Advances in Animal and Veterinary Sciences
Research Article
Tocotrienol and Yeast Selenium Improve the Adverse Effect of Florfenicol in Broiler Chickens
Hosny Abdelfadil Ibrahim1, Mohamed Hasan Khairy1, Ali Mohamed Asy2, Eman Abdelmoneim Abozeid2*
1Department of Pharmacology, Faculty of Vet. Medicine, Zagazig University, Zagazig, Egypt; 2Department of Biochemistry, Animal Health Research Institute, Benha-Branch, ARC, Dokki, Giza, Egypt.
Abstract | Prolonged use of florfenicol can lead to detrimental side effects in poultry. This work focuses on the role of tocotrienol and yeast selenium to mitigate these side effects in broilers’ kidneys. One hundred and fifty, apparently healthy, one-day-old, unsexed broiler chicks were used, fed on a well-balanced ration throughout the experimental period. Chicks were divided into five equal groups, 30 chicks each. Group (1) control group chicks fed to a balanced diet only. Group (2) Chicks treated with florfenicol (20 mg /kg BW, in drinking water) per bird for 3 successive days and the florfenicol was administered in other groups by the same dose and for the same period of time. Group (3) chicks treated with florfenicol and tocotrienol (170 mg/kg BW, orally) for 7 successive days. Group (4) Chicks treated with florfenicol and yeast selenium (0.15 mg /kg BW, orally) for 7 successive days. Group (5) Chicks treated with a combination of florfenicol, tocotrienol and yeast selenium. Blood samples and the kidney of chicks were collected on the 18th, 25th and 35th day of age. Chickens treated with florfenicol exhibit an increase in renal malondialdehyde (MDA), as well as a decrease in renal superoxide dismutase (SOD) and reduced glutathione (GSH). Tocotrienol and yeast selenium decrease the MDA and increase SOD and GSH in renal tissue. Tocotrienol returns serum uric acid to its normal level. Tocotrienol and yeast selenium don’t not significant change the growth performance parameters in broilers. A combination of tocotrienol and yeast selenium improved the renal histopathological changes caused by Florfenicol. It was concluded that tocotrienol and yeast selenium alone or their combination improve antioxidant effects and mitigate lipid peroxidation in broilers’ kidneys Therefore, tocotrienol and yeast selenium can improve the safety of using florfenicol in broilers.
Keywords | Tocotrienol, Yeast selenium, Florfenicol, Antioxidant, Kidney, Broilers.
Received | April 18, 2021; Accepted | May 14, 2021; Published | July 15, 2021
*Correspondence | Eman Abdelmoneim Abozeid, Department of Biochemistry, Animal Health Research Institute, Benha-Branch, ARC, Dokki, Giza, Egypt; Email: eman.abozeid89@gmail.com
Citation | Ibrahim HA, Khairy MH, Asy AM, Abozeid EA (2021). Tocotrienol and yeast selenium improve the adverse effect of florfenicol in broiler chickens. Adv. Anim. Vet. Sci. 9(8): 1275-1282.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.8.1275.1282
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Abozeid et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Redox reactions (reduction-oxidation) are processes in which an electron or hydrogen atom is transferred from one cell to another (Pamplona and Costantini, 2011). Reactive oxygen species and reactive nitrogen species induce lipid peroxidation of lipid cell
membranes and lipoproteins because they are highly containing polyunsaturated fatty acids (El Sebaei et al., 2021). Florfenicol is a broad-spectrum synthetic antibacterial agent and it is one of the amphenicol families used in poultry farms for the treatment of most gram-positive and gram-negative bacteria (Tavakkoli et al., 2013). Florfenicol inhibits protein synthesis by binding to the 50S subunit ribosome (Dowling, 2013). Florfenicol produced oxidative stress effect on chick’s kidney through inhibition of the expression of related factors in Nrf2-ARE which has a great role in the prevention of oxidative stress (Wang et al. 2020).
Antioxidants are molecules that may protect cells from the damage caused by unstable molecules known as free radicals (Hamid et al., 2010). Natural (biological) antioxidants are categorized as a water-soluble antioxidant (hydrophilic) as vitamin C, glutathione, lipoic acid and uric acid and fat-soluble antioxidant (hydrophobic) as vitamin E, carotenoids and coenzyme Q. Water-soluble antioxidants catch free radicals present in the blood plasma and cell cytosol, while lipid-soluble antioxidants prevent lipid peroxidation to protect cell membranes (Surai, 2007). Tocotrienol is a strong antioxidant, with an unsaturated isoprenoid chain, it can protect cytochrome P-450 from oxidative damage 6.5 times more than tocopherol and it is the main component of palm oil (Serbinova et al., 1991). Tocotrienol induces Nrf-2 and HO-1 against damage effect occurred by H2O2 in case of oxidative stress and Nrf-2 which has a great role to regulate cellular antioxidant defense mechanism (Tan et al., 2015). Tocotrienol decreases the harmful effect on the proximal renal tubule by elevation reduced glutathione (GSH) level in the renal cortex, improving reabsorption function and lowering renal lipid peroxidation (Khan et al., 2010).
Selenium yeast is one of the selenium types introduced as feed additives for 3 decades it consists of individual yeast related to Saccharomycetaceae or admixture of more yeast which results from fermentation process or special culture (Schrauzer, 2006). Glutathione peroxidase is an enzyme activated by selenium to a modified enzyme (glutathione peroxidase 4), it occurred by substituting sulfur in the glutathione molecule. This modified enzyme transformed hydrogen peroxide into harmless water and oxygen. Body oxidation resistance is enhanced more by organic selenium than inorganic selenium (Suchý et al., 2014). Organic selenium addition to broiler’s diets displayed a significant increase in renal superoxide dismutase activity and a significant decrease in renal MDA level (Dalia Mohamed et al., 2017). Prolonged use of florfenicol can lead to detrimental side effects in poultry. This work focuses on the role of tocotrienol and yeast selenium to mitigate these side effects in broilers’ kidneys.
MATERIALS AND METHODS
Animals Expermintal birds
One hundred and fifty, one-day-old, unsexed Cobb broiler chicks were used and purchased from El-Watania Poultry Company-Cairo-Egypt. The birds were allocated in separate units of metal wire-floored battery for five successive weeks.
Drugs
Florfenicol 10% (Floricol®) was obtained from Pharma Swede Co., Egypt. Each mL contains 100 mg of florfenicol base. The recommended dose was (20 mg/kg b.wt. BW, per bird) for 3 successive days. Tocotrienol 50% (Tocovid®) from Hovid Company, Malaysia. The recommended dose was (170 mg/kg BW, orally) for 7 successive days. Yeast selenium 2000 ppm (Bio-SEL 2000®) from IBEX International Co. LTD, Egypt. The recommended dose was (0.15mg /kg BW, orally) for 7 successive days.
Experimental design
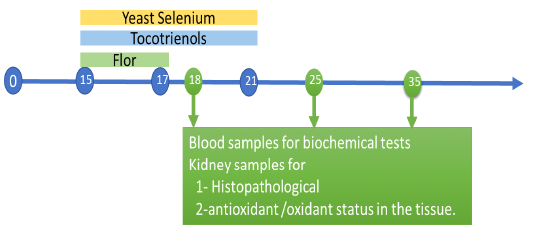
One hundred and fifty, apparently healthy, one-day-old, unsexed broiler chicks of a commercial breed (Cobb breed) were used. After thorough cleaning and disinfection, the chicks were housed in a constant environmental and hygienic condition, fed on a well-balanced ration throughout the experimental period (five successive weeks). Chicks were divided into five equal groups, 30 chicks each. Group (1) control group chicks fed to a balanced diet only. Group (2) Chicks treated with florfenicol (20 mg /kg BW, in drinking water) per bird for 3 successive days from the 15th day till 17th day old and the florfenicol was administered in other groups by the same dose and for the same period of time. Group (3) chicks treated with florfenicol and tocotrienol (170 mg/kg BW, orally) for 7 successive days from 15th and 21d st day old. Group (4) Chicks treated with florfenicol and yeast selenium (0.15 mg /kg BW, orally) for 7 successive days from 15th and 21d st day old. Group (5) Chicks treated with a combination of florfenicol, tocotrienol and yeast selenium. Blood samples and the kidney of chicks were collected on the 18th, 25th and 35th day of age.
Blood samples were collected from the jugular vein or wing vein of five chicks of each group. The kidney of chicks was divided into two parts. One part was fixed in 10 % formalin solutionfor histopathological examination and another part was stored at -20°C for determination of antioxidant /oxidant status in the tissue.
Determination of growth performance parameters
Live body weight, body weight gain, feed consumption,
Table 1: Effect of oral administration of tocotrienol, yeast selenium on growth performance parameters (body weight, body weight gain, feed consumption, feed conversion ratio) in broilers exposed to the adverse effect of florfenicol. (Mean ± SEM).
| Growth Performance Parameters | Groups | Experimental periods | |||||
| One-day old |
1st week |
2nd week |
3rd week |
4th week |
5th week |
||
|
Body weight (gm) |
1 |
44.10±.80a |
141.35±5.28a |
352.00±10.29a |
694.00±15.46a |
1087.27±16.81a |
1520.18±28.67a |
| 2 |
44.90±.73a |
123.15±5.40a |
352.64±10.33a |
674.86±20.74a |
1092.18±21.35a |
1698.81±11.51a |
|
| 3 |
45.05±.63a |
154.35±4.34a |
366.83±7.75a |
687.07±16.66a |
1075.75±25.79a |
1565.42±36.38a |
|
| 4 |
44.55±.54a |
145.70±4.42a |
358.00±7.04a |
697.50±14.48a |
1098.55±23.02a |
1581.64±42.45a |
|
| 5 |
42.60±.65a |
149.10±3.26a |
359.29±8.85a |
710.86±19.45a |
1139.63±33.18a |
1597.54±32.34a |
|
|
Growth Performance Parameters |
Groups | Experimental Periods | ||||
|
1st week |
2nd week |
3rd week |
4th week |
5th week |
||
|
Body Weight Gain |
1 |
97.25±5.54a |
209.29±10.68a |
348.43±21.04a |
387.82±24.54a |
432.91±37.06a |
| 2 |
78.25±5.22a |
222.94±9.95a |
314.00±25.42a |
432.27±29.02a |
606.64±69.93a |
|
| 3 |
109.30±4.488a |
211.33±7.33a |
320.13±18.29a |
383.67±33.35a |
489.67±28.49a |
|
| 4 |
101.150±4.29a |
213.18±7.97a |
339.00±15.59a |
418.81±24.99a |
483.09±56.51a |
|
| 5 |
106.500±3.519a |
211.82±8.54a |
351.86±21.99a |
425.00±40.69a |
457.91±45.48a |
|
|
Feed Consumption (gm) |
1 |
152.64 ±12.21a |
330.91 ± 26.47a |
577.94 ± 46.24a |
677.13 ± 54.17a |
789.45 ± 63.16a |
| 2 |
116.76 ± 9.34a |
335.19 ± 26.82a |
487.33 ± 38.99a |
691.85 ± 55.35a |
758.44 ± 220.68a |
|
| 3 |
175.37 ± 14.03a |
321.83 ± 25.75a |
506.16 ± 40.49a |
628.95 ± 50.32a |
835.96 ± 66.88a |
|
| 4 |
151.98 ± 12.16a |
355.67 ± 28.45a |
601.76 ± 48.14a |
832.82 ± 66.63a |
993.43 ± 79.47a |
|
| 5 |
161.12 ± 12.89a |
324.64 ± 25.97a |
559.74 ± 44.78a |
688.46 ± 55.08a |
790.63 ± 63.25a |
|
|
Feed Conversion Ratio |
1 |
1.57 ± 0.13a |
1.58 ± 0.13a |
1.66 ± 0.13a |
1.75 ± 0.14a |
1.82 ± 0.15a |
| 2 |
1.49 ± 0.12a |
1.50 ± 0.12a |
1.55 ± 0.12a |
1.60 ± 0.13a |
1.72 ± 0.14a |
|
| 3 |
1.60 ± 0.13a |
1.52 ± 0.12a |
1.58 ± 0.13a |
1.64 ± 0.13a |
1.71 ± 0.14a |
|
| 4 |
1.50 ± 0.12a |
1.67 ± 0.13a |
1.78 ± 0.14a |
1.99 ± 0.16a |
2.06 ± 0.16a |
|
| 5 |
1.51 ± 0.12a |
1.53 ± 0.12a |
1.59 ± 0.13a |
1.62 ± 0.13a |
1.73 ± 0.14a |
|
ab Mean values within the same row with different superscript letter are statistically different at P ≤ 0.05. SEM = Standard Error of Means. 1) control group 2) Florfenicol treated group 3) Florfenicol treated group with tocotrienol 4) Florfenicol treated group with Yeast selenium 5) Florfenicol treated group, Tocotrienol with Yeast selenium.
and feed conversion ratio were determined. Each chick was weighed at the beginning of the experiment. Then weighed at the end of each week till the end of the experiment. Then we got the average live body weight.
At the beginning of each day, a known amount of feed was applied for each group, at the end of the day the residual amount of feed was weighed and subtracted from the applied amount to obtain the total feed consumed during the day. The total feed consumption per day was divided by the number of birds of each group to obtain the average daily feed consumption per bird per group. Then we got the average weekly feed consumption per group.
The body weight gain per week was calculated by subtracting the body weight between two successive weights. Then got the average body weight gain. The feed conversion ratio was calculated by dividing feed consumption (gm) over body weight gain (gm) in the same period of time (week) (Wagner et al., 1983).
Biochemical measurement (Kidney function tests)
Blood samples were collected and sera were separated. The sera were stored in the freezer at (-20C) till examination to detect serum uric acid (Haisman and Muller, 1977) and serum creatinine (Henry, 1974).
Evaluation of antioxidant and oxidant status in renal tissue
Kidney tissues were collected on the 18th, 25th and 35th day of age. These tissues were prepared into 10% kidney tissue homogenate samples with normal saline to measure SOD activity (Nishikimi et al., 1972), GSH concentration
Table 2: Effect of oral administration of tocotrienol, yeast selenium on blood biochemical parameters in broilers exposed to the adverse effect of florfenicol. (Mean ± SEM).
| Blood biochemical parameters | Experimental Periods | |||
| Groups | 18 day | 25 day | 35day | |
|
Uric Acid (gm/dl) |
1 |
6.15±.32bc |
4.85±.03a |
2.85±.09b |
| 2 |
7.25±.20a |
3.95±.23b |
3.00±.06b |
|
| 3 |
5.25±.31c |
3.85±.14b |
3.90±.34ab |
|
| 4 |
7.45±.49a |
4.90±.40a |
2.70±.52b |
|
| 5 |
6.50±.23ab |
4.95±.09a |
3.60±.06ab |
|
|
Creatinine level (gm/dl) |
1 |
0.74±.05a |
0.73±.03a |
0.66±.03a |
| 2 |
0.82±.06a |
0.81±.05a |
0.61±.03ab |
|
| 3 |
0.81±.03a |
0.87±.03a |
0.51±.04b |
|
| 4 |
0.84±.03a |
0.77±.04a |
0.61±.02ab |
|
| 5 |
0.78±.03a |
0.77±.08a |
0.56±.02b |
|
ab Mean values within the same row with different superscript letter are statistically different at P ≤ 0.05. SEM = Standard Error of Means. 1) Control group 2) Florfenicol treated group 3) Florfenicol& Tocotrienol treated group 4)Florfenicol & yeast selenium treated group 5) Florfenicol & Tocotrienol with Yeast selenium treated group.
Table 3: Effect of oral administration of tocotrienol and yeast selenium on renal antioxidant /oxidant status in broilers exposed to adverse effect of florfenicol. (Mean ± SEM) (n=5)
| Groups | Renal antioxidants | Renal oxidant | |||||||
| SOD | GSH | MDA | |||||||
| 18 days | 25 days | 35 days | 18 days | 25 days | 35 days | 18 days | 25 days | 35 days | |
| 1 |
73.58±8.07ab |
51.05±8.76b |
78.86±3.63a |
3.92±.012a |
4.54±.20a |
4.88±.25b |
5.15±.86a |
3.10±1.79a |
4.30±.35b |
| 2 |
47.33±9.14c |
41.83±5.68c |
53.55±.26b |
3.12±.34b |
3.58±.11b |
3.49±.12c |
4.40±.69a |
4.30±.029a |
5.98±.014a |
| 3 |
57.02±1.06b |
82.41±15.29a |
88.315±3.76a |
3.95±.029a |
4.29±.01a |
4.90±.01b |
4.50±.43a |
5.09±.42a |
4.05±.17b |
| 4 |
80.19±.41a |
97.56±8.89a |
80.19±8.45a |
4.44±.014a |
4.47±.23a |
5.45±.21a |
5.05±.17a |
5.68±.043a |
4.17±.19b |
| 5 |
68.15±1.11ab |
89.52±3.066a |
83.29±7.15a |
4.33±.15a |
4.52±.12a |
5.69±.080a |
4.65±.14a |
3.88±.21a |
3.50±.43b |
ab Mean values within the same row with different superscript letter are statistically different at P≤ 0.05. SEM = Standard Error of Means. 1) Control group 2) Florfenicol treated group 3) Florfenicol& Tocotrienol treated group 4)Florfenicol & yeast selenium treated group 5) Florfenicol & Tocotrienol with Yeast selenium treated group. (SOD: Superoxide dismutase, GSH: Reduced glutathione, MDA: malondialdehyde).
(Beutler et al., 1963) and MDA level (Ohkawa et al., 1979).
Histopathological examination of kidney tissue
Kidney samples were fixed in10% formalin solution for 48 hours for histopathological examination (Bancroft and Stevens, 1977).
Statistical analysis
Statistical analyses were conducted with the Statistical Package for Social Science (SPSS Inc. Released, 2009) to determine if variables differed between groups, according to Snedecor and Cochran (1989). The Shapiro-Willk test was used to test the normal distribution of the data before statistical analysis was performed. Analysis of variance was conducted by one-way ANOVA and compare between means were conducted by Duncan’s multiple range test (Duncan, 1955). Probability values of less than 5 % (P < 0.05) were considered a significant.
RESULTS
Growth performance parameters
All treated groups (Florfenicol, Florfenicol with tocotrienol, Florfenicol with yeast selenium and Florfenicol, tocotrienol with yeast selenium) showed no significant (P>0.05) differences in body weight, body weight gain, food consumptions and feed conversion ratio when compared with the control as shown in Table (1).
Blood Biochemical results (Kidney function test)
Florfenicol treated group showed a significant (P<0.05) increase in serum uric acid on the 18th day old. Other treated groups returned serum uric acid levels to normal. On the 35th day old, the tocotrienols either alone or in combination with yeast decreased the creatinine level as shown in Table (2).
Evaluation of antioxidant and oxidant status in renal tissue
Florfenicol increased the MDA level in renal tissue on the 35th day of age but significantly (P<0.05) decrease SOD activity and GSH concentration in renal tissue when compared with the control group. Florfenicol with tocotrienol, Florfenicol with yeast selenium and combinations of florfenicol, tocotrienol with yeast selenium displayed a significant (P<0.05) decrease in MDA level on the 35th day of age as well as significant (P<0.05) increases in SOD and GSH in renal tissue when compared with the florfenicol treated group as shown in Table (3).
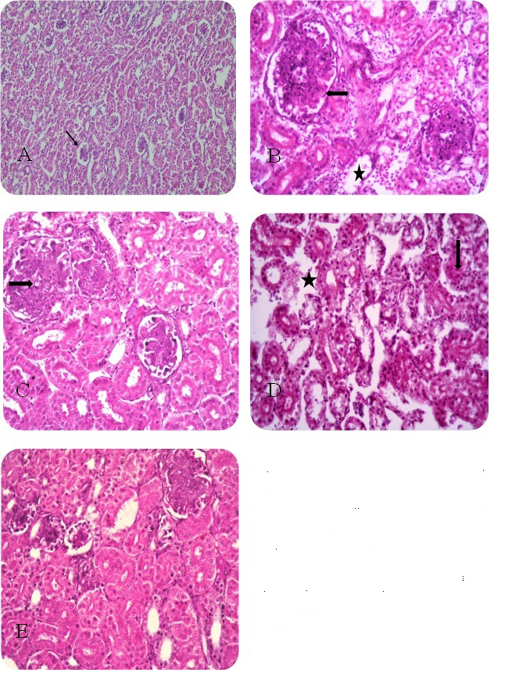
Figure 1: Renal histopathologicail finding of sacrified chicks A) Control negative group. B) Florfenicol treated group C) Florfenicol with tocotrienol treated group D) Florfenicol with selenium yeast treated group E) Florfenicol with tocotrienol and selenium yeast treated group.
Histopathological examination of renal tissue
As shown in Fig. (1A) the examined kidney sections revealed normal renal tissue. Kidney sections of the florfenicol treated group showed proliferation of the parietal layer of the Bowmen’s capsule as well as a proliferation of the glomerular tuft is evident and coagulative necrosis of the renal tubular epithelium with lytic necrosis as shown in Fig. (1B). Kidney sections of florfenicol, tocotrienol treated group displayed necrobiotic changes with coagulative necrosis in the renal tubular epithelium represented by homogenous eosinophilic cytoplasm and Karyolysis of the nuclei and hyper-cellularity of the glomerular tufts as shown in Fig. (1C). The examined kidney sections of broilers treated with florfenicol and yeast selenium showed interstitial edema with degenerative changes of the renal tubular epithelium (granular and vacuolar) as shown in Fig. (1D). Treatment of broilers with a combination of florfenicol, tocotrienols and yeast selenium revealed degenerative changes of the renal tubular epithelium with the normal architecture of the glomerular tuft and little evidence of necrotic changes in the renal tubular epithelium as shown in Fig. (1E).
DISCUSSION
Prolonged use of florfenicol can lead to detrimental side effects in poultry. This work focuses on the role of tocotrienol and yeast selenium to mitigate these side effects in broilers’ kidneys. Serum uric acid level in all groups (except florfenicol treated group) returned to the normal level. Our results in yeast selenium were in agreement with (Jankowski et al., 2011; Hada et al., 2013; Moghaddam et al., 2017). On the other hand, Zia et al. (2017) revealed a significant decrease in serum uric acid in chickens.
Florfenicol increased the MDA level in renal tissue on the 35th day of age but significantly decrease SOD activity and GSH concentration in renal tissue when compared with the control group. This result was explained by Wang et al. (2020) reported that florfenicol induced oxidative stress effect on chick’s kidney through inhibition of the expression of related factors in Nrf2-ARE which has a great role in the prevention of oxidative stress. Nrf2 signaling is stimulated in oxidative stress animals to regulate the expression of downstream genes and resist oxidative stress damage (Albarakati et al. 2020). A similar result has been observed by Wang et al. (2020). Stressful chickens showed significant changes in oxidative stress and antioxidant biomarkers as MDA and GSH concentrations (Eladl et al., 2019).
