Advances in Animal and Veterinary Sciences
Research Article
Safety and Quality Evaluation of Various Grades of Egyptian Beef Luncheon Sausage and Burger Patties: A Comparative Study
Mai M. Zohdy1, Hoda A.M. Aiedia1, Mohamed M.T. Emara2, Taha M. Nouman2, Mai A. Mohamed2*
1Department of Food Hygiene, Animal Health Research Institute, Cairo University; Egypt; 2Department of Food Hygiene and Control, Faculty of Veterinary Medicine, Cairo University, Egypt.
Abstract | The current study was carried out to compare the safety and quality between the high price (premium) and low price (economic) meat products that are commonly distributed in the Egyptian markets. To achieve this goal, 200 samples from the various grades of Egyptian beef luncheon sausage and burger patties were collected randomly from different Egyptian markets and subjected to physicochemical, microbiological, and histological examinations. Results revealed that both grades of examined luncheon samples had higher moisture content than its permissible limits. However, the protein contents were lower than the established limit in both grades of all examined products. Results also showed that all examined samples showed fatty acids profile was nearly similar to that of chicken and buffalo meat than that of beef. Moreover, the results revealed that the samples that exceeded the permissible limits of TBARS and TVBN values were higher in premium than those of economic products for both luncheon and burger patties. Conversely, the economic luncheon showed a higher percentage of samples that exceeded the permissible limit of residual nitrite when compared to the premium one. Both grades of all examined products contained higher levels of monosodium glutamate (MSG) than that of the established level, moreover; all examined bacterial groups exceeded their stated limits in most examined samples. Histological examination revealed that all examined products contained a low percentage of muscular tissue and higher amounts of fat, heart muscle, connective tissue, skin, bone, cartilage, and carbohydrates. This study proved that both chemical and histological examinations provide an effective tool to detect the various methods of adulteration in meat products.
Keywords | Quality, Safety, Sausages, Patties, Adulteration
Received | April 01, 2021; Accepted | April 15, 2021; Published | July 01, 2021
*Correspondence | Mai A Mohamed, Department of Food Hygiene and Control, Faculty of Veterinary Medicine, Cairo University, Egypt; Email: mayota_vet2008@yahoo.com
Citation | Zohdy MM, Aiedia HAM, Emara MMT, Nouman TM, Mohamed MA (2021). Safety and quality evaluation of various grades of egyptian beef luncheon sausage and burger patties: a comparative study. Adv. Anim. Vet. Sci. 9(8): 1194-1202.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.8.1194.1202
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Mohamed et al., This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
In the last decade, world consumption of meat products has been increased due to their rapid preparation as fast foods and lower price when compared with raw meat. Although meat products are considered a good source of essential amino acids, vitamins, and trace elements, there was a potent correlation between their consumption and the incidence of various diseases as tumor, heart, lung, liver, and urinary diseases (Etemadi et al., 2017). Moreover, adulteration of meat products is another challenge. Besides being illegal, it may pose potential health problems and economic waste for the consumers and breaking trust in the meat industry. Adulteration of food is defined as the act of purposely corrupting its quality parameters by combining or replacing inferior substances or by the removal of some valuable ingredients (The Columbia Encyclopedia, 2002). Mechanically recovered poultry meat “MRPM” (Mohamed et al., 2016; Malak et al., 2020), poultry skin (FSIS, 1999), starches (Totosaus, 2009), and heart (Krishnan and Sharma, 1990) are considered the most common forms of adulteration of meat products.
MRPM is characterized by its higher amount of fat, calcium, connective tissue, haem pigments, bone residues, undesirable aroma, color oxidation and higher microbial load (Serdaroğlu et al., 2005). Therefore, using MRPM as a raw material for the production of meat products may deteriorate their chemical, microbial, and sensory quality attributes. Moreover, high ingestion of meat products containing MRPM may cause various health hazards to the consumers as hypercholesterolemia (Serdaroğlu et al., 2005) and gastroenterological side effects (Mohamed et al., 2016). Furthermore, the addition of heart muscles during the processing of meat products may lead to a reduction in their shelf life, since heart muscle contains higher amounts of blood and iron (Biel et al., 2019), which may accelerate fat and color oxidation.
