Advances in Animal and Veterinary Sciences
Research Article
Incidence of Infertility in Female Buffaloes due to some Reproductive Disorders
Mahmoud Hamouda1*, Ahmed Saber2, Abdullkarem Al-Shabebi1
Department of Pathology, College of Veterinary Medicine, King Faisal University, Saudi Arabia; Department of Pathology, Animal Reproductive, Research Institute, Egypt.
Abstract | The present study was designed to study the prevalence of common reproductive disorders that play a role in infertility in female buffaloes. A total of 340 reproductive tractsof female buffaloes was examined. Ovaries, oviducts and uteri were inspected for gross lesion and examined histologically. Ovarian lesions were recorded in 59 animals out of 340 (17.35%) as follows: follicular cysts in 8 animals (2.35%), luteal cysts in 9 animals (2.64%), cystic corpus luteum in 3 animals (0.88%), persist corpus luteum in 18 animals (5.29%), ovarian hypoplasia in one animal (0.29%), oophritis in one animal (0.29%), ovarian hydrobursitis in 2 animals (0.59%),ovario bursal adhesions in 3 animals (0.88%), teratoma in 2 animals (0.59%), fibroma in one animal (0.29%), paraovarian cysts in 11 (3.24%). Hydrosalpinx was recorded in 5 animals (1.47%). Acute endometritis was recorded in 3 animals (0.88%). Chronic endometritis was recorded in 26 animals (7.65%). Adenomyosis was recorded in 2 animals (0.59%). Regarding to the effect of season on the frequency of disorders, the lesions in the summer season either ovarian (ᵡ2=8.11,P>0.05) or uterine (ᵡ2=13.83,P>0.01) were of higher incidence than other seasons. In a conclusion, the current study disclosed that reproductive disorders seem to be an important value with possible subsequent infertility in female buffaloes.
Keywords | Buffaloe, Infertility, Uterus, Ovary.
Received | July 14, 2020; Accepted | July 22, 2020; Published | September 01, 2020
*Correspondence | Mahmoud Hamouda, Department of Pathology, College of Veterinary Medicine, King Faisal University, Saudi Arabi; Email: mhamouda@kfu.edu.sa
Citation | Hamouda M, Saber A, Al-Shabebi A (2020). Incidence of infertility in female buffaloes due to some reproductive disorders. Adv. Anim. Vet. Sci. 8(11): 1188-1193.
DOI | http://dx.doi.org/10.17582/journal.aavs/2020/8.11.1188.1193
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2020 Hamouda et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Buffaloes are considered one of the suitable domestic animals that can tolerate and survive the unsuitable environmental condition as hot humid climate. Buffaloes are typed as river and swamp groups. The productivity of buffaloes remains low due to poor management, nutrition and breeding (Pasha and Hayat, 2012). The main problem of Buffaloes’ production is infertility. The cause of infertility is numerous, varied and complicated hence so many factors are concerned in successful reproduction. Various infertility problems of buffalo can be classified as follows: anestrus, endometritis, repeat breeding, dystocia, uterine torsion and prolapse. Ovarian abnormalities are one of these problems and include developmental anomalies, inflammatory conditions and neoplasms. Such alterations impair the development of ovarian follicles and corpus luteum and subsequently estrous cycle and pregnancy (Azawi and Ali, 2015). Ovarian hypoplasia associated with a deficiency of germ cells occurs infrequently in buffaloes (Dobson, 1986). Periophoritis and oophoritis were reported from abattoir studies on buffalo genitalia (Kumar and Singh, 1985).Ovariobursal adhesions results from adhesion between the mesosalpinx and mesovarium, often including the fimbriae and ovary (Saxena et al., 2006). Ovarian cysts have been recorded in buffaloes (Charoennam et al., 2019). Persistent CL in buffalo genitalia may be due tometritis, pyometra and embryonic death (Russo, 2010). Ovarian neoplasms have been described in buffaloes. The origin of neoplasms is epithelial or germ cells or ovarian stroma. Cyst adenoma have been recorded in Indian buffaloes (Kumar and Singh, 1984). The germ cell tumours have been recorded in buffaloes and described as teratoma (Mandal and Singh, 1976), while other forms were described as dermoids (Dwivedi and Singh, 1971). Sex cord stromal tumours are derived from the cellular components of the ovary. The most common sex cord tumour in buffaloes is a granulosa cell tumour (Gorakh, 1980). Mesenchymal tumours, include fibromas, hemangiomas, leiomyomas and their malignant counterparts have been recorded (Talerman and Vang, 2014). The oviduct abnormalities were identified as other etiological causes of infertility and include congenital defects, salpingitis, hydrosalpinx, pyosalpinx, adhesions, oviduct occlusions and pachysalpinx (Patra et al., 2012; Shivhare et al., 2012).
Uterine infections in dairy animals cause infertility in acute cases and sub-fertility in chronic cases. Consequently, uterine infection reduces the conception rate, increase calving to conception interval and contributes to increased culling rate (Sheldon et al., 2009). Endometritis, parametritis and pyometra were recorded by several authors as causative agents of infertility in buffaloes (Moss et al., 2002). As buffaloes are of poor breeder, the present study was formulated to elucidate the incidence of the most common affections of the female reproductive tract to control the economic losses due to such conditions.
MATERIALS AND METHODS
Animals
Female genital organs (ovaries, Fallopian tubes and uteri) of 340 adult breeding non-pregnant buffaloes (3-6 years) with previous history of infertility (anestrous and repeat breeding) were collected from slaughterhouses at Giza Province (Egypt) during the period of 24 months (from March 2017 to February 2019).
Gross Pathology
Gross inspection of ovaries, Fallopian tubes and uteri was conducted. Ovaries were *examined for the size and the presence of abnormal structures; Fallopian tubes were examined for the size and abnormal contents, while uteri were examined for any abnormal changes.
Microscopic Examination
Specimens from affected ovaries, Fallopian tubes and uteri were taken and fixed in formal saline. The specimens processed and the slides were stained with Hematoxylin and Eosin (Suvarna et al., 2019). The obtained slides were examined under a compound light microscope (Olympus CX31).
Statistical Analysis
Statistical analysis was carried out by using the SPSS 16.0 program (SPSS 16.0 for Windows Evaluation Version Release 16.0; 06 September 2007). The chi - square test was used in the evaluation of different variables. P< 0.05 was considered statistically significant for seasonal variations in the incidence of lesions.
RESULTS
Ovarian lesions were observed in 59 animals out of 340 (17.35%). The frequency of each ovarian lesion is not significantly affected by the season as a factor (P>0.05). However, the total ovarian lesions are significantly affected or dependent upon the season (ᵡ2=8.11, P>0.05). Uterine lesions were observed in 31 animals out of 340 (9.12%). The frequency of each uterine lesion is not significantly affected by the season as a factor (P>0.05). However, the total uterine lesions are significantly affected or dependent upon the season (ᵡ2=13.83, P>0.01).
Follicular cysts
The cysts were observed in 8 animals out of 340 (2.35%). They were either unilateral (5 cases) or bilateral (3 cases). They were spherical and bulging from the ovarian *surface. They appear single or multiple like grapes. The size ranged from 2.1-7 cm. The wall was thin, semitransparent and well vascularized (Figure 1a). The contents were either straw colored fluid or viscous in nature. Histologically, the ovum and the cells of the cumulus oophorus were degenerated or completely absent. The granulose cells were decreased in number and most of them were degenerated or completely absent (Figure 1b). The theca interna was highly cellular and vascular, while theca externa appeared fibrous.
Luteal Cysts
The cysts were observed in 9 animals out of 340 (2.64%). They were unilateral either right or left. They were spherical and bulging from the ovarian surface. There was no ovulation fossa. The size ranged from 1.3-8 cm. The wall was thick, tense and opaque. The contents were either viscus or gelatinous. Histologically, the ovum and the cells of the cumulus oophorus were degenerated or completely absent. The granulosa cells were hypertrophied and hyperplastic and can be identified into two types of cells, large polyhedral cells with pale, vacuolar cytoplasm (granulosa luteal cells) (Figure 1c) and small cells which appeared more deeply stained (theca luteal cells). The outer wall of cysts was consisted of a thick layer of connective tissue.
Cystic Corpus Luteum
The cysts were observed in 3 animals out of 340 (0.88%). They were spherical and bulging from the ovarian surface. The size ranged from 1.5-2 cm. The wall was thick and the colour was dark brown. The genital organs were normal in the three cases. Histologically, they are quite similar to corpora luteal gravidities except for the presence of a central cavity.
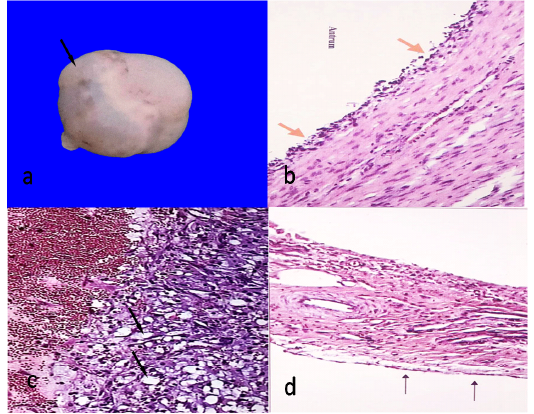
Figure 1: Follicular cyst, 7cm in diameter showing semitransparent membrane (arrow)(a)Histological structure of follicular cyst showing degenerated granulosa cells (arrows)(HEX100)(b)Histological structure of luteal cyst showing vacuolated granulosa cells (arrows)(HEX100)(c)Ovarian hypoplasia, stroma of connective tissue with blood vessels covered with flattened epithelium (arrows)(HEX100)(d).
Persistent Corpus Luteum
The cysts were observed in 18 animals out of 340 (5.29%). The size and shape are similar to corpora luteal gravidities. Small, capsulated and dark brown structure was embedded in the thick fibrous stroma of the cortex. All cases associated with endometritis (16 cases) and metritis (2 cases). Histologically, irregular masses of normal lutein cells separated by connective tissue septa.
Ovarian Hypoplasia
Ovarian hypoplasia was observed unilaterally in one case out of 340 (0.29%). It was small, white, flattened soft mass replacing the ovary of left side. Histologically, Connective tissue band containing thick walled blood vessels covered with flat epithelial cells (Figure 1d).
Fibroma
Fibroma was observed in one case out of 340 (0.29%). It was firm and spherical attaching the ovary with mesovarian ligament. Histologically, whorled of fibroblasts and collagen fibers running in different directions without criteria of malignancy (Figure 2a).
Teratoma
Teratoma was observed unilaterally in two cases out of 340 (0.59%). It was firm and spherical and cystic type. The size ranged from 1.5-6 cm. The wall was thick and the contents were brown jelly materials with hair (Figure 2b). Histologically, There was a cavity lined with keratinized stratified squamous epithelium, under which, there was a thick layer of connective tissue containing hair follicles, sweat glands and sebaceous glands without criteria of malignancy (Figure 2c).
Ovariobursal Adhesions
Ovariobursal adhesions were recorded unilaterally in three cases out of 340 (0.88%). The adhesion in two cases appeared as a fibrous band between the ovary and the bursa. While the third case the ovary was completely encapsulated and adhered to the corresponding bursa. Histologically, there was granulation tissues intermingled with few smooth muscle cells.
Ovarian Hydrobursitis
Ovarian hydrobursitis was noticed unilaterally in two cases out of 340 (0.59%). The cyst had a semi-transparent wall with clear serous fluid and encompasses the ovary (Figure 2d). Histologically, the cyst lined by flattened epithelium and surrounded with a thin layer of connective tissue.
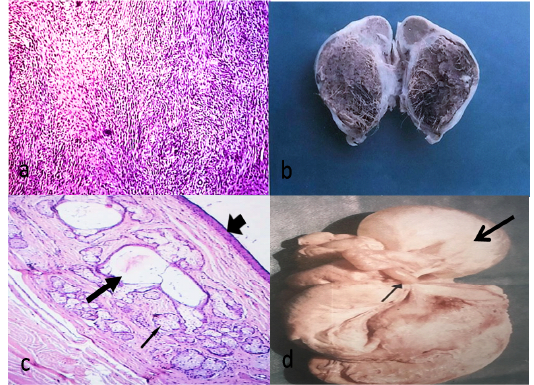
Figure 2: Fibroma, fibroblasts waving in different directions (HEX100)(a)Cystic type of ovarian teratoma about 6.5X4 cm containing hairy material and brownish jelly-like fluid(b) Histological structure of teratoma showing stratified epithelium (arrow head), sweat glands (thick arrow) and sebaceous glands (thin arrow)(HE X40)(c)A complicated case, adhesion (thin arrow), ovarian hydrobursitis (thick arrow) (d).
Oophritis
Oophritis was observed unilaterally in one case out of 340 (0.29%). The ovary appeared congested. Histologically, the ovarian stroma was heavy infiltrated with lymphocytes associated with dilated blood vessels and lymphatics.
Hydrosalpinx
Hydrosalpinx was recorded in 5/340 (1.47%). It was unilateral in 4 cases and bilateral in one case. Hydrosalpinx was simple type and appeared as a thin transparent wall, distended with a clear fluid (Figure 2d). The distention may be segmented (two cases) and involved the whole length in three cases. Histologically, the lining epithelium was low columnar to cuboidal and the primary as well as the secondary folds appeared shorter or may be absent.
Paraovarian Cysts
The cysts were observed in 11 animals out of 340 (3.24%). They were unilateral either right or left. They were spherical with thin and transparent wall (Figure 3a). They either attached to mesovarian or mesosalpinx. The size ranged from 0.5-3 cm and the contents were a clear serous fluid. Histologically, the cysts were lined with one layer of cuboidal or flattened epithelial cells.
Acute Endometritis
Acute endometritis was recorded in 3/340 (0.88%). The endometrium was severely congested and some areas were necrosed and covered with greenish exudate. Histologically, the endometrium was desquamated and the endometrial glands seemed to be degenerated as well as the stromal blood vessels were severely congested associated with large amount of neutrophils, which appear in some cases in a focal manner (abscess) (Figure 3b).
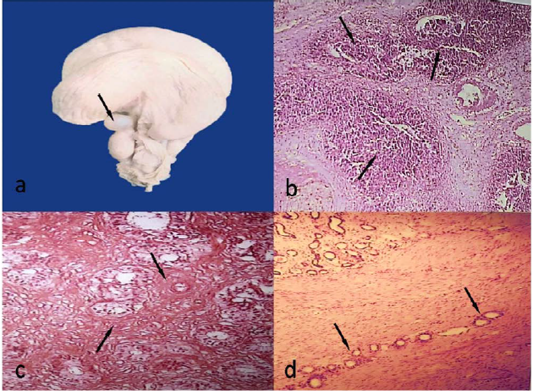
Figure 3: Paraovarian cyst, 1.8cm in diameter (arrow)(a)Acute endometritis, multiple abscesses (arrows) (HEX40)(b)Chronic endometritis, massive fibrosis around glands and blood vessels (arrows)(HEx100)(c)Adenomyosis, uterine glands migrating throughout myometrium (arrows) (HEX40)(d).
Chronic Endometritis
Chronic endometritis was recorded in 26/340 (7.65%). The endometrium was corrugated and dirty yellowish in colour. Some areas were completely destructed and ulcerated. Histologically, there were massive fibroblasts around the endometrial glands (Figure 3c) as well as large amounts of lymphocytes, macrophages and plasma cells.
Adenomyosis
Adenomyosis was recorded in 2/340 (0.59%). The two cases were associated with chronic endomteritis. Histologically, there were endometrial glands migrating throughout myometrium (Figure 3d).
DISCUSSION
Infertility in buffaloes has been attributed to either hereditary or environmental factors. In the present study, ovarian affections were a major pathological findings followed by uterine affections (17.35% and 9.12%) respectively. Regarding to the effect of season on the frequency of disorders, the lesion in the summer season either ovarian (ᵡ2=8.11, P>0.05) or uterine (ᵡ2=13.83, P>0.01) were of higher incidence than other seasons. Similar findings were reviewed in female buffaloes by Ponraj et al. (2017). This high incidence was due to suppression of ovarian activity and increasing embryonic deaths during periods of increasing of daylight length (Campanile et al., 2007). Follicular cysts were recorded in 8 cases (2.35%). The condition is due to failure or insufficiency of luteinizing hormone (LH) during oestrus period (Acland, 2001). Luteal cysts were recorded in 9 cases (2.64%).These cysts were believed to involve either insufficiency of luteinizing hormone (LH) or possibly immaturity of follicles at normal LH surge leading to failure of ovulation (Jones et al., 1997). The corpus luteum was recorded in 3 cases (0.88%) and it is considered a physiological pattern of ovarian function. Persistent corpus luteum cysts were recorded in 18 (5.29%) andusually associated with endometritis.The persistence of corpus luteum in cattle was due to metritis, pyometra and late embryonic death (Struve et al., 2013).The recorded hypoplasia was unilateral and this finding could be responsible for infertility, however, bilateral hypoplasia lead to sterility and resulted from hereditary by impaired migration of primordial germ cells (Venhoranta et al., 2013). Oophritis was recorded in one case (0.29%). Similar incidence in buffaloes was recorded in abattoir studies (Kumar and Singh, 1985). The etiology of such inflammation was either to ovarian manipulation or ascending infection from the uterus (Agarwal et al., 2005). The ovariobursal adhesion was recorded in two cases (0.88%). On the other hand, Sharma et al. (1993) reported the cases of Ovariobursal adhesion (19.17%). This condition is commonly due to mishandling of the ovary during rectal palpation of the ovary, manual enucleation of the corpus luteum, rupture of ovarian cysts or infection. It affects fertility through interference with tubal motility and lead to irregular oestrus.Ovarian hydrobursitis was recorded in two cases (0.59%). It was found that ovarian
Table 1: Effect of season on ovarian and uterine lesions in female buffaloes
|
Lesions |
Total animals (340) |
Seasons | ||||||
| Spring | Summer | Autumn | Winter |
χ2 test |
P | |||
|
Follicular cysts |
8 | (2.35%) | 1 | 3 | 4 | 0 | 5.63 | 0.13 |
| Luteal cysts | 9 | (2.64%) | 1 | 4 | 3 | 1 | 3.31 | 0.35 |
|
Cystic corpus luteum |
3 | (0.88%) | 0 | 2 | 0 | 1 | 3.36 | 0.31 |
| Persistent corpus luteum | 18 | (5.29%) | 6 | 5 | 0 | 7 | 6.17 | 0.1 |
|
Ovarian hypoplasia |
1 | (0.29%) | 0 | 0 | 0 | 1 | 2.78 | 0.42 |
| Fibroma | 1 | (0.29%) | 1 | 0 | 0 | 0 | 3.01 | 0.39 |
|
Teratoma |
2 | (0.59%) | 0 | 2 | 0 | 0 | 6.16 | 0.11 |
| Ovariobursal adhesions | 3 | (0.88%) | 1 | 1 | 1 | 0 | 2.14 | 0.54 |
|
Ovarian hydrobursitis |
2 | (0.59%) | 0 | 1 | 0 | I | 2.01 | 0.57 |
| Oophritis | 1 | (0.29%) | 0 | 0 | 0 | 1 | 2.79 | 0.43 |
| Paraovarian cysts | 11 | (3.24%) | 2 | 4 | 1 | 4 | 2.22 |
0.53 |
| Total | 59 | (17.35%) |
13/59 (22.03%) |
23/59 (38.98%) |
9/59 (15.25%) |
14/59 (23.73%) |
8.11 |
0.04* |
| Hydrosalpinx | 5 | (1.47%) | 0 | 2 | 2 | 1 | 2.39 |
0.49 |
| Total | 5 | (1.47%) | 0 | 2(40%) | 2(40%) | 1(20%) | ||
| Acute endometritis | 3 | (0.88%) | 2 | 1 | - | - | 3.69 | 0.29 |
| Chronic endometritis | 26 | (7.65%) | 10 | 10 | 1 | 5 | 9.27 | 0.03* |
| Adenomyosis | 2 | (0.59%) | 1 | 1 | 0 | 0 | 2.01 | 0.57 |
| Total | 31 | (9.12%) |
13/31 (41.94%) |
12/31 (38.71%) |
1/31 (3.23%) |
5/31 (16.13%) |
13.83 |
0.003** |
| Overall | 95 | (27.94%) |
26/95 (27.37%) |
37/95 (38.95%) |
12/95 (12.63 %) |
20/95 (21.05%) |
||
=*Significant at p<0.05; ** Significant at P<0.01
hydrobursitis causes infertility in dromedary female camels and is associated with inflammatory genital conditions (Benaissa et al., 2014). Teratoma was recorded in two cases (0.59%). Similar incidence was noticed in buffaloes (Raja and Srilatha, 2008). The etiology of tumor is poorly known, but rapid proliferation of germ cells is one postulated reason (Mac Lachlan, 1987).Teratoma is considered as a cause of infertility through disrupting the normal ovarian function (Pande et al., 2016). Paraovarian cysts were found in the broad ligament between the ovary and the Fallopian tube (3.24%). A nearly similar incidence was recorded in buffaloes (Potekar et al., 1982). They originate from the embryologic remnants of the mesonephric and paramesonephric ducts. They are of little significance in animal infertility. Hydrosalpinx was recorded in 5/340 (1.47%). This condition may occur as a secondary due to segmental aplasia of the paramesonephric duct or adhesion at the proximal or distal ends of the oviduct (Ponraj et al., 2017). The accumulated fluid creates unsuitable media for embryo implantation (Shivhare et al., 2012). The incidence of acute and chronic endometritis was 0.88% and 7.65% respectively. Endometritis is the most frequent cause of infertility in buffalo, especially under field conditions. The main cause of endometritis is non-specific opportunist pathogens that contaminate the uterus during Postpartum peroid (subclinical endometritis) (Bajaj et al., 2016). Other conditions are retention of placenta, abortion, dystocia, induction of parturition, genital prolapse, uterine inertia and traumatic lesions in the genital tract.Adenomyosis was recorded in 2 cases (0.59%) and associated with endometritis. Similar findings were recorded in buffaloes and cattle (Korzekwa et al., 2013). This condition was usually associated with endometritis and so resulting in infertility.
CONCLUSION
The current study disclosed that reproductive disorders seem to be an important value with possible subsequent infertility in female buffaloes in Egypt.
AcKNOWLEDgEMENT
The authors are thankful to the Deanship of Scientific Research, King Faisal University, Saudi Arabia for support this work.
CONFLICT OF INTEREST
None of the author has any conflict of interest to declare.
AUTHOR CONTRIBUTIONS
Mahmoud hamouda, principal author wrote the manuscript. Ahmed Azab examined and described gross and microscopic changes. Abdulkarem Al-Shabebi analyzed data statistically. All authors revised the manuscript and approved.
REFERENCES






