured shrimp, probably because it is rich in natural microorganisms (Xu and Pan, 2013). Bacteria that have been used successfully as probiotics include Bacillus (Wang et al., 2008). Studies have shown that administration of these bacteria as probiotics in the shrimp Litopenaeus monodon and Fenneropenaeus indicus as well as in the fish Labeo rohita led to improved growth and survival rate were improved, as well as enhancement in immunity and disease resistance (Nayak et al., 2007). Among these, Bacillus bacteria have been used widely as putative probiotics. Especially B. subtilis, a Gram positive nonpathogenic, spore-forming bacterium, is now being used for oral bacterial therapy, prophylaxis of gastrointestinal disorders, improving pond water quality and the survival of animals in aquaculture (Irene et al., 2005). In addition, the clinical effects of B. subtilis as an immunostimulatory agent in a variety of diseases have been documented (Green et al., 1999). However, studies on the use of live bacterial cells as probiotics to improve the immune system and antioxidant capacity are scarce (Rengpipat et al., 2000).
Oxidative stress results from either increased exposure or production by the organism of Reactive Oxygen Species (ROS) is also implicated in the immune response mechanism to both prokaryotic and eukaryotic pathogens (Adema, 1991). Many studies have also examined antioxidants pressures in aquatic invertebrates, but few have focused on crustaceans (Holmblad and Soderhall, 1999). With respect to shrimps, most of these studies have evaluated the effect of pollutant or environmental parameters on oxidative stress (Li et al., 2008). However, very few have reported the effect of pathogen infection on antioxidant defenses in penaeid shrimps (Hsiesh et al., 2008). However, there are very few articles reporting on the effects of dietary additives on oxidative status of cultured shrimps (Chiu et al., 2007). Hence, to investigate the suitability of synbiotics as a dietary supplement, we included it in diets at different levels and fed to larval stage of Litopenaeus vannamei.
The present study aims to evaluate the effect of dietary supplementation of synbiotics on water quality, microbial population, growth performance of larval of L. vannamei for 60 days, proximate analysis of larval extract of L. vannamei, non-specific immune response of larval extract of L. vannamei, oxidative status of larval extract of L. vannamei, cytokine gene expression of larval extract of L. vannamei and susceptibility to experimental infection by V. harveyi in L. vannamei juveniles after end of feeding (60 days).
MATERIALS AND METHODS
The study was conducted at the Private Marine Fish Farm (Medhat El-Sherif for Marine culture), at Borg-El Arab city, Alexandria governorate, Egypt, during a typical shrimp culture cycle of Litopenaeus vannamei from May to September 2018.
Ethical approval
All handlings of shrimp were directed according to the guidelines for animal care and use for scientific purposes built up by the Ethics Committee of the Faculty of Veterinary Medicine, Kafrelsheikh University, Egypt.
Experimental design
The experiment was conducted in nine indoor tanks (capacity 1000 L) for ten weeks using 9000 white shrimp (L. vannamei) in post-larval stage (PL12) with mean initial weight (0.5 ± 0.02 g/ piece). They were obtained from Barket-Ghalioun fish farm (National farm belonging to the Egyptian army), Kafr El-Sheikh Governorate. The post-larvae were acclimatized to laboratory conditions for two week to reduce stress of transfer and associated subsequent mortalities (Wabete et al., 2006); where shrimp were fed a commercial diet containing 40% dietary protein level of shrimp feed (Alar company, Egypt). After the accommodation period, shrimps of apparently uniform size were distributed into three experimental groups of 3000 post-larvae / each group allotted into three replicates of 1000 post-larvae / each replicate (experimental tanks). Salinity of all tanks was adjusted from 25‰ - 35‰ by adding sea water over a period of six hours. The first experimental group (G-I) was kept as control (feed on basal diets with continues change of water and aeration). The second experimental group (G-II) (synbiotic culture treatment) was fed on 50 % basal diets + fermented rice bran with Bacillus subtilis without change of water and high aeration). However, the last experimental group (G-III) was fed on fermented rice bran with Bacillus subtilis (without basal diets and change of water and high aeration). The experimental dietary regimens were offered at a decreasing rate of 10- I.5% of body weight adjusted weekly based on growth monitoring. Shrimp were fed to apparent satiation four times daily. Water quality parameters were measured continuously along the experiment period and kept up by siphoning out shrimp faeces and exchanging culture media at a rate of 10% daily. Each tank was continuously oxygenated by injection of high pressure air and supplied with seawater pumped.
Experimental diet preparation
Bacillus subtilis used in the system of synbiotics in the current study was commercially purchased as Sanolife PRO-W ® (INVE Company, Belgium) which contains 2×1011 CFU/gram used by 80 gm /Hectare in Shrimp culture. For the treated group, rice bran was added to water salinity 35‰ by the rate of 1:5 mixed with Bacillus subtilis and leave for 48 hours with continuous agitation and stir without aeration to fermentation. The PH was maintained to be 6-7 using sodium bicarbonate. The probiotic concentration in the feed was checked after diet formulation by counting Bacillus subtilis strains on MRS plates using serial dilution. The fermented rice mash with Bacillus subtilis was then stored in 5-litre boxes at 20°C until use.
Physico-chemical analyses of water samples
Water quality parameters were observed routinely throughout the trial time frame. Temperature and dissolved oxygen were recorded twice daily (08:00 am and 03:00 pm) with OxyGuard Handy Gamma. The pH and salinity were recorded once daily (08:00 am) with a pH meter (Hach Lange HQ 40D) and conductimetry (WTW cond 315i), respectively. Total ammonia nitrogen (NH4+-N) and nitrite-nitrogen (NO2--N) were analysed twice weekly by spectrophotometric method according to Bendschneider and Robinson (1952).
Sampling of water and bacterial quantification
Following procedures applied by Viliamil et al. (2003), rearing water samples were collected, pooled and analyzed during various growth stages along the experiment. Serial dilution and plating of samples from 10-1 to 10-7 was carried out to detect the total bacterial count (TBC) using nutrient agar (NA), and total Vibrio count (TVC) using trypticase soy agar (TSA) supplemented with 7% NaCI, as number of colony forming units per ml cfu/ml for water samples. Dominant colony types on TSA plates were visually selected on the basis of different colony appearance and abundance.
Growth performance of L. vannamei feed on synbiotics
Along the experimental work, the shrimp samples were weighted and the total weight gain (TWG), average daily gain (ADG), specific growth rate (SGR), survival rate (SR%), feed conversion ratio (FCR), and protein efficiency ratio (PER) were calculated in all groups according to El-Shinawy et al. (1999) according to the following equations:
Total weight gain (TWG) (g) Final body weight –Initial body weight (Annet, 1985).
Average Daily Gain (g/fish/day) = TWG (g)/trial period (d)
Specific growth rate (SGR % / day) = [Ln Final body weight- Ln Initial body weight] × 100/trial period (d) (Pouomonge and Mbonglang, 1993)
SR= Total number of fish at the end of the experiment × 100/absolute number of fish at the start of the experiment.
FCR= feed consumption (g)/Live weight gain. (De Silva and Anderson, 1995).
PER=Live weight gain (g)/protein intake (g). (De Silva and Anderson, 1995).
Proximate composition analysis
Initial body composition analysis was conducted using prior frozen 100 shrimp samples. At the end of the feeding experiment, 10 shrimp larvae samples from each treatment were netted, and their total weights (g) were recorded. Shrimp larvae were rinsed with sterile sea water for few seconds, ground into homogeneous slurry, oven-dried at 60 °C / 48 h, reground and analyzed for total protein, lipid, ash and carbohydrate content, moisture and fatty acid profiles following the procedures outlined by previous records (Lowry et al. (1951); Bligh and Dryer (1959); AOAC (1980, 1998); Travelyan and Harrison (1952); Jain and Singh (2000) and Folch et al. (1957) respectively).
Determination of non-specific immune response
Shrimp small sized larvae samples were collected every 2 weeks, washed in sterile seawater (SSW) three times and homogenized at a ratio of 0.10 g / 0.90 ml SSW to obtain the 10th concentration (Mathew, 1996; Leano et al., 1998). Lysozyme activity was measured by previously described method (Paglia and Valentine, 1967), in light of the ability of lysozyme to lyses Gram positive lysozyme delicate bacterium; Micrococcus lysodeikticus. Phenoloxidase activities (PO) was measured spectrophotometrically (Stat Lab, Germany) by recording the formation of dopachrome produced from L-dihydroxyphenylalanine (L-DOPA, Sigma) as previously described (Hernandez Lopez et al., 1996).
Measurement of oxidative stress parameters
Antioxidant defenses were measured by the level of production of antioxidant enzymes such as Superoxide Dismutase (SOD), Catalase (CAT) and lipid peroxidation (LP). Ten shrimps per tank, in the inter-moult stage, were randomly sampled in order to measure oxidative stress parameters. Individual shrimps were caught and homogenized. 10 μl of the extracted samples were immediately diluted in 100 μl pre-cooled Tris buffer 10mM, 1mM DTPA, 1mM PMSF, pH 7.4 and 25 μl was further diluted in 25 μl trisodium citrate buffer 30 mM, 0.34 M sodium chloride, 1 mM EDTA. Samples were then immediately frozen and stored at -80°C until assays were required to be conducted. Superoxide dismutase (SOD) activity was assayed with minor modifications according to (Peixoto et al., 2006). Nitrotetrazolium blue chloride (NBT) was used as detection molecule instead of cytochrome. Activity was reported by its ability to inhibit 50 % reduction of NBT. Catalase (CAT) activity was performed utilizing spectrophotometric assurance of hydrogen peroxide (H2O2) which frame stable complex with ammonium molybdate (Claiborne, 1985). Lipid peroxidase (Malondialdehyde assays) was determined according to the method of Abdelkhalek et al., 2015.
Cytokines expression
At the end of the feeding trial, 10 shrimp larvae samples /treatment were taken, pooled and homogenized using a glass homogenizer and stored at −80 °C (Perez-Sanchez et al., 2011). Total RNA was extracted using Trizol reagents (Invitrogen, UK) according to the manufacturer’s manual. The reverse transcription (RT-PCR) was performed as described by Chi et al. (2014) using SYBR green method in an iQ5 iCycler thermal cycler (Bio-Rad). The reactions were set on a 96-well plate by mixing, for each sample, 1 μL of diluted (1/20) cDNA, 5 μL of 2x concentrated iQ TM SYBR Green Supermix (Bio-rad), containing SYBR Green as a fluorescent intercalating agent, 0.3 μM forward primer and 0.3 μM of reverse primer. The thermal profile for all reactions was 3 min at 95 °C and then 45 cycles of 20s at 95 °C, 20s at 60 °C and 20s at 72 °C. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was included as an internal reference for normalization of gene expression data. Data analysis was conducted using the 2−ΔΔCT method (Livak and Schmittgen, 2001). Specific primers used for interleukin 4 (IL-4) was (F:5`CTATTAATGGGTCTCACCTCCCAACT`3; R:5`CATAATCGTCTTTAGCCTTTCCAAG`3), interleukin 12 (IL-12) was (F: 5`CAGCCTTGCAGAAAAGAGAGC`3; R: 5`CCAGTAAGGCCAGGCAACAT`3) and heat shock protein (HSP) was (F: 5`GTGACGCGAAGATGGACAAGTC`3; R: 5`CACCGTAAGCTACAGCCTCGTC`3).
Challenge test (experimental infection after breeding on synbiotic system)
The challenge test was conducted to study the performance of synbiotics treatment in increasing V. harveyi resistance in white shrimp. Three hundred pieces (100 pieces / group) of shrimp of L. vannamei shrimp PL75 (average weight 5.8 ± 0.17 g/piece) were used from survive samples in original experiment. They were transported from tanks and kept in full prepared glass aquaria (90 x 50 x 35 Cm) supplemented with aerated seawater (with a salinity of 35%), and acclimated in the wet lab for 1 weeks before experimentation began. Virulent live Vibrio harveyii strain used in the challenge experiment, was obtained from department of poultry and fish diseases Fac. of Vet. Alex. Univ. where it was previously isolated from diseased syndrome in private shrimp hatchery. Shrimps were challenged by immersion with 1.96×107 cfu ml-1 according to Lin et al. (2013). Settled shrimp wastes were cleaned daily by siphoned of the aquarium’s water, which was replaced by aerated water from the water storage tank. Mortality by V. harveyii infection was identified by checking the characteristic V. harveyii of infected shrimps. The mortality of shrimps was recorded daily for a period of 14 days and the dead ones were immediately removed from the experimental aquarium. The protection against V. harveyii after challenge was calculated as the relative percentage survival (RPS) (Alabi et al., 1999).
RPS = 1 - mortality of immunized shrimp (%) ÷ mortality of control (%) × 100
Statistical analysis
The obtained data were statistically analyzed using SPSS 15 statistical software and were further tested using a multiple comparison (Tukey) test at P<0.05.
RESULTS AND DISCUSSION
Physico-chemical analyses of water samples
As demonstrated in Table 1, dissolved oxygen had not shown any significant difference (p>0.05) between treatments and mean DO value was between 5.50-5.57 mg/L. PH value varied significantly (p<0.05) among the treatments and lowest pH was recorded in G-III. A significant difference (p>0.05) was observed in the TAN values among the treatments, but the highest TAN value of 1.51 mgL-1 was recorded in G-III treatment. A significant difference (p<0.05) was recorded in nitrite values with the highest value of 0.83 mg L-1 were recorded in G-III. The water temperature was recorded between 26.36-26.63°C and had not shown any significant difference (p>0.05) between the treatments.
Despite differences in water quality parameters observed in the current study among treatments, all of them were within the optimum range for L. vannamei culture (Mishra et al., 2008; Fast and Lester, 2013). Although water temperature and dissolved oxygen had not shown any significant difference between treatments; pH value was varied significantly among the treatments with lowest recorded pH value in G-III. The reduction of pH value in the synbiotic treatments may be attributed to the higher respiration rates of the heterotrophic community which increased the carbon dioxide concentration mainly in limited water exchange treatments (Wasielesky et al., 2006). Recorded pH values are partially similar to previous records (Esparza-Leal et al., 2010; Maicá et al., 2012).
Significant difference was observed in the TAN and nitrite values among the treatments with the highest values in G-III synbiotic treatment. These results may be attributed to feed decomposition and animal excretions resulting in increased ammonia, which is toxic to shrimp. Consequently, algae and heterotrophic bacteria may directly assimilate ammonia to build cellular proteins, and nitrifying bacteria can oxidize ammonia to form nitrite and nitrate (Cohen et al., 2005; Ebeling et al., 2006; Ray et al., 2010; Vinatea et al., 2010). In contrary, successful reduction of ammonia in limited/zero-exchange culture systems were demonstrated by the manipulation of the carbon to nitrogen ratio, implicating the assimilation of the inorganic nitrogen compounds and the production of microbial biomass (Avnimelech, 2009; Megahed, 2010; Vinatea et al., 2010; Gaona et al., 2011; Gao et al., 2012; Xu et al., 2013; Krummenauer et al., 2014).
Total bacterial count (TBC) and total vibrio count (TVC)
Total bacterial and total vibrio counts could be a measure to assess the health status of shrimp (Emerenciano et al., 2013). In the current study, as demonstrated in Figure 1, the total bacterial load in rearing water during larval culture recorded the highest significant value in G-III (feed on fermented rice bran with B. subtilis without basal diets and change of water and high aeration). Similarly, significant high TBC count was noted in Biofloc system; depending over the growth of heterotrophic microbes existing within the culture system (Hari et al., 2004; Azim et al., 2008). On the contrary, the total vibrio count recorded the highest significant value in G-I (feed on basal diets with continues change of water and aeration) and the lowest significant value in G-III. This might be attributed to the consumption of a large fraction of the biofloc available in the culture tank (no change of water) or changes in the composition and abundance of microbial organisms in the food chain (Emerenciano et al., 2012). These results mirror that heterotrophs dominate over vibrio in the water column in biofloc system (Ju et al., 2008). To clarify the role of synbiotic in disease perspective, a pathogen-heterotrophs interaction should be investigated. In biofloc-managed shrimp culture, concentrations increase than the normal levels (Kim et al., 2014).
Table 1: Water quality parameters (mean ± S.E) observed in the present study.
| Groups |
DO (mgL-1) |
pH |
TAN (mgL-1) |
NO2- (mgL-1) |
Temperature (°C) |
| G-I |
5.57 ± 0.08 a |
8.12 ± 0.04 a |
1.23 ± 0.02 c |
0.13 ± 0.02 b |
26.36 ± 0.12 a |
| G-II |
5.50 ± 0.13 a |
7.87 ± 0.10 ab |
1.36 ± 0.06 b |
0.72 ± 0.14 ab |
26.41 ± 0.16 a |
| G-III |
5.21 ± 0.37 a |
7.59 ± 0.14 b |
1.51 ± 0.09 a |
0.83 ± 0.19 a |
26.63 ± 0.17 a |
Results are presented as means ± SD of triplicate observations. Means in the same column with different superscripts are significantly different (P <0.05); G-I: Control (feed on basal diets with continues change of water and aeration); G-II: Feed on 50 % basal diets + fermented rice bran with B. subtilis (without change of water and high aeration); G-III: Feed on fermented rice bran with B. subtilis (without basal diets and change of water and high aeration).
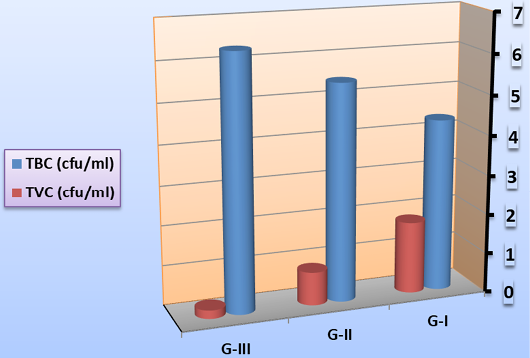
Figure 1: Mean total bacterial count and total vibrio count (cfu/ml) in rearing water during larval culture on symbiotic.
One of the guaranteed focal points of synbiotic cultures is the low occurrence of pathogenic events or of high pathogen loads in correlation with traditional cultures, perhaps in view of interspecific interactions, or of competition for substrate or essentials nutrients between pathogens and biofloc-forming microorganisms (Crab et al., 2010; Emerenciano et al., 2013). This impact might be particularly apparent when bioflocs are enriched with probiotic bacteria, resulting in increasing survival and development of V. parahaemolyticus infected L. vannamei in a BFT culture system (Krummenauer et al., 2014).
Growth performance of L. vannamei feed on synbiotics
Growth performance and feed utilization of the cultured shrimp larvae, reared in the current experiment, were significantly affected by dietary supplementation of the synbiotic compared to those of shrimp fed the Control diet (Table 2). Final body weight, weight gain and specific growth rate of shrimp fed basal diet (G-I) were significantly increased compared to those in G-II (feed on 50% basal diets + fermented rice bran with B. subtilis) and G-III (feed on fermented rice bran with B. subtilis (without basal diets and change of water and high aeration). Offering dietary basal diet at level of 40 % protein to shrimp, resulted in significantly higher protein efficiency ratio (PER) and lower feed conversion ratio (FCR) in synbiotic treated groups. Significantly higher feed intake (FI) was observed in shrimp fed the basal diet than in G-I than in G-II and G-III. The highest significant survival rate was demonstrated in G-I.
Table 2: Mean values of the growth performance of L. vannamei in the four treatments at the end of the 60 days experimental period.
| Treatment | G-I | G-II | G-III |
| Initial individual weight (g) | 0.52 ± 0.02 | 0.50 ± 0.02 | 0.50 ± 0.02 |
| FBW ± SD | 5.71 ± 0.5 a | 5.52 ± 0.4 b | 5.36 ± 0.4 c |
| WG (g) | 5.21 | 5.02 | 4.86 |
| ADG (g) | 0.087 | 0.084 | 0.081 |
| SGR (%/ day) | 4.06 | 4 | 3.95 |
| FCR | 2.69 | 2.79 | 2.88 |
| PER | 1.44 | 3.23 | 1.19 |
| SR (%) | 87.78 | 76.67 | 57.33 |
Results are presented as means ± SD of triplicate observations. Means in the same column with different superscripts are significantly different (P <0.05); G-I: Control (feed on basal diets with continues change of water and aeration); G-II: Feed on 50 % basal diets + fermented rice bran with B. subtilis (without change of water and high aeration); G-III: Feed on fermented rice bran with B. subtilis (without basal diets and change of water and high aeration).
In the current experiment, it could be noticed that offering dietary basal diet at level of 40 % protein to shrimp, resulted in significantly higher protein efficiency ratio (PER) and lower feed conversion ratio (FCR) in synbiotic treated groups. Increased protein efficiency ratio may be attributed to that utilization of synbiotics consider increasingly productive conversion of ingested food into structural protein (Hai and Fotedar, 2009). The result match with previous reports (Xu and Pan, 2013, 2014; Khanjani et al., 2016).
However, the shrimp in G-I feeding the basal diet showed better growth performance parameters compared to the synbiotic treatments (G-II and G-III). The current results are in accordance with earlier reports (Murthy and Naik, 2002). The diminished growth in synbiotic treatments might be due to poor digestion and a catabolic effect resulted from higher proportion of probiotics (Murthy and Naik, 2002). Previous studies have reported that biofloc system with addition of external carbon source improves the digestive enzyme activity improving growth of shrimps (Xu and Pan, 2012). With the use of synbiotics, it may be possible to produce greater benefits than the application of individual probiotic (Merrifield et al., 2010; Zhang et al., 2010). Other reports, in contrast to the current study, had shown improved growth in shrimp as a result of dietary supplementation with synbiotics; that might be credited to physiological and biological changes in the gastrointestinal condition, elevated digestive enzyme activities, possible improvements of intestine morphology, enhancement of the digestion and nutrient absorption of shrimp by the synthesis of vitamins, cofactors and enzyme, or via synbiotics fermentation by endogenous gut microbes to produce short chain fatty acids (Gatesoupe, 1999; Li et al., 2007; Wang, 2007; Daniels et al., 2010; Ai et al., 2011; Lesmanawati, 2013). Moreover, the synbiotics effect might also be potentially influenced by the type of species and the environment. The nature of synbiotics may be attributed to stimulation of the growth of beneficial bacteria such as lactic acid bacteria; which is beneficial residents of the fish’s intestinal ecosystem by producing bacteriocins inhibiting the growth of certain fish pathogens and thus positively affect the host’s microflora (Ringø et al., 2010). The growth performance was significantly improved by supplementing the basal diet with synbiotics in various fish species; rainbow trout (Oncorhynchus mykiss) fingerlings (Rodriguez- Estrada et al., 2009; Mehrabi et al., 2012), Zebra fish larvae (Nekoubin et al., 2012), Caspian kutum fry (Haghighi et al., 2010), Japanese flounder (Ye et al., 2011).
Proximate composition of L. vannamei feed on synbiotics
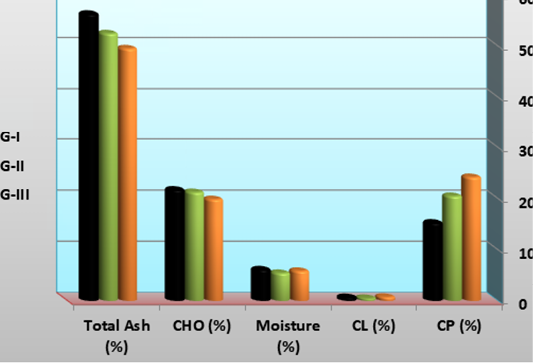
Chemical composition of the experimental shrimp larval body; crude protein (CP), crude lipid, moisture percentage, carbohydrate and ash content were summarized in and Figure 2. There was significant increase in crude protein and lipid in G-I than those of G-II and G-III. However, G-I recorded the most significant lowest values of moisture, carbohydrate and ash contents. Martínez-Córdova et al. (2014) reported that the proximate composition of synbiotic might differ according to several factors such as cultured species, aeration intensity, salinity, temperature, dissolved oxygen, carbon source, as well as microbial community developed (heterotrophic, mixotrophic or autotrophic). Higher body protein and lipid content in the fish fed the basal diets implies this fact that, the ingested food was converted more effectively into the structural protein and lipid subsequently resulted in more muscle as it is a desirable aspect in fish farming. The results disagree with previous reports where synbiotics supplementation specifically increased the carcass protein with no significant difference in lipid and ash content; in biofloc reared shrimp larvae Emerenciano et al. (2013), rainbow trout (Mehrabi et al., 2012), Japanese flounder (Ye et al., 2011) and Caspian kutum fry (Haghighi et al., 2010). Other researchers reported that biofloc could provide amino acids, fatty acids and vitamins for shrimp (Logan et al., 2010; Emerenciano et al., 2012). Increased ash content of the shrimp body composition in the present study might be attributed to continuous availability of abundant minerals and trace elements especially phosphorus from the biofloc (Xu and Pan, 2012).