Other treated groups showed significant increases in renal SOD activity and GSH concentration with a significant decrease in renal MDA level on the 35th day of age in comparison with the florfenicol treated group. The obtained result in tocotrienol is explained by Palozza et al. (2006) who reported that tocotrienol inhibit 2, 2-azobis 2-amidinopropane (AAPH) which induced MDA production. In addition Awadin et al. (2019) mentioned that the antioxidant activity of Vitamin E may be attributed to protecting phospholipid cell membrane. More explanation to the antioxidant effect of tocotrienol in kidney observed by Khan et al. (2010) who mentioned that tocotrienol decreases the harmful effect on the proximal renal tubule by improving reabsorption function and elevation GSH level in the renal cortex with lowering renal lipid peroxidation on the kidney. The same results were observed by, (Khan et al., 2011; Budin et al., 2013). The antioxidant effect of yeast selenium is explained by Li et al. (2020) who investigated that yeast selenium improved Nrf2 which has a great role to regulate cellular antioxidant defense mechanisms and its target genes of HO-1, GSH-px, MnSOD, and CAT so prevented oxidative damage occurred in the kidney. These results in yeast selenium agreed with (Petrovič et al., 2006; Li et al., 2016; Mohamed et al., 2017).
In the present study, the treatment of broilers with florfenicol revealed proliferation of the parietal layer of the Bowmen’s capsule as well as a proliferation of the glomerular tuft is evident and coagulative necrosis of the renal tubular epithelium with lytic necrosis. Our findings are in accordance with, Isa et al. (2020) who recorded that kidney sections of broiler chicks showed diffused hemorrhages and coagulative necrosis after administration of chloramphenicol (250 mg/kg BW, orally) for seven weeks to broiler chicks. On the other hand, Wang et al. (2020) reported that kidney sections of chickens treated with florfenicol in different doses (0.15, 0.3, 0.6, 1.2, and 1.8 g/L) for five days showed damage, deformity, cell atrophy and increased intercellular space.
In this study, the treatment of broilers with florfenicol and tocotrienols displayed necrobiotic changes with coagulative necrosis of the renal tubular epithelium represented by homogenous eosinophilic cytoplasm and Karyolysis of the nuclei with hyper-cellularity of the glomerular tufts. On the other hand, Budin et al. (2013) reported that the kidney of rats fed to a diet supplemented with (200mg/kg BW) of tocotrienol for 28 days showed normal renal corpuscles and renal tubules with normal lining epithelium.
The current study, treatment of broilers with florfenicol and yeast selenium revealed interstitial edema with degenerative changes of the renal tubular epithelium (granular and vacuolar). On the same line, Mirjana et al. (2004) reported that chickens fed to (20 or 30 mg/kg of selenized yeast orally) for 10 days showed fatty drops and intracellular edema in the renal tubules. On the other hand, Ilham et al. (2016) illustrated that juvenile barramundi fish fed to a diet supplemented with 2 mg /kg of organic selenium for 60 days showed a normal kidney without any histopathological changes.
In the present study, the treatment of broilers with a combination of florfenicol, tocotrienols and yeast selenium revealed degenerative changes of the renal tubular epithelium with the normal architecture of the glomerular tuft and little evidence of necrotic changes in the renal tubular epithelium.
CONCLUSION
Tocotrienol, yeast selenium alone and their combination improve the antioxidant effect, mitigate lipid peroxidation, ameliorate renal histopathological finding and returned serum uric acid to normal levels in broilers’ kidneys. Therefore, tocotrienol and yeast selenium can improve the safety of using florfenicol in broilers.
ACKNOWLEDGMENTS
We thank Prof. Dr. Fatma Darwish, head department of Pathology of Animal Health Research Institute, Benha-Branch, Egypt for her valuable support and help during practical histopathology work.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHOR CONTRIBUTIONS
Hosny Abdelfadil Ibrahim and Mohamed Hasan Khairy conceived and designed research. Ali Mohamed Asy carried out all experiments and performed data analysis. Eman Abdelmoneim Abozeid and Ali Mohamed Asy drafted the manuscript, made critical contribution to the discus- sion and revised the manuscript. All authors read and ap- proved the final manuscript.
REFERENCES