Advances in Animal and Veterinary Sciences
Research Article
Histological and Immunohistochemical Comparison of Mature Rat Testes with Glycerol, Propanediol and Dimethylsulphoxide as Cryoprotectants
Ibtisam Khalaf Abd Ali 1, Khalid Kamil Kadhim 2*, Anam Rashid Al-salihi 3
1Department of Basic Medical Sciences, Faculty of Nursing, University of Baghdad; 2Department of Anatomy and Histology, Faculty of Veterinary Medicine, University of Baghdad; 3Department of Human Anatomy, Faculty of Medicine AL-Nahrain University, Iraq.
Abstract | Cryopreservation induced morphological changes of the testicular tissues with different cryoprotectants have been assessed in present study. The testicular tissues of thirty mature male rats (six rats for each group) were used. Group A was control (Fresh sample), samples from group B were cryopreserved using only the freezing media. The others groups were assigned to three freezing protocols using either dimethylsulphoxide (DMSO) group (C), Glycerol (D) or propanediol (PrOH) group (E) as a cryoprotectant. Light and transmission microscopic analysis were carried out on testicular tissue. Immunohistochemical analysis was used for detection of DNA damage. Normal structures were seen in the fresh control group (A), in contrast to the group (B) which showed sever cryoinjury. Different tissue changes in cryoprotectant groups, however, these alterations were vary according to the type of cryoprotectant used. The cryoinjury changes were extreme in propanediol group (E) followed by glycerol group, while the DMSO group showed lighter changes. The damages including irregularities and shrinkage of seminiferous tubules, disruption of interstitial tissue and increase in the intertubular space, abnormal arrangement of testicular cords and desquamated cells in the tubular lumen. The results of DNA fragmentation test revealed that frozen group with DMSO had the significantly lowest rate of tissue damage compared to glycerol and PrOH. These results were confirmed by the ultrastructure observations, that represented by discontinuity of the cell membrane, detachment of the spermatogonia and spermatocytes from the basement membrane and from the supporting cells, swelling of mitochondria and disruption of nuclear membrane and shrinkage of nucleus with dense clumped chromatin. In conclusion, storage of testicular tissue of mature rat using DMSO gave the best results, with little structural alterations of the tissue. Whereas the other cryoprotectant (Glycerol and propanediol) damaged DNA integrity compared to DMSO.
Keywords | Spermatogonia, Cryoprotectant, Cryopreservation, Testicular tissue
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | February 08, 2015; Revised | November 16, 2015; Accepted | November 18, 2015; Published | December 07, 2015
*Correspondence | Khalid Kamil Kadhim, University of Baghdad Iraq; Email: khalidkamkad@yahoo.com
Citation | Ali IKA, Kadhim KK, Al-salihi AR (2016). Histological and immunohistochemical comparison of mature rat testes with glycerol, propanediol and dimethylsulphoxide as cryoprotectants. Adv. Anim. Vet. Sci. 4(1): 35-45.
DOI | http://dx.doi.org/10.14737/journal.aavs/2016/4.1.35.45
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2016 Ali et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Cryopreservation of testicular tissue has become a part of gamete preservation in wild animal post-mortem. Genome resource banking is currently urged as an important tool for sustaining the germ line of endangered wild felid species (Tsutsui et al., 2003; Chatdarong et al., 2007). Testicular sperm are cryopreserved in the form of whole either testicular tissue or sperm suspension (Crabbe et al., 1999). However, freezing as a suspension is less common than freezing as tissue pieces because sperm entrapped in seminiferous tubules are difficultly isolated, resulting in lower number of sperm retrieval (Silber et al., 1995; Allan and Cotman, 1997). During freezing/thawing procedure, the extracellular space becomes hypertonic due to the removal of water as ice crystals develop. Intracellular water, therefore, moves outward across the cell membrane due to the differential osmotic gradient, and cells dehydrate and shrink. This is the opportunity when certain cryoprotective compounds come into play permeating the cells and protecting them against high solute concentration or ice crystal damage (Fuller and Paynter, 2004; Pegg, 2007). For successful cryopreservation of tissue, such as testis tissue, the majority of essential cells need to be viable for the tissue to survive and retain its function. Multiple approaches have been used to assess tissue/cell viability and extent of cryogenic injuries. These approaches commonly include histopathological examination of tissue sections for morphological changes. Using light microscopy, for instance, such objective criteria as seminiferous cord/tubular diameter or cell density within tubule cross sections can be measured, or semiquantitative morphometric analyses applied to subjectively score such criteria as health or integrity of tissue compartments (Abrishami et al., 2010; Travers et al., 2011). Transmitted electron microscopy is not widely used, but it can be invaluable in the examination of ultrastrucural components most likely to be affected by testis tissue cryopreservation, including cytoplasm integrity, nuclear membrane, and various organelles. For detection of DNA fragmentation provides insight into the extent of cell damage (Keros et al., 2007). The focus of recent studies has turned towards preserving gonadal tissue for future use (Travers et al., 2011; Yildiz et al., 2013). The objective of this study was to compare and evaluate three types of cryoprotectants protocols (DMSO, glycerol and propandiol) on the testicular tissue using rats as a model by means of light, transmission electron microscope and immunohistochemical staining.
MATERIALS AND METHODS
Animals
Thirty mature male rats (Mus Musculus) were used in this experiment. Mature fertile rats were (10-16) weeks old with 300-350 gm of weight. Animals were obtained from the Animal House at the National Center for Drug Control and Research / Ministry of Health and housed at the animal house in the High Institute of infertility diagnosis and assistant reproductive technology/Al-Nahrain University. They were kept under normal environmental condition for one week before euthanized.
Samples Collection and Processing
The animals were euthanized by exposure to high dose of ether/chloroform (inhalation). Scrotum and the testes were collected. The testes weight were recorded individually.
Experimental Design
Collected testes (n=30) from mature fertile male rats were divided into five equal groups:
The tissue samples were evaluated by light microscopy, transmission electron microscopy, and immunohistochemical staining against 8-OHdG antibody.
The Cryopreservation of testis occurs in the following steps:
- The freezing media was prepared as following:
a- The Hams F-12 culture medium Bottle (Sigma–Aldrich, N6658, USA) PH range 7.2-7.4
b- Culture media was filtered by use of 0.22 μm millipore filters (Sartorious). The cryoprotectant was added to it at Concentration 15% glycerol (BDH), 15% DMSO (Sigma) and 15%1, 2 PrOH (Merck). When using DMSO or 1, 2 PrOH as cryoprotectant the 0.1M of sucrose (BDH) was added to the solution (Hovatta et al., 1996).
c- The freezing medium was sterilized by using 0.22 μm millipore filters then U.V. light (Daihan Lab Tech).
- The 1.8 ml cryovial was filled with 1.5 ml of freezing media.
- The testis was dissected from mature fertile and immature rats under dim yellow light then it was washed by using culture media Liquid
- The samples were transported to the cryovial tubes that contain 1.5 ml of freezing media supply with one type of cryoprotectant, then the cryovials were placed in the cryovial holders and remained for about 10 minute in the refrigerator when using DMSO as cryoprotectant and at room temperature when using glycerol and 1,2 PrOH as cryoprotectant for equilibration.
- Cryovial holders that contain cryovials were suspended in the liquid nitrogen vapor for 30 minutes before plunged in the liquid nitrogen.
- After six weeks of cryopreservation. The thawing processes to the sample was done through rapidly transfer cryovial from liquid nitrogen to the water bath (Kotterman) 37ºC until melting ice, for at least 5 minutes. The cryoprotectants were removed from sample by descending concentration gradually (10%, 5% of 0.05 M sucrose when use glycerol as cryoprotectant, and 10% of 0.05 M sucrose when use DMSO or PrOH. Finally the samples washed by culture media only for about 5 minute for each concentration.
Light Microscopy
The specimens were fixed in 10% neutral buffered formalin for overnight and processed with the routine histological procedure for dehydration, clearing, impregnation with paraffin and blocking, sectioning at (5-6 μm) thickness, finally, the sections were stained with H&E (Bancroft and Stevens, 1982).
Slides were examined under light microscope for evaluation of the histological changes during the experiment. The slides were photographed by light microscope provided with TV-Based computer.
Transmission Electron Microscopy
The specimens were fixed in 4% gluteraldyhyde post-fixed in 1% osmium tetroxide and processed with the routine histological procedure for transmission electron microscope (Shaklai and Tavasoli, 1977). The sections (5-6 μm thickness) were analysed using a transmission electron microscope Philips CM-100.
Immunohistochemical Detection of 8-OHdG
Procedure of Immunohistochemistry: Monoclonal mouse specific HRP/DAP kit (abcam, Code ab64259, manufactured by Santa Cruz Biotechnology) is recommended for detection of 8-OHdG by immunohistochemistry was used. Immunohistochemical detection of 8-OHdG was done for fixed paraffin sections followed the procedure used by Ishikawa et al. (2007).
Assessment of Immunohistochemical Staining: Images were captured using microscope contain TV-Based computer, at least ten images were captured for each sample. Assessments of immunohistochemical staining were done by using Aperio positive pixel count algorithms to analyse digital slides. This algorithm has a set of default input parameters when first selected. These inputs have been pre- configured for brown colour quantification in the three intensity ranges (weak, positive, and strong). The algorithm is applied to an image by using Image Scope software v10 (Aperio).
Statistical analysis: The mean difference between groups was performed using one way ANOVA test, through SPSS version 20.
RESULTS AND DISCUSSIONS
Histological Observation
The paraffin section of testis in control group (A), showed normal tissue morphological and structural, the seminiferous tubules with normal arrangement surrounded by collagenous connective tissue (tunica albuginea) with myoid cells. The interstitial irregular connective tissue that separated seminiferous tubules was highly vascularized and contained large polyhedral cells (leydig cells) with eosinophilic cytoplasm and spherical nucleus. The seminiferous epithelium composed of spermatogonia, spermatocyte, spermatid and supporting (Sertoli) cells. Numbers of spermatozoa in the central lumen of seminiferous tubule were also observed (Figure 1 A). Sertoli cells (Sustentacular) they were tall columnar cells, that their apical membranes reached the lumen. The cells located at the base of the epithelium and they had large, basal, oval pale nucleus, but these cells were relatively few in numbers. The seminiferous epithelium further contains cells undergoing spermatogenesis; the least mature cells (spermatogonia) were located at the base of the epithelium, the A spermatogonia were dome-shaped cells. They have flattened, oval nuclei
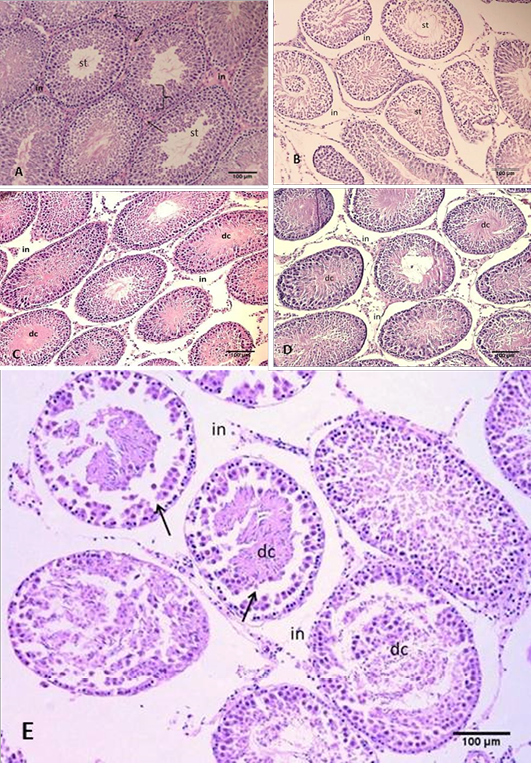
Figure 1: Section of the testis
(A) Control group, showing normal arrangement of the seminiferous tubules, seminiferous epithelium (arche), interstitial connective tissue (in) with leydig cells (arrows) and spermatozoa in the tubal lumen (st); (B) Group B using freezing media only, showing irregular arrangement and shrinkage of the seminiferous tubules. Notice intertubular space and gaps between seminiferous tubules; (C) Group C cryopreserved with DMSO, showing space in the interstitial connective tissue and gaps formation (in), desquamated cells (dc) inside tubular lumen; (D) Group D cryopreserved with glycerol, showing the damage of the interstitial connective tissue and gaps formation (in), desquamated cells (dc) inside tubular lumen; (E) Group E cryopreserved with 1,2 PrOH, showing sever disruption of the interstitial connective tissue, notice; Clear gaps between seminiferous tubules (in), detachments of the spermatogonia from the basement membrane (arrows), desquamated cells within tubular lumen (dc). H&E
stains darkly (type A dark spermatogonia) or lightly (type A pale spermatogonia). Type B spermatogonia have dark round nuclei. More matured cells (spermatocytes), at the middle zone of the germinal epithelium there were largest cells of the seminiferous epithelium (primary spermatocytes). While the secondary spermatocytes were relatively smaller than the primary spermatocytes, they lie nearer the lumen. The spermatids were spherical, polygonal or elongated cells towards the lumen, and the most mature cells, the spermatozoa were situated at the lumen (Figure 2 A).
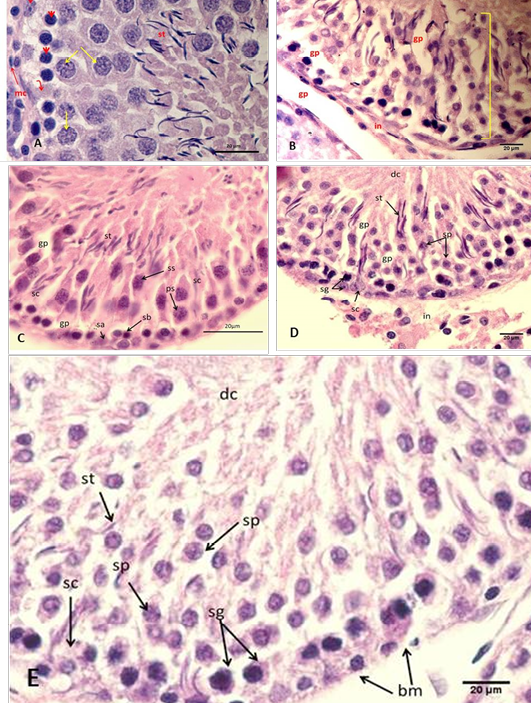
Figure 2: Section of the testis
(A) Control group A, showing normal arrangement of testicular cord, Sertoli cell (arched arrow), spermatogonia type A (elbow arrow), spermatogonia type B (head arrow), spermatocytec (yellow arrows), myoid cell (mc) with and spermatozoa (st); (B) Group B using freezing media only, showing irregular arrangement of the seminiferous epithelium (arch). Notice: disruption of interstitial tissue and gaps between cells (gp), desquamated cells (dc); (C) Group C cryopreserved with DMSO, showing detachment of epithelia of the testicular cord with each other, where spermatogonia A (sa), spermatogonia B (sb), Sertoli cell (sc), primary spermatocyte (ps), secondary spermatocyte (ss), spermatozoa (st), gaps between cells (gp); (D) Group D cryopreserved with glycerol, showing detachment of epithelia of the testicular cord with each other; (E) Group E cryopreserved with 1,2 PrOH, showing sever detachment of epithelia of the testicular cord with each other and disruption of the basement membrane (bm), where spermatogonia (sg), Sertoli cell (sc), spermatocyte (sp), spermatozoa (st), desquamated cells (dc). H&E
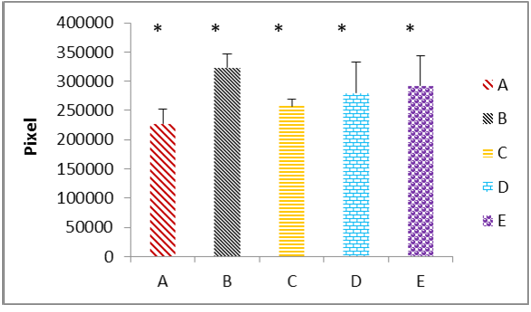
(A) the fresh control group; (B) group cryopreserved with freezing media only; (C) group cryopreserved with DMSO; (D) group cryopreserved with glycerol; (E) group cryopreserved with propanediol. The data represented as mean ± SD, (*) mean significant different at (P ≤ 0.05).
These normal histological observations of the testes were in agreement with Cartner and Hiatt (2007), Naraghi et al. (2010), Murdakai et al. (2011) and Nosseir et al. (2012) in rat.
In present work, after six weeks of cryopreservation, the paraffin sections of testis stained with H&E stain showed morphological and structural alteration in seminiferous tubule and the component of interstitial tissue. However, these alterations differ according to the type of cryoprotectants that used. Zdravkovic et al. (2006) and Thuwanut and Chatdarong (2012) have been reported that freezing retards autolytic morphological and histological changes caused by autolytic enzymes released from lysosomes. The results in this study showed that the main prominent histological changes observed in sections from group (B) that used only freezing media were the seminiferous tubules presented irregularities and shrinkage, severe disruption of interstitial tissue and increase in the intertubular space. Severe rupture of the layers of the connective tissue fibers was also observed (Figure 1 B). Furthermore, there were characteristic gaps within the seminiferous epithelium due to the cytoplasmic shrinkage. The testicular cords showed severe detachment of the spermatogonia and spermatocytes from the basement membrane and from the supporting cells. In addition the lumen of the seminiferous tubules contained desquamated cells as noticed in (Figure 2 B) that was not seen in the control group. These observations after freezing /thawing of testicular samples were in line with Jezek et al. (2001) who showed that freezing-thawing procedure had no significant effect on tubular diameter; however, it caused a `folding’ of the lamina propria and notable damage to Sertoli cells and spermatogenic cells that displayed occasional nuclear damage, vacuolization, and shrinkage/swelling of the cytoplasm. Regards this phenomena, Fuller (2004) has been reported that during freezing samples the extracellular ice formation causes elevated solvent concentrations, this leads to cell dehydration; which can permanently damage cell membranes. It’s concluded that the effects of freezing on the live cells are explained in two main theories, One emphasizes is the direct mechanical puncturing of the cell membranes by ice crystals. And the other is the effects of osmotic changes due to ice formation (Mazur, 1999; Pegg, 2007).
According to the histological observation of group (C) samples that cryopreserved by DMSO as a Cryoprotectant, there were no major differences compared with control group (A). These samples contain less damage. They were well-preserved testicular tissue. The main prominent histological changes observed after cryopreservation by DMSO were mild disruption of interstitial tissue and mild increase in the intertubular space (Figure 1 C). Furthermore, the testicular cords showed mild detachment of cells from the basement membrane and contained less desquamated cells confined to the center of the testicular cords. However, the other cells components seemed well preserved as noticed in (Figure 2 C). Bagchi et al. (2008) have been suggested that some cryoprotectants such as DMSO inhibit nucleation by increasing the high viscosity of intracellular water. In contrast, DMSO had a deleterious effect on adult rat testes as reported by Unni et al. (2012).
In the present study, the paraffin sections obtained from group (D) that tissue cryopreserved by glycerol as cryoprotectant showed moderate morphological and structural changes. These changes characterized by moderate disruption of interstitial tissue between the layers of the connective tissue fibers, some of the seminiferous tubules presented irregularities and shrinkage (Figure 1 D). Furthermore, detachment of spermatogonia from the basement membrane and desquamated cells in the lumen of seminiferous tubules were observed (Figure 2 D). On the other hand, Abrishami et al. (2010) have been concluded that glycerol was a better cryoprotectant for pig tissues. These differences may be related testicular architecture, morphology, or lipid composition according to the suggestion of Keros (2007). However, Fuller (2004) has been reported that none permeating cryoprotectant, increase and promote cellular dehydration by increasing the extracellular solute concentration thereby reducing intracellular crystallization.
Based on our observations, group (E) that tissue samples subjected to 1, 2 PrOH as Cryoprotectant, they displayed the severest morphological and structural changes. There were shrinkage and irregularities of seminiferous tubules in addition to severe disruption of interstitial tissue and increase in the intertubular space (Figure 1 E). Furthermore, typical changes were observed in cell to cell connections between the seminiferous tubules epithelia, mainly in the basal compartment and destruction of the basement membrane in addition to severe detachment of spermatogonia (Figure 2 E). Woods et al. (2004) had been reported that tissue freezing is a cryobiological challenge due to several cell types in the tissue and is influenced by cell-to-cell interaction that limit cell functional integrity. However, these different results of the PrOH compared with the other
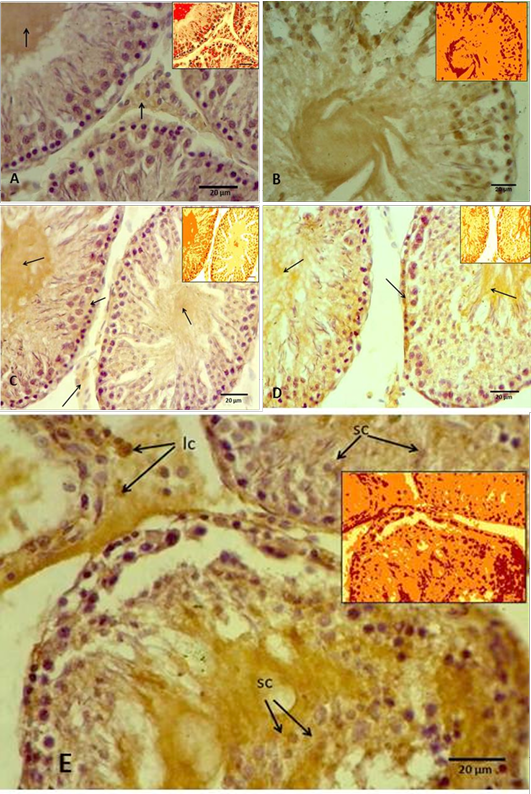
(A) Control groups A, showing slight antigen-antibody reaction as a brown area (arrows) in the interstitial connective tissue and in the tubular lumen, while the spermatogenic cells showing negative reaction. Inset showing Aperio positive pixel jmage; (B) Group B, showing intensely positive staining as a brown area in the most of seminiferous tubule components as a positive reaction. Inset showing Aperio positive pixel jmage; (C) Group C, showing weak positive signals for anti-8-OHdG antibody reaction (arrows). Inset showing Aperio positive pixel jmage; (D) Group D, showing intensely positive staining for anti-8-OHdG antibody reaction in the basal layer of the germ cells and the lumen of the seminiferous tubule (arrows). Inset showing Aperio positive pixel jmage; (E) Groups E, showing strong positive signals for anti-8-OHdG antibody reaction in the germ cells, including spermatogonia and spermatocytes (sc), and even leydic cells (lc) in the interstitial connective tissue. Inset showing Aperio positive pixel jmage.
cryoprotectants may be belong to its biophysical properties and low permeating to the cells and protecting them against high solute concentration (Bagchi et al., 2008).
Immunohistochemical Observations
Immunohistochemicall procedure were detected the damage in different types of testicular cells that caused by cryopreservation. The results revealed that the positive reaction of 8-OHdG was the nuclei which stained almost homogeneously, the pattern of stain is diffuse and color of stain is grey to brown. The cells labelled by the antibody display a staining almost entirely confined to the nucleus and with a diffuse pattern. Rarely cytoplasmic staining is observed. In this study appeared that the rat’s testicular sections of control and cryoprescrved testes were stained with anti-8-OHdG antibody, and in both sections showed variable distribution of 8-OHdG, but with the cryopreserved group displaying significantly higher staining compared to the control group. The statistical analysis to these sections through the Aperio positive pixel count algorithms showed significantly (P≤ 0.05) higher value in the group (B) compared with the other groups. In contrast, group (C) represented significantly (P≤ 0.05) lower values than cryoprescrved groups (D and E). Whereas the control group (A) has been shown the lowest value at all as illustrated in Figure 3.
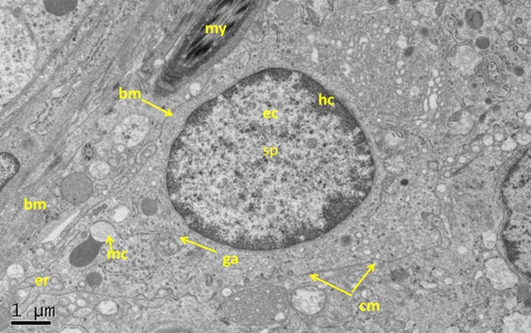
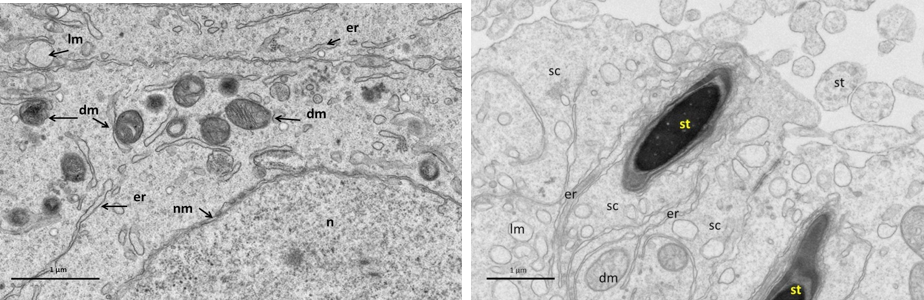
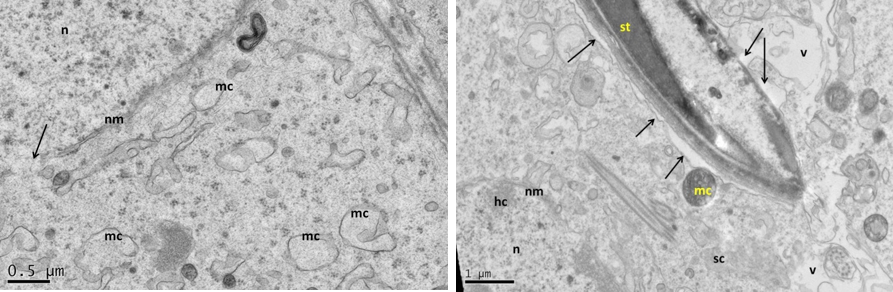
Figure 5: Electron micrograph (TEM) of rat testes from fresh control group (A) Showing (Left) normal cellular components
Notice intact cytoplasmic membrane (arrows), where light spermatogonia A (nsa) with dome shaped nucleus sit on the basement membrane (bm), B spermatogonia (nsb) with rounded nucleus, nucleus of sertoli cell (nsc). (Right) dark spermatogonia A (sp) rounded nucleus (with intact nuclear membrane) resting on the basement membrane where the normal myoid cell (my) underneath, golgi apparatus (ga), peripherally heterochromatin (hc), euchromatin, cytoplasm membrane (cm), mitochondria (mc).
In control group (A), where the tissue sections showed normal morphological and normal components of seminiferous tubule, there were weak positive signals for anti-8-OHdG antibody were detected. However, the positive signals showed variable intensity, weak positive signals appeared in the centre of the seminiferous tubule and around numerous germ cells. The positive signals increased intensity in the interstitial tissue, whereas no positive signals were observed in nucleus of the germ cells (Figure 4 A). In contrast, the results of group (B) that samples were preserved using only freezing media, the sections showed intensely positive staining, these include the most of the seminiferous epithelium (Figure 4 B). However, the spermatogonia and spermatid seemed more resistance to the cryoinjury effects. Thuwanut and Chatdarong (2012) have been reported that freezing of the testicular samples lead to karyolysis in spermatocyte and cell desquamation in the tubules lumen.
In cryopreserved group, the tissue sections displaying significantly higher staining intensity compared to the control group (A), vary according to the type of the cryoprotectant that used. Tissue sections obtained from the samples cryopreserved by DMSO as cryoprotectant showed varying degrees of positive intensity for 8-OHdG in the seminiferous tubules and the interstitial tissue of the testis. The weak positive signals appeared around the germ cells and in the interstitial tissue of the testis. While the nucleus of the germ cells, the spermatocyte and spermatid showed negative reaction. However, the centre of the tubules appeared more intensely positive, and some of the primary spermatocyte in basal layer had weak positive signals (Figure 4 C). Thuwanut and Chatdarong (2012) have been showed that Dimethyl sulphoxide is providing a greater protection of DNA integrity than other cryoprotectant in testicular tissue. Because it has low molecular weight and high tissue penetration offering better results than PrOH (Yildiz, et al., 2013). Penna et al. (2012) have been shown that one of the changes is retraction of apical processes of Sertoli cells which affects specialized intercellular junctions and this explained releasing the germ cells to the tubular lumen. While in present work, tissue samples subjected to glycerol as cryoprotectant displayed greater intensity of the positive signals compared with the previous groups. These strong positive signals concentrated mainly in the nucleus of the secondary spermatocyte cells and in the interstitial tissue of the testes. In addition, weak positive signals distributed among the germ cells and in the lumen of the seminiferous tubules (Figure 4 D). This agrees with previously reported data supporting that glycerol had the lowest rate of tissue survival compared to DMSO (Yildiz et al., 2013).
Whereas in this study, tissue samples cryopreserved by 1, 2 Pr OH showed stronger positive signals in both seminiferous tubules and the interstitial tissue more than in control, DMSO, and glycerol group (Figure 4 E). Generally the spermatogonia and spermatid seemed more resistance to the cryodamage than the other types of cells. Jezek et al. (2001) have been reported that uses the cryoprotectant
Near nucleus (Left), and at its luminal processes (Right) showing; normal cellular components. Notice intact nuclear membrane (nm) around normal distribution of euchromatin (n), normal junction between sertoli cell (sc) and developing spermatid (st) within sertoli cell processes, normal light mitochondria (lm) and dark one (dm), endoplasmic reticulum (er).
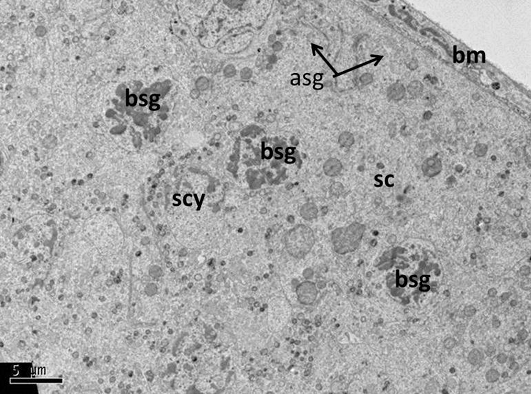
Figure 7: Electron micrograph (TEM) of rat testes from group (B) cryopreserved with freezing media only showing sever cellular changes
No clear demarcation between cells due to damage of cell membrane, the cells nuclei had dense clumped chromatin. Where A permatogonia (asg); B spermatogonia (bsg); Sertoli cell (sc); basement membrane (bm).
medium may be not optimal for all types of cells. The optimal conditions to protect a particular cell will also vary and depends on factors such as the molecular weight and permeability of the cell membrane to the compound. Thus an optimal cryoprtectant agent should be defined for each cell type separately. However, it is difficult to find the optimal cryopreservation conditions for all testicular tissues.
Ultrastructural Observation
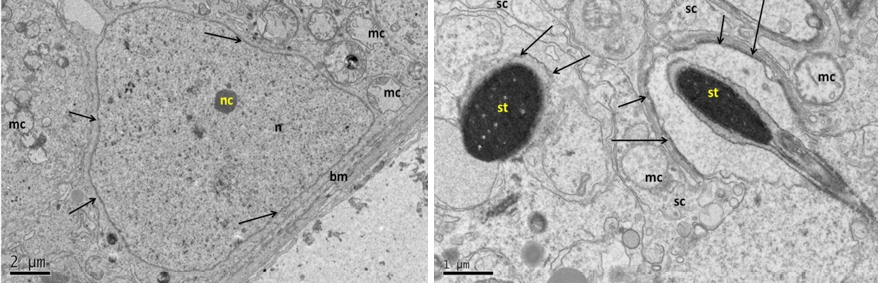
Generally, the ultrastructural analyses of testicular sections of the control group (A) were evaluated as being structurally normal interstitial tissue between the seminiferous tubules, myoid cells, and normal arrangements of the cellular components. Two types of A spermatogonia were recognized, the light one (light dome shaped nucleus) and dark one (round dark nucleus) (Figure 5). Clear attachments of spermatogonia and sertoli cells to the basement membrane were observed. Also cell-to-cell connections were well preserved between spermatocytes or spermatid with sertoli projections. Good integrity of the cytoplasm membrane and normal distribution of the cytoplasmic organelles including the Golgi apparatus, rough endoplasmic reticulum and the size and shape of mitochondria and its cristae. There were clear dark and light mitochondria in the cytoplasm of the sertoli cell. Integrity of the nuclear membrane, the nuclei were regular-shaped, normal morphology of euchromatin and heterochromatin, in addition to the normal nucleolus (Figure 5, 6). These normal ultrastructural features of the testicular tissue were in line with previous study in rat testes (Shokri et al., 2012). However, the presence of various sizes lipid droplets as a lighter spots in the cytoplasm of murine Sertoli cells that reported by Chui et al. (2010) were not detected in present study. Otherwise, sections belong to group (B) demonstrated major types of cryoinjury after freezing-thawing procedure. It caused notable damage to Sertoli cells, spermatogonia and spermatocytes. The damage includes intercellular junctions also. In this group, the cells loss greater part of the cell membrane and replaced by huge gaps between adjacent cells. The mitochondria showed severe swelling and there were no cristae recognized inside it. Presences of cytoplasmic vacuolation were clearly observed in this group. Greater part of the nucleus membrane had also showed severe disruption. Dense clumped chromatin of the nucleus and missing its marginal arrangement. In addition to the dense clumped nucleoli (Figure 7, 8). Penna et al. (2012) suggested that these changes correspond to metabolic alterations of the Sertoli cell. Whereas Jezek et al. (2001) have been shown that spermatogonia after thawing were showed shrinkage due to osmotic damage that lead to loss of
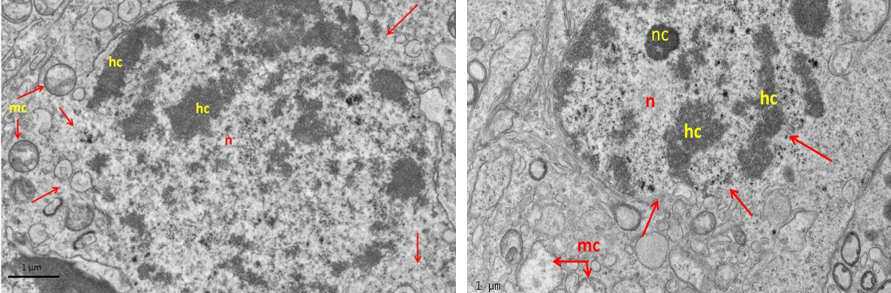
Figure 8: Electron micrograph (TEM) of spermatogonia (Left) and spermatocyte (Right) from group (B) cryopreserved with freezing media
It shows sever cryo-injury of cell components represented by dense clumped chromatin (hc) of the nucleus (n); disruption of the nuclear membrane (arrows); and swelling of the mitochondria (mc).
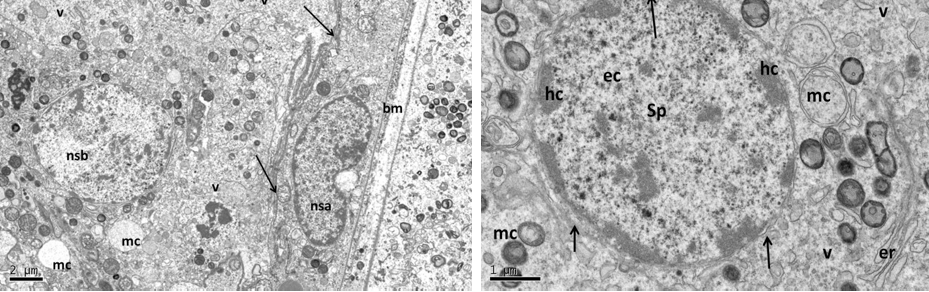
Figure 9: Electron micrograph (TEM) of rat testes from group (C) cryopreserved with DMSO showing (Left) Light cellular changes
Notice intact cytoplasmic membrane (arrows), where light spermatogonia A (nsa) with dome shaped nucleus (intact nuclear membrane) sit on the basement membrane (bm), B spermatogonia (nsb) with rounded nucleus, vacuolated cytoplasm (v). (Right) spermatogonia B (sp) rounded nucleus, intact nuclear membrane (arrows), peripherally heterochromatin (hc), euchromatin, mitochondria (mc).
At the cell base (Left) showing; intact nuclear membrane (arrows) around normal distribution of euchromatin (n), with small nucleolus (nc), intact basement membrane (bm),and swelling mitochondria (mc) the (cristae in some of them were lost). At the apex of sertoli cell (Right photo) showing the luminal processes of sertoli (sc), surrounded the developing spermatid (st), notice normal junction between them (arrows).
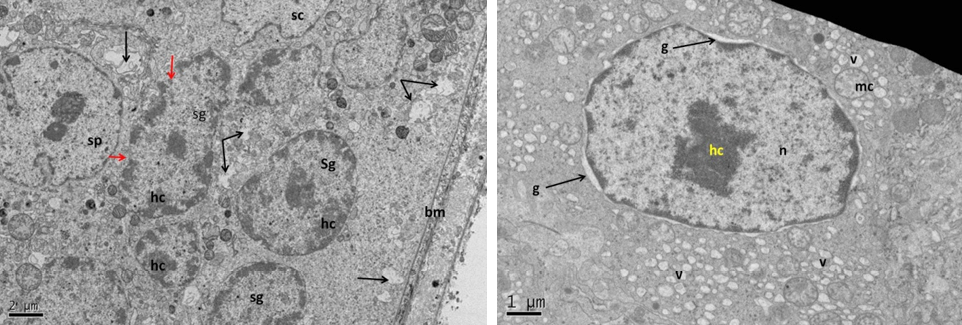
Figure 11: Electron micrograph (TEM) of rat testes from group (D) cryopreserved with glycerol showing (Left) cellular changes
Notice vacuolation of the cytoplasm (arrows), shrinkage of the nucleus of spermatogonia (sg), spermatocytes (sp) and Sertoli cell (sc), where heterochromatin had clumping appearance (hc). (Right) spermatogonia showing shrinkage nucleus (n), with clumped chromatin (hc), surrounded by gap (g), swelling mitochondria (mc), vacuolated cytoplasm (v) and no clear cell membrane.
At the cell base (Left) showing; nucleus of Sertoli cell (n); nuclear membrane (nm) with disruption area (arrow), notice destruction of the mitochondria. The same cell (Right photo) showing detachment of the developing spermatid (st) with Sertoli cell processes (arrows), vacuolation of the cytoplasm (v), dense clumping heterochromatin (hc) in the nucleus (n).
membrane integrity which followed with cell membrane damage.
Sections belong to group (C) showed changes at the level of cell components of the epithelium of the seminiferous tubules, but it seemed minor changes compared to group (B). These changes were represented by swelling of the mitochondria. However, some of these had clear cristae. No changes in the cell membrane and cell to cell connection but there was vacuolation of the cytoplasm. In addition to light clumped chromatin in the nucleus. However, they showed mostly normal distribution. Normal nuclei shape with nuclear intact membrane, particularly in spermatid that showed normal appearance (Figure 9, 10). In contrast to the earlier study Unni et al. (2012) who reported that both spermatogonia and spermatid but not spermatocytes are best preserved against cryodamage by DMSO.
The ultrastructural analyses of samples cryopreserved by glycerol (group D) showed greater changes at the level of cell components compared with the group (C). These changes were represented by discontinuity of the cytoplasmic membrane and clear empty space between neighboring cells. Swelling of the mitochondria and vacuolation of the cytoplasm were seen. Abnormal nuclei shape with interrupted nuclear membrane and mild clumped chromatin (Figure 11, 12). Vacuolization in the cytoplasm of the spermatogenic cells has been reported as in the present work as a type of injury resulting from cryopreservation and it’s an early sign of testicular damage (Penna et al., 2012). However, Unni et al. (2012) have been shown that glycerol is preserved the integrity of the spermatid but not the other cells.
The sections cryopreserved by propandiol (group E) were
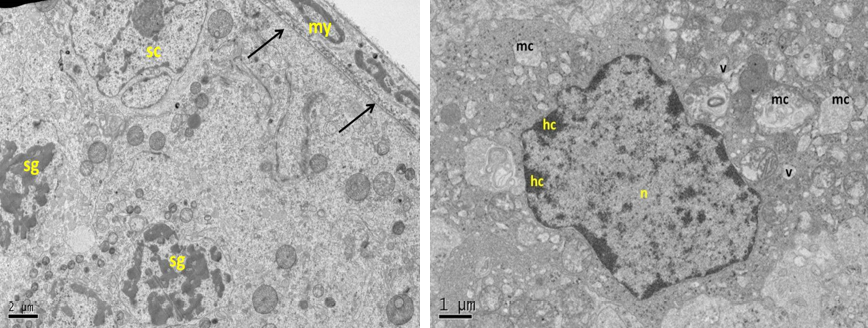
Figure 13: Electron micrograph (TEM) of rat testes from group (E) cryopreserved with 1, 2 prOH showing (Left) cellular changes
Notice sever damage to the cell membrane (no clear demarcation between cells); shrinkage of the nucleus with clumping chromatin of spermatogonia (sg), and Sertoli cell (sc), basement membrane (arrows) and intact myoid cell (my). (Right) spermatogonia showing shrinkage nucleus (n), with clumped chromatin (hc), swelling mitochondria (mc), vacuolated cytoplasm (v) and no clear cell membrane.
subjected to severe ultrastructure cellular changes when compared with the previous cryopreservative groups, including the sertoli cells and the other spermatogenic cells. These changes were represented by disruption of the cytoplasmic membrane, loss connection between neighboring cells and swelling of the mitochondria. The nuclei were shrinkage with severe interrupted nuclear membrane with dense clumped chromatin (Figure 13). Fuller (2004) and Fuller and Paynter (2004) have been shown that the degrees of cryoprotection are varying in different cell types depending on the cryoprotectants properties and those of cell membranes. The side effect of the cryoprotectant is additional cytotoxic effects. Thus, tissue tolerance to cryoprotectants is limited and overexposure may cause damage (Pegg, 2002).
We have demonstrated that dimethylsulphoxid is well cryoprtatent agent for testicular tissue of mature rats compared with glycerol and propanediol that tissue showed more sensitivity to them. However, that even if many cells showing light changes after cryopreservation with DMSO, preservation of all tissue components is not guaranteed.
ACKNOWLEDGEMENTS
We gratefully acknowledge the University of AL-Nahrain /Faculty of Medicine for their help by providing us the lab animals and histological tissue processing which enabled this work to be carried out.
Conflict of interest
The authors declare that they have no competing interests.
Authors’ Contribution
Khalid K Kadhim: planning for work, follow- up workflow, Reading and writing results.
Ibtisam k Abd Ali: Collection of tissue sample, sample processing and lab technique.
Anam R Al-salihi: assist in the part of lab work and reading result
REFERENCES