Fatty acids profiles of L. vannamei feed on synbiotics
Fatty acids profiles of fresh shrimp fillets reared in the current experiment are demonstrated in Table 3 and Figures 3, 4 and 5. Shrimp in G-I, feeding on basal diets, recorded the highest levels of saturated (SFAs), polyunsaturated and monosaturated fatty acids.
It could be noticed that polyunsaturated fatty acids (PUFAs) constitute the majority of the fatty acids composition (36.23% of total fatty acids), followed by saturated (33.07% SFAs) and monounsaturated fatty acids (30.70% MUFAs). The total polygenes content including eicosapentaenoic acid 20:5 (n-3) (EPA) and docosahexaenoic acid 22:6 (n-3) (DHA) were the most prominent polyunsaturated fatty acids.
Table 3: Fatty acids profiles (%weight) of the Post-larval extract of L. vannamei feed on diet and synbiotics (mg- g-1 dry weight) at the end of the 60 days experimental period.
| Fatty acids profiles | G-I | G-II | G-III |
| C14:0 | 10.3 | 7.5 | 5.5 |
| C16:0 | 62.5 | 31.0 | 23.6 |
| C16:1n-7 | 43.1 | 28.3 | 23.6 |
| C18:0 | 1.8 | 0.5 | 0.8 |
| C18:1n-9 | 12.8 | 4.4 | 1.3 |
| C18:2n-6 | 5.6 | 4.4 | 1.7 |
| C20:4n-6 | 5.3 | 0.3 | 0.3 |
| C20:5n-3 | 45.0 | 49.3 | 43.2 |
|
Σ SAT |
74.6 | 38.8 | 29.9 |
|
Σ MUFA |
55.9 | 32.7 | 24.6 |
|
Σ PUFA |
55.9 | 53.9 | 45.2 |
|
Σ ω-3 |
45.0 | 49.3 | 43.2 |
|
Σ ω-6 |
10.9 | 4.7 | 2.0 |
| Total Fatty Acids | 186.3 | 125.4 | 99.6 |
SAT: saturated fatty acids; MUFA: monounsaturated fatty acids; PUFA: polyunsaturated fatty acids.
The improved characteristic larvae can be clarified by the contribution of natural food (microorganisms) from the synbiotic system. Under our culture conditions, we can easily understand that synbiotic potentially represents an important food source, especially for PLs and PUFAs. But it is important to note that the proximal composition or nutritional value of biofloc changes according to the culture conditions and is closely related to the microflora of the biofloc environment (Xu et al., 2012). Moreover, the outdoor tanks were exposed to daylight and the biofloc aggregate was a mixture of micro-algae and bacteria. Microalgae biomass is well known to be rich in PUFA and could be an important source of essential FA for aquatic animals (Olvera-Novoa et al., 1998).
In the current study, the total lipid content of the synbiotic was close to 7 represented by Postlarvae (NRC, 2011). FA (n-3) represented the most elevated extent of all FAs in the PLs fractions. The essential FAs for shrimps found in biofloc aggregates were dominated by EPA (10% of the dry matter), DHA (1.7% of DM) and ARA (1.5% of DM). The dietary requirements for these 3 essential FAs for P. japonicas, P. monodon and L. vannamei were found to be 0.5 to 1.1% (EPA and DHA) and 0.5% (ARA), respectively (NRC, 2011). These FAs play a crucial role in the reproduction of peneid shrimps (Coman et al., 2011). More broadly, the latter FA is believed to play an important role in reproduction in many cultured marine fish and crustacean species (Mazorra et al., 2003).
Non-specific immune response in larval extract
As summarized in Figure 6, lysozyme and phenoloxidase activities were significantly increased in G-II and G-III which is supplemented with B. subtilis in their diets (significantly higher (P<0.05) than control group). The results indicated that the immune response of shrimp was strongly stimulated by ingestion of B. subtilis. Similar results were obtained by Rengpipat et al. (2000) with Bacillus in P. mondon and by Mariel et al. (2004) with Bacillus in P. vannamei. Tseng et al. (2009) also found that shrimp fed at a higher concentration of the probiotic exhibited a significant increase in phenoloxidase activity, phagocytic activity and clearance efficiency compared with the control shrimp. The bacterial biomass mixed with feed might help in colonization of the intestine to perform competitive exclusion mechanism in shrimp (Rengpipat et al. 2000), and colonization with specific microbiota may play a role in balancing the intestinal mucosal immune system, which may contribute towards the induction and maintenance of immunological tolerance or inhibition of the deregulated responses induced by pathogens in the host (Gatesoupe, 1999).
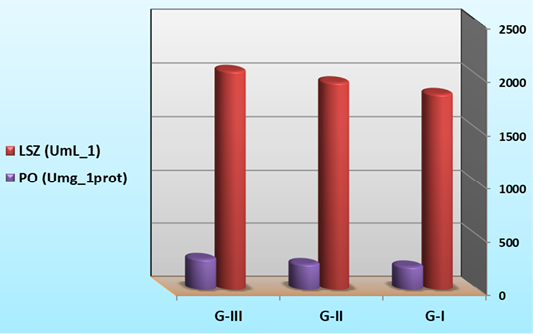
Figure 6: Immune parameters of L. vannamei feed on synbiotics at the end of the 60 days experimental period.
Lysozyme, being a cationic enzyme with antibacterial activity (Chipman and Sharon, 1969), can be elevated in fish blood by infections or invasion of foreign materials, such as immunostimulants, vaccines and probiotics (Siwicki and Studicka, 1987). For instance, Bacillus subtilis, and Lactobacillus acidophilus (Aly et al., 2008), Pediococcus acidilactici (Ferguson et al., 2010; Standen et al., 2013) supplemented to tilapia feed led to the enhancement of lysozyme activity. Similarly, both live and heat-inactivated Psychrobacter sp. resulted in significant upregulation of g-type lysozyme gene in Atlantic cod (Gadus morhua) kidney leucocytes in-vitro (Caipang et al., 2010; Lazado et al., 2010). Elevation in the lysozyme activity, following different probiotics and prebiotics, has also been recorded in snakehead (Channa striata) fingerlings (Talpur et al., 2014), common carp (Cyprinus carpio) (Jian and Wu, 2004) and large yellow croaker (Pseudosciaena crocea) (Jian and Wu, 2003). The pro-phenoloxidase system was involved in encapsulation and melanization, which functions as a non-self-recognition system (Johansson and Soderhall, 1989).
There is some information available to date regarding the interaction between synbiotics and non-specific immune response of shrimp. In the present experiment, the immune response, were significantly improved by supplementing the basal diet with synbiotics. This is in agreement with results of some studies that have revealed the effects of synbiotics in improved health status of the Japanese flounder (Paralichthys olivaceus) which was more pronounced in fish fed the synbiotics than those fed pre- and probiotics alone. Synbiotics, the combined application of probiotics and prebiotics, is based on the principle of providing a probiont with a competitive advantage over competing endogenous populations; thus, effectively improving the survival and implantation of the live microbial dietary supplement in the gastrointestinal tract of the host (Gibson and Robefroid, 1995). With the use of synbiotics, it may be possible to produce greater benefits than the application of individual probionts (Merrifield et al., 2010). According to Soleimani et al. (2012), dietary supplementation of synbiotics improved the innate immune response, stress resistance in Caspian roach fry. Stimulation of the immune response of fish through dietary supplements is of high interest for commercial aquaculture (Staykov et al., 2007). The innate immune system is very important in this regard because aquatic animals are continually vulnerable to numerous opportunistic pathogens and this part of immune response provides the first line of defense for the host (Magnadóttir, 2006). The use of natural immunostimulants is a promising area in aquaculture because they are biodegradable, biocompatible and safe for both the environment and human health (Ortuno et al., 2002). It is clear from the present study that dietary supplementation of synbiotics can modulate the innate immune responses of the shrimp L.vannamei. Similarly, Ye et al. (2011) reported that lysozyme activity was significantly higher in Japanese flounder fed a synbiotics diet (FOS + Bacillus clausii, MOS+ Bacillus clausii or FOS + MOS + Bacillus clausii) than in fish fed the control diet. Similar to our results, dietary FOS has been reported to stimulate the innate immune responses, such as serum total immunoglobulin and serum lysozyme activity in roach (Soleimani et al., 2012). The immunostimulatory nature of synbiotics may be attributed to stimulation of the growth of beneficial bacteria such as lactic acid bacteria (Zhang et al., 2010).
Oxidative stress parameter
Dietary supplementation of synbiotic by B. subtilis could affect the antioxidative parameters of shrimp extract as summarized in Table 4 and Figure 7. The SOD activities of G-II and G-III (1011 / CFUg_1 B. subtilis feed-treated) were significantly higher than G-I (feed on basal diet). There was no significant difference in the CAT activity (P<0.05) among the all synbiotic and control group. On contrary, the malondialdehyde concentrations were significantly lower (P<0.05) in the synbiotic -treated groups than the control. To our knowledge, very few studies with penaeid shrimps have reported probiotic effects on antioxidant defenses and oxidative stress status. Recently, Chiu et al. (2007) reported that Lactobacillus plantarum administered at 107 CFU.g-1 of feed was able to induce immune modulation and enhanced immunity ability of L. vannamei, increasing its resistance to V. alginolyticus infection. At this stage where further investigations on the subject are still required to elucidate the possible mode of action, it could however be hypothesized that the observed effect was as a result of the following: (i) a lower production of ROS due to a limitation of the infection level and/or (ii) a higher antioxidant status in the shrimp fed a probiotic diet. Given the observations by Chiu et al. (2007), it would be interesting to study the relationship between anti-oxidative status and immune response under the particular conditions of the current study.
The association of several biomarkers is therefore regarded as the best optional way to suitably evaluate oxidative stress status. In marine aquatic organisms, it has been reported that the main antioxidant enzymes are generally with higher activity in biotransformation organs as digestive tissues (Livingstone et al., 1992; Lemaire and Livingstone, 1993). We have also, in this study, measured CAT and SOD activity in the larval extract, since the blood cells of invertebrates are the primary effectors in host defence and are involved in various immune processes such as phagocytosis (Söderhäll and Cerenius, 1998).
Assaying of antioxidant enzymes can provide an indication of the antioxidant status of the organisms and can serve as biomarkers of oxidative stress (Kohen and Nyska, 2002). One of the most important biochemical parameters for antioxidant effects is the SOD level of tissues. SOD is an enzyme-metabolizing superoxide radical, and its level is directly related to CAT activities. Studies of the enhancement of the antioxidant ability of shrimps arising from the administration of Bacillus are scarce. In the present study, a promising antioxidative response stimulation of P. vannamei was shown with probiotic B. subtilis treatment. The superoxide anion (O2-) can react with NO to generate peroxynitrite, which may lead to the formation of hydroxyl (OH) radicals (Koppenol et al., 1992). Lipid peroxidation, considered being a complex process that is self-propagating and causes destruction, increases the rigidity of cellular membranes. The widely used assay for lipid peroxidation is MDA formation, which represents the final product of lipid peroxidation. The concentration of MDA is direct evidence of the toxic processes caused by free radicals and the MDA level was considered to be suitable indicator of the extent of lipid peroxidation (Nogueira et al., 2003). The decrease in the MDA level might be an indicator of an increase in the enzymatic and nonenzymatic antioxidants of defence mechanisms. During the present investigations, a significantly lower MDA concentration in the larval extract fed with probiotics was observed compared with the control group. The results revealed that dietary supplementation of B. subtilis increased the activities of SOD and CAT, and consequently reduced lipid peroxidation and superoxide anion in the larval extract of shrimp (P. vannamei).
Larval extract of L. vannamei exhibited SOD and CAT activities in the same range of values to the ones reported in previous studies carried out in healthy (control) shrimps of the species, P. monodon and L. vannamei (Li et al., 2008). These results clearly indicate that the healthy shrimps were not exposed to any oxidative stress resulting in detectable response(s) such as those of anti-oxidative defence systems and or specific tissue damage. Healthy shrimps fed the probiotic diet did not exhibit any obvious differences regarding SOD and CAT activity and MDA levels compared to control animals with regards to the conditions. On the other hand, the probiotic fed shrimps presented lower activities of SOD and CAT. This observation could be explained by a lower stimulation of these two antioxidant enzymes. Indeed, the reduction of antioxidant enzymatic activities were, in some cases, associated with decreased oxidative stress and free radical activities (These were based on the fact that the lower oxidative stress, the fewer antioxidant enzymes were produced (Rahmat et al., 2006). With regards to MDA, probiotic shrimps also exhibited lower levels than the controls but without any significant difference. However, we can assert that the measure of MDA, as a biomarker of oxidative damage to lipids, presents weaknesses with regard to its sensitivity, as recently reviewed by Lykkesfeldt (2007).
Cytokine genes expression assay
At the end of the feeding trial, larval extract of L. vannamei was checked for cytokine gene expression; encoding IL-4, IL-12 and HSP using RT-qPCR (Figures 8A, B and C). Results of IL-4 and IL-12 genes indicated that levels of expression of these genes were significantly decreased in G-I (feed on basal diet) compared to those G-II (feed on 50% basal diets + fermented rice bran with B. subtilis) and G-III (feed on fermented rice bran with B. subtilis without basal diets and change of water and high aeration). However, results of HSP gene recorded that G-I were significantly increased compared to G-II and G-III; where there was downregulation of HSP.
Cytokines are protein mediators produced by immune cells. They play an important role in the immune system by contributing to cell growth, differentiation and defense mechanisms (Nayak, 2010) against improper conditions that may induce stress which negatively affects fish health and performance (He et al., 2011). The expression of different types of cytokine genes in various hosts can be up-regulated by probiotics (Nayak, 2010). Of these cytokine genes, heat shock protein (HSP), in particular HSP70 gene expression has been found to be related to stressful conditions in aquaculture (Rollo et al., 2006). This gene has been generally reported to be down-regulated in fish fed probiotics; coinciding with enhanced growth rate (Avella et al., 2010b, 2011). Whole-body HSP70 expression can be significantly reduced in various fish species, including clown fish larva (Amphiprion ocellaris) and zebrafish treated with Lactobacillus rhamnosus (Avella et al., 2010b, 2012), as well as in common sole (Solea solea) treated with Enterococcus faecium IMC 511 (Avella et al., 2011). Additionally, Avella et al. (2010a) reported that gilthead sea bream larvae fed live prey enriched with probiotics (mixture of Bacillus) showed reduced level of HSP70 gene expression, which indicated a positive role of this mixture in reducing the severity of cellular stress as suggested by Aly et al. (2008). In line with these findings, the present study showed lowered HSP gene expression in larval extract of L.vannamei fed synbiotics, when compared with the control group. However, Lactobacillus fructivorans and L. plantarum succeed to induce high total body HSP70 expression in sea bream (Rollo et al., 2006). Bacillus subtilis C-3102 also lead to up-regulation in the liver HSP70 gene expression of common carp (Cyprinus carpio) at day 10, but reduced thereafter, while did not affect intestinal HSP70 expression level (He et al., 2011).
Table 4: Antioxidation factors of L. vannamei feed on synbiotics at the end of the 60 days experimental period (Umg_1 protein).
| Groups |
SOD U mg_1 protein |
CAT Umg_1 protein |
MDA Umg_1 protein |
| G-I | 11.29 ± 3.55 b | 1.00 ± 0.05 | 8.67 ± 0.81a |
| G-II | 20.41 ± 2.91 ab | 1.29 ± 0.21 | 5.71 ± 0.72 b |
| G-III | 27.34 ± 5.51 a | 1.05 ± 0.03 | 4.83 ± 0.67 b |
Results are presented as means ± SD of triplicate observations. Means in the same column with different superscripts are significantly different (P <0.05). G-I: Control (feed on basal diets with continues change of water and aeration); G-II: Feed on 50 % basal diets + fermented rice bran with B. subtilis (without change of water and high aeration); G-III: Feed on fermented rice bran with B. subtilis (without basal diets and change of water and high aeration); SOD: superoxide dismutase; CAT: catalase; MDA: malondialdehyde.
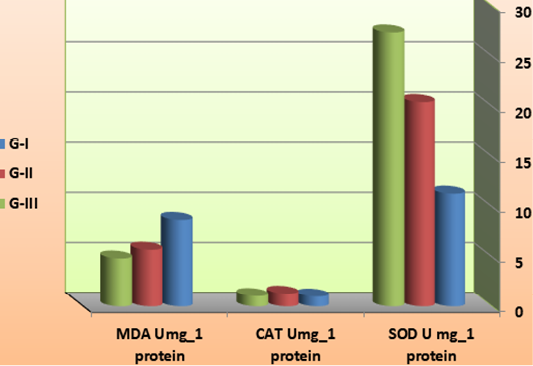
Figure 7: antioxidation factors of L. vannamei feed on synbiotics at the end of the 60 days experimental period.
Interleukin-4 (IL-4) is one of the essential cytokines in mediated immune responses. It has been shown to regulate the production of many immune cytokines, particularly in humans and mammals, while little work has been done in fish (Li et al., 2007). The present results showed that IL-4 gene expression was up-regulated in the larval extract of L.vannamei fed synbiotics compared to the control group. This finding is in agreement with the results of Perdigon et al. (2002) who confirmed that up-regulation of IL-4 was shown in mice after 5 days of oral administration of Lactobacillus delbrueckii sp. bulgaricus or Lactobacillus casei.
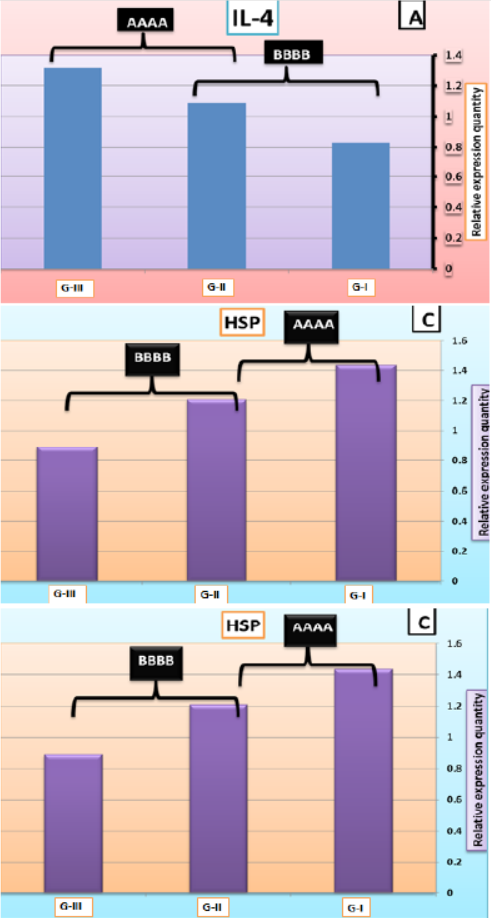
Figure 8: IL-4 (A), IL-12 (B), and HSP (C) mRNA quantification in the larval extract of L. vannamei feed on synbiotics at the end of the 60 days experimental period. Values are mean + SE, n = 3.
IL-12 is a pro-inflammatory critical cytokine, playing a major role in regulating the adaptive immune response, which coordinates the ensuing immune response (Wang and Secombes, 2013). The available information indicates that a number of probiotics can effectively modulate the secretion of IL-12 in large amounts in many animals (Von Der Weid et al., 2001; Christensen et al., 2002; Nayak, 2010). The present study showed that IL-12 gene expression was up-regulated in the larval extract of L.vannamei fed synbiotics compared to the control group. Similarly, Kato et al. (1999) reported that oral administration of Lactobacillus casei strain induced the production of pro-inflammatory cytokine IL-12 in mouse.
Challenge experiment of Vibrio harveyii
At the end of the feeding trial, shrimp larvae were challenged with V. harveyii by immersion with 1.96×107 cfu ml-1. The cumulative mortality and protective efficacy of L. vannamei was illustrated in Figure 9. Cumulative mortality was significantly increased and protective efficacy was significantly decreased in G-I (feed on basal diet) compared to those G-II (feed on 50% basal diets + fermented rice bran with B. subtilis) and G-III (feed on fermented rice bran with B. subtilis without basal diets and change of water and high aeration).
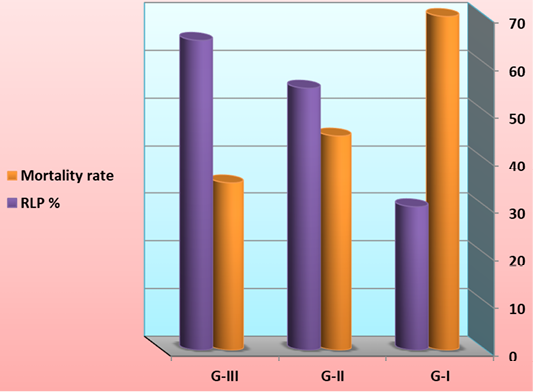
Figure 9: Cumulative mortality and protective efficacy of L. vannamei feed on synbiotics at the end of the 60 days experimental period and challenge with V. harveyii after end of the experiment by immersion with 1.96×107 cfu ml-1.
One of the claimed advantages of synbiotic cultures is the low occurrence of pathogenic events or of high pathogen loads in comparison with traditional cultures, possibly because of interspecific interactions, or competition for substrate or essentials nutrients between pathogens and biofloc-forming microorganisms (Crab et al., 2010; Emerenciano et al., 2013). This effect may be particularly evident when bioflocs are enriched with probiotic bacteria, where Krummenauer et al. (2014) found effective in increasing survival and growth of Vibrio parahaemolyticus-infected L. vannamei in a BFT culture system. It is important to provide healthy shrimp with higher production and probiotics has a great deal of potential (Gomez-Gil et al. 2000). Effects of commercial probiotic on aquaculture has been investigated by researchers, and some of these research have not shown any positive effects on growth parameters or survival rate or any promising result on the cultural condition.
Although shrimp L. vannamei growth and FCR during the 60 days of the trial were extremely good once the infection by Vibrio was noticed. We assume that the difference in shrimp survival rate between the three feeds is related to the diet composition. Preliminary information suggests that supplementation of the synbiotic may have contributed to the improved survival. Although significant reduction in mortality was noticed treatments once the shrimp were infected by the V. harveyii. This improved survival of rate of shrimp postlarvae has to be linked to their lower SOD and CAT ratio compared to the shrimps from the control. Larva of shrimp feed on synbiotics exhibited a better antioxidant status too, marked by a higher concentration is considered to be one of the most important components of the antioxidant defences of living cells, neutralizing hydroxyl radicals against which there is no enzymatic neutralization (Surai, 2002). Thus, our result indicates that synbiotics rearing acts in such a way as to increase the antioxidant status of shrimps and to reduce oxidative stress, leading to a better resistance to handling stress undergone during their transfer into the hatchery and the reproduction process (Wabete et al., 2004). Similarly, application of synbiotics was found to enhance survival of Zebrafish larvae (Nekoubin et al., 2012), rainbow trout (Mehrabi et al., 2012) and Caspian kutum fry (Haghighi et al., 2010).
The improved resistance in the present study was similar to that reported for cobia (Salze et al., 2008); White Sea bream larvae (Dimitroglou et al., 2010) and Kutum fry (Akrami, 2010). It has been suggested that greater resistance to challenges might be due to improved microvilli alignment, as has been reported in (Dimitroglou et al., 2010), which may increase the protective function of the mucin barrier and affect ion regulation (Salze et al., 2008); however, future studies are required to test this speculative hypothesis. This study corroborates the functionality of synbiotics in the diet of roach which positively affects growth performance, immune response, and beneficial intestinal microbiota and stress resistance.
CONCLUSION
From the overall data of the current study, it could be concluded that the synbiotic containing probiotic B. subtilis in shrimp (L. vannamei) diets can significantly improve the growth by enhancing the immune response (Phenoloxidase and Lysozyme) and antioxidative activity (CAT, SOD and MAD activity). Moreover, the synbiotic has positively affected water quality and biofloc composition. The concurrent increases of non-specific responses of the shrimp fed the synbiotic supplemented diets may suggest that the antioxidant defense system and innate immune system could work synergistically to improve the physiological performance of the shrimp leading to higher disease resistance against bacterial challenge. This system can play a key role in developing a sustainable aquaculture via better water quality, maintenance decrease in feed requirements and higher production to achieve more profit in shrimp farming. Consequently, the synbiotic could be practically used as a viable alternative dietary supplement.
Authors Contribution
All authors contributed equally.
Conflict of interest
The authors declare no conflicts of interest.
REFERENCES