Substitution of higher quality meat with lower quality raw materials results in the production of highly perishable products, which requires the addition of higher levels of nitrites to extend the shelf life of such products. However, methaemoglobinaemia and cancer of different organs were reported as a result of overconsumption of products containing nitrites (Aschebrook-Kilfoy et al., 2011). Moreover, the processors may add large amounts of monosodium glutamate (MSG) as a flavor enhancer to give the typical meat aroma (Bellisle, 1999) to overcome the flavor problems of low-quality meat material. Many studies reported that MSG had toxic and genotoxic effects (Khatab and Elhaddad, 2015) on humans if consumed at high doses. The addition of high amounts of carbohydrates as starch also acts as another way for meat product adulteration. Starch is added in various meat products as a functional additive at certain limits, where it may be added as an emulsion stabilizer or an extender at a rate not higher than 5 and 10%, respectively (Pospiech et al., 2014). However, some un-honest meat processors may add starch at a level that may reach to 25% of the total weight of the products to increase their bulk and reduce their cost. Higher amounts of added starch may harm all the technological properties of the products e.g. dry texture and bad binding (Totosaus, 2009).
To the best of our knowledge, the determination of fatty acids profile, nitrite, and MSG contents as methods for detection of meat products adulteration is limited. Therefore, the main aim of the current study was to evaluate the suitability of these parameters in addition to the histological examinations as an accurate tool to identify the different types of adulterants in luncheon sausage and burger patties.
MATERIALS AND METHODS
Samples
A total of 200 random samples of beef emulsion luncheon sausage and burger patties (100 samples for each) were collected from different local meat processing plants in Egypt at different production batches. Both luncheon sausages and burger patties were classified according to their price into premium and economic products (50 samples for each). Each sample was represented by three packages (500g each) from the same production date. Samples were transferred immediately in a cooling ice box to the laboratory of the Animal Health Research Institute where, they were subjected to physicochemical, microbiological, and histological examinations.
Examinations
Chemical examination
Moisture, protein, and fatty acids analysis: The procedures described by AOAC (2003) were followed up for determination of moisture and protein contents of each sample. However, for determination of fatty acids, the lipid of each sample was extracted and saponified according to IUPAC (2000). After that, the fractionation of fatty acids was carried out by using gas chromatography (ACME model 6100 GC, Young LTN Instrument Co., Korea) adjusted at temperatures of 110- 210°C. The actual content of fatty acids was determined by the aid of standard fatty acids analysis following the equation established by ISO/12966-2 (2011). Moisture, protein, and fatty acids contents were expressed in percentage.
Thiobarbituric-Acid Reactive Substances (TBARS) and Total Volatile-Base Nitrogen (TVBN) values: TBARS value (mg Mal. /Kg) was evaluated using the method stated by Du and Ahn (2002), while those described by Kearsley et al. (1983) were followed up for measurement of TVBN value (mg/100 gram).
Determination of residual nitrite and Monosodium glutamate (MSG): The color development-dependent reaction described by AOAC (2000) was carried out for determination of residual nitrite content (ppm). The MSG content (mg/100gram) was estimated by using HPLC (Series 1050, Hewlett-Packard. Les Ulis. France) at Meat Hygiene laboratories, Faculty of Veterinary Medicine, Benha University following the methodology established by Lateef et al. (2012).
Bacteriological examination
The Sample preparation and tenfold serial dilution were carried out following the procedures established by ISO/6887-1 (2017). The methodology established by Morton (2001), Scott et al. (2001) and Feng et al. (2002)
Table 1: Moisture, protein and fatty acid contents (%) of premium and economic luncheon sausages
| Luncheon | ||||||
| Premium | Economic | |||||
| Min | Max | Mean | Min | Max | Mean | |
|
Moisture |
58.57 | 64.91 |
61.98±0.58a |
58.59 | 63.11 |
61.31±0.35a |
| Protein | 13.56 | 16.08 |
14.85±0.12a |
6.19 | 13.33 |
8.15±0.64b |
| Lauric acid (C12:0) | 0.07 | 0.15 |
0.11±0.01a |
0.04 | 0.21 |
0.12±0.03a |
| Myristic acid (C14:0) | 2.50 | 3.34 |
2.91±0.15a |
0.52 | 1.64 |
1.05±0.23b |
| Myristoleic acid (C14:1) | 0.63 | 1.11 |
0.70±0.09a |
0.10 | 0.31 |
0.20±0.05b |
| Palmitic acid (C16:0) | 16.23 | 20.31 |
14.98±0.55a |
11.06 | 18.77 |
13.78±0.25b |
| Stearic acid (C18:0) | 20.94 | 21.53 |
21.34±0.09a |
7.57 | 18.34 |
18.12±2.33b |
| Oleic acid (C18:1) | 35.67 | 38.77 |
37.23±0.63a |
34.64 | 42.63 |
38.7±1.71a |
| Linoleic acid (C18:2) | 1.43 | 5.83 |
3.64±0.96a |
3.80 | 19.09 |
11.36±3.34a |
Values represent the mean of three independent replicates ± standard error
a-b: Values with different superscript within the same row differ significantly at P<0.05
Table 2: Moisture, protein and fatty acid contents (%) of premium and economic beef burger patties
| Beef burger patties | ||||||
| Premium | Economic | |||||
| Min | Max | Mean | Min | Max | Mean | |
| Moisture | 57.96 | 65.84 |
61.48±0.77a |
50.16 | 63.34 |
57.24±1.09b |
| Protein | 11.53 | 17.16 |
13.83±0.47a |
7.82 | 13.39 |
11.02±0.60b |
| Lauric acid (C12:0) | 0.08 | 0.13 |
0.11±0.01a |
0.01 | 0.16 |
0.08±0.03a |
| Myristic acid (C14:0) | 1.98 | 3.50 |
2.77±0.29a |
1.94 | 3.10 |
2.46±0.20a |
| Myristoleic acid (C14:1) | 0.57 | 1.21 |
0.62±0.11a |
0.29 | 0.51 |
0.39±0.04b |
| Palmitic acid (C16:0) | 14.20 | 18.39 |
15.86±0.58a |
13.25 | 16.98 |
11.99±0.25b |
| Stearic acid (C18:0) | 18.09 | 22.05 |
20.06±0.84a |
27.13 | 31.91 |
29.51±1.02b |
| Oleic acid (C18:1) | 36.17 | 37.56 |
36.89±0.26a |
31.21 | 34.12 |
34.69±0.56b |
| Linoleic acid (C18:2) | 1.53 | 12.66 |
7.09±2.44a |
2.07 | 7.21 |
4.61±1.13a |
Values represent the mean of three independent replicates ± standard error
a-b: Values with different superscript within the same row differ significantly at P<0.05
were carried out for enumeration of total aerobic plate counts (APC), anaerobic mesophilic bacteria and fecal coliform counts, respectively. However, isolation of Staph aureus and E. coli were performed according to the procedures described by Bennet and Ga (2016) and ISO/16649‐2 (2001), respectively.
Histological examination
Meat product samples were soaked in 10% neutral formalin saline for 24 hours for fixation, then washed under tape water, and dehydrated in upgrading ethanol. The dehydrated samples were cleared in xylene, and finally embedded in paraffin wax at 56ºC for 24 hours (Migaldi et al., 2016). The prepared paraffin blocks (1×1×1 cm) were cut at 4 µm by slide microtome, deparaffinized, and stained by Hematoxylin and Eosin (H&E) (Banchroft et al., 1996), Trichrome Green for staining of connective tissue (Official Standard, 1989), and Periodic Acid Schiff (PAS) for staining of carbohydrates (Sheehan and Hrapchack, 1987).
Statistical analysis
The chemical and microbiological analyses were carried out in three replicates and the collected data were presented as mean ± standard error (SE). The results were analyzed by using SPSS statistics 23.0 for windows, where the Independent Sample T-test was used to compare the results between the premium and economic products for both beef luncheon sausages and burger patties. Significant differences were determined by using the Least Square Difference test (LSD) procedure at (P<0.05) level.
RESULTS AND DISCUSSION
Chemical examination
Moisture, protein, and fatty acids: The results of the chemical analysis revealed that there was a non-significant (P>0.05) difference in moisture content among premium and economic luncheon, while there was a significant (P<0.05) increase in protein content of premium luncheon when compared with the economic product (Table 1). Conversely, moisture and protein contents of premium burger patties were significantly (P<0.05) higher than those of economic patties (Table 2). The results also showed that moisture contents of both luncheon grades and premium patties were slightly higher than the limit (60%) established by EOS/1114 (2005) and EOS/1688 (2005) for luncheon sausages and burger patties, respectively. However, the protein contents of all examined luncheon and patties samples were lower than the value (15%) regulated by EOS specified for each product. Higher moisture and lower protein contents may be explained by the substitution of high-quality meat with low-quality raw materials as MRPM and heart during the processing of meat products. It has been reported that MRPM contained lower protein levels (Mohamed et al., 2016), while heart muscle contained higher moisture levels (Biel et al., 2019) when compared with beef. Moisture and protein results were in agreement with those reported by Malak et al. (2020) who found large variations in both moisture and protein values of Egyptian luncheon collected from different processing plants.
The fatty acids results of luncheon showed that levels of myristic, myristoleic, palmitic, and stearic acids were significantly (P<0.05) higher in premium luncheon than those of economic one (Table 1). On the other hand, there were non-significant (P>0.05) differences in lauric, oleic, and linoleic acids contents between premium and economic luncheon. The results also revealed that there was a significant (P<0.05) increase in myristoleic, palmitic, and oleic acids contents, while a significant (P<0.05) decrease in stearic acid content of the premium patties when compared with those of the economic one (Table 2). However, there were non-significant (P>0.05) differences in other examined fatty acids profile among premium and economic patties. The fatty acids profile of different grades for both luncheon and patties showed obvious deviation than the normal fatty acids profile of beef, where all examined samples had nearly the same levels of lauric acid that is present mainly in poultry meat and MRPM (Jantawat and Dawson, 1980). Moreover, the maximum levels of linoleic acid were 5.83, 19.09% for premium and economic luncheon and 12.66, 7.21% for premium and economic patties, respectively, which were extremely higher than the level reported in beef (about 2.5%) (Leheska et al., 2008). Higher levels of linoleic acid may be related to the addition of chicken skin and/or MRPM, which contain higher amounts of such acid (Peña-Saldarriaga et al., 2020).
The previous studies reported that oleic, stearic, palmitic, myristic and myristoleic acids were the most abundant fatty acids of beef with mean values of about 31, 14, 23, 3.50 and 0.80 %, respectively (Lemos et al., 2016). Based on these results, all examined luncheon and patties samples showed lower contents of palmitic, myristic, and myristoleic acids, while higher contents of oleic and stearic acids than those reported in beef. This means that the examined meat products may be formulated with a mixture of cheaper beef alternatives as chicken skin that contains higher amounts of oleic acids (Feddern et al., 2010) and buffalo meat that contains higher amounts of both oleic and stearic acids (Tamburrano et al., 2019).
TBARS and TVBN
Data in Table (3) showed that the premium luncheon had significantly (P<0.05) lower TBARS and significantly (P<0.05) higher TVBN values when compared with the economic product. However, there were non-significant (P<0.05) differences in TBARS and TVBN values among premium and economic burger patties (Table 3). According to Warriss (2000), 10, 58, 17, and 34 % of the premium and economic luncheon, as well as premium and economic patties had unacceptable levels of TBARS, respectively. Moreover, the percentages of samples that exceeded the permissible limit of TVBN stated by EOS/1522 (2005) were 34, 12% for premium and economic luncheon and 10 and 8% for premium, and economic patties, respectively. These results indicated that both grades may be processed with high perishable raw materials as MRPM, where it contains more polyunsaturated fatty acids and hemoproteins, which are more susceptible to both chemical and biochemical oxidation. Furthermore, the high microbial load of MRPM and the effect of the separation process on the integrity of protein are considered the main causes for the elevation of TVBN value of MRPM (Viuda-Martos et al., 2012).
Residual nitrite and MSG
The results clarified that there were non-significant (P<0.05) differences in residual nitrite and MSG between the different grades for both luncheon and burger patties. Nitrite is added mainly in cured meat products as luncheon for several functions as the development of cured color, inhibition of Clostridium botulinum growth, and prevention of lipid oxidation (Alahakoon et al., 2015). However, higher addition of nitrite may result in formation of nitrosamine, which has a carcinogenic effect (Aschebrook-Kilfoy et al., 2011), therefore; its level in the finished product is regulated and should not exceed 80 ppm in luncheon (Codex, 1995). Based on this regulation, luncheon samples that exceeded the permissible limit of residual nitrite were 12 and 50% for premium and economic products, respectively. Although burger is not a cured product and the addition of nitrite during its processing is not permitted by Codex (1995), all examined samples contained variable amounts of nitrite with a mean value of 28.57 and 29.56 ppm for
Table 3: TBARS, TVBN values, residual nitrite and monosodium glutamate (MSG) contents of premium and economic beef luncheon sausages and burger patties
| Luncheon | |||||||||
| Permissible limits (PL) | Premium | Economic | |||||||
| Min | Max | Mean | % of samples exceeded PL | Min | Max | Mean | % of samples exceeded PL | ||
| TBARS (mg mal./kg) | *1.00 | 0.17 | 1.23 |
0.55± 0.07a |
10.00 | 0.27 | 1.57 |
0.91± 0.11b |
58.00 |
| TVBN (mg/100g) | **20.00 | 14.70 | 22.40 |
19.27± 0.69a |
34.00 | 8.82 | 20.30 |
13.568± 0.92b |
12.00 |
| Nitrite (ppm) | ***80.00 | 51.74 | 85.25 |
69.39± 2.28a |
12.00 | 42.39 | 125.80 |
79.89± 6.24a |
50.00 |
| MSG (mg/100g) | ***500.00 | 915.40 | 8379 |
2395.67± 119.83a |
100.00 | 883.10 | 2154 |
1248.30± 150.49a |
100.00 |
| Beef burger patties | |||||||||
| TBARS (mg mal./kg) | *1.00 | 0.47 | 1.57 |
0.75±0.05a |
17.00 | 0.109 | 1.76 |
0.783± 0.12a |
34.00 |
| TVBN (mg/100g) | **20.00 | 10.46 | 23.38 |
13.67±1.09a |
10.00 | 10.50 | 20.72 |
14.81± 0.83a |
8.00 |
| Nitrite (ppm) |
***Not permitted |
11.83 | 27.557 |
28.57±1.55a |
100.00 | 6.50 | 41.56 |
29.56± 3.03a |
100.00 |
| MSG (mg/100g) | ***500.00 | 752.30 | 1156.90 |
946.00± 54.98a |
100.00 | 595.60 | 1443.10 |
1052.89± 105.92a |
100.00 |
Values represent the mean of three independent replicates ± standard error
a-b: Values with different superscript within the same row differ significantly at P<0.05
* Warriss (2000) **EOS/1522 (2005) for frozen meat ***Codex (1995)
Table 4: Bacteriological counts (log10 cfu/g) of premium and economic beef luncheon sausages and burger patties
| Luncheon | |||||||||
| Permissible limits (PL) | Premium | Economic | |||||||
| Min | Max | Mean | % of samples exceeded PL | Min | Max | Mean | % of samples exceeded PL | ||
| Mesophilic bacteria | *4.00 | 4.08 | 6.94 |
5.82±0.26a |
100.00 | <2.00 | 6.39 |
3.44±0.61b |
42.00 |
| Anaerobic bacteria | *<2.00 | 3.48 | 7.55 |
5.58±0.40a |
100.00 | <2.00 | 5.20 |
3.28±0.49b |
84.00 |
| S. aureus | *<2.00 | <2.00 | <2.00 |
<2.00±0.00a |
0.00 | <2.00 | <2.00 |
<2.00±0.00a |
0.00 |
| Fecal coliform | *2.00 | <2.00 | <2.00 |
<2.00±0.00a |
0.00 | <2.00 | <2.00 |
<2.00±0.00a |
0.00 |
| E. coli | ***<2.00 | <2.00 | <2.00 |
<2.00±0.00a |
0.00 | <2.00 | <2.00 |
<2.00±0.00a |
0.00 |
| Beef burger patties | |||||||||
| Mesophilic bacteria | **5.00 | 4.00 | 6.67 |
5.45±0.24a |
66.00 | 4.51 | 6.26 |
5.55±0.14a |
84.00 |
| Anaerobic bacteria | **2.00. | 4.00 | 6.68 |
5.42±0.29a |
100.00 | 5.11 | 6.52 |
5.93±0.13a |
100.00 |
| S. aureus | **2.00 | <2.00 | 5.30 |
1.17±0.54a |
34.00 | <2.00 | 2.48 |
0.65±0.30a |
16.00 |
| Fecal coliform | **2.00 | 0.48 | 1.43 |
0.94±0.11a |
0.00 | <2.00 | 2.38 |
1.36±0.28a |
34.00 |
| E. coli | ***<2.00 | <2.00 | 2.60 |
0.73±0.32a |
36.00 | <2.00 | 2.00 |
017±0.17a |
10.00 |
Values represent the mean of three independent replicates ± standard error
a-b: Values with different superscript within the same row differ significantly at P<0.05
*EOS/1114 (2005) for traditional Egyptian luncheon **EOS/1688 (2005) for frozen beef burger ***ICMSF (2011)
premium and economic products, respectively.
The results also presented that the level of MSG was extremely higher than its established level stated by Codex (1995) in all examined luncheon and patties. This indicated that both premium and economic products may be adulterated, where higher residual nitrite may be added to decrease the oxidation and spoilage of poor raw materials used in the product’s formulation. In addition, to magnify the meat aroma in poorly formulated meat products, meat processors may add high amounts of MSG to give flavor similar to that of meat (Bellisle, 1999).
Bacteriological examination
The mean values of bacterial counts showed that premium luncheon had a significant (P<0.05) increase in aerobic mesophilic and anaerobic bacterial counts when compared with the economic one, however; the other examined bacteria were under detectable limits in both grades (Table 4). The results also revealed that there were non-significant (P>0.05) differences in all examined bacterial groups between both grades of burger patties. The bacteriological results clarified that all examined samples were heavily contaminated with various types of bacteria, where all examined patties and premium luncheon samples exceeded the permissible limit of anaerobic bacterial counts established by EOS/1688 (2005) and EOS/1114 (2005) specified for each product, respectively. Moreover, the samples that exceeded the stated limit of aerobic mesophilic bacterial counts were 100, 42, 66, and 84% for premium & economic luncheon and premium & economic patties, respectively. In addition, the premium patties samples that exceeded the limits established for S. aureus and E. coli were 34 and 36%, respectively, while those of economic patties were 16 and 10%, respectively. Although, the counts of fecal coliforms were within acceptable limit in all examined premium patties, 34% of examined economic product exceeded the permissible limit.
Higher aerobic mesophilic count of all examined samples may act as an indication of a bad sanitary condition during processing (Ma et al., 2014), fecal coliform and E. coli counts may indicate post-processing fecal contamination (Martin et al., 2016), whereas S. aureus count may point to poor handling and uncontrolled temperature condition (Kadariya et al., 2014). Furthermore, the addition of MRPM during the processing of meat products leading to a reduction of their safety and quality, where various types of bacteria as E. coli, S. aureus, Salmonella, Campylobacter were isolated from MRPM causing health risks to the consumers (Akramzadeh et al., 2020).
Histological examination
Histological analysis of luncheon sausages and burger pat
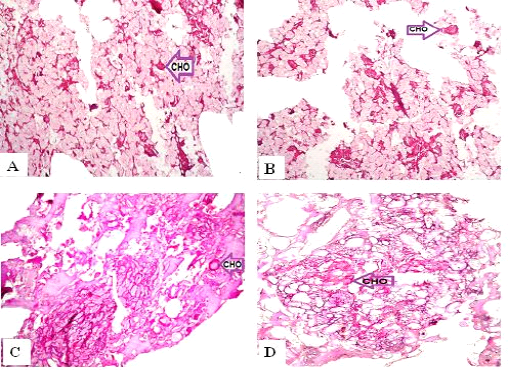
Figure 1: H&E sections of luncheon sausages; A: D (premium luncheon); E: G (economic luncheon); M, muscle; F, fat; H, heart; B, bone; S, skin; C, cartilage
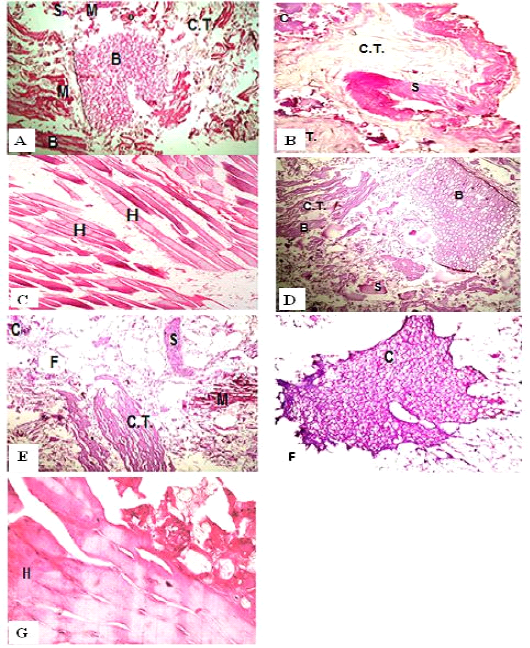
Figure 2: H&E sections of burger patties; A:C (premium burger patties); D:G (economic burger patties); M, muscle; F, fat; H, heart; B, bone; C.T., connective tissue; S, skin; C, cartilage
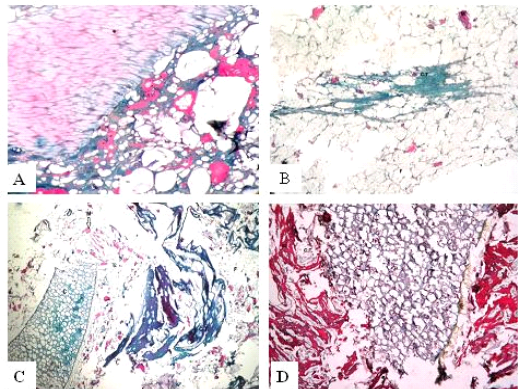
Figure 3: Trichrome sections of luncheon sausages and burger patties; A (premium luncheon); B (economic luncheon); C (premium burger patties); D (economic burger patties); muscle stained red; connective tissue stained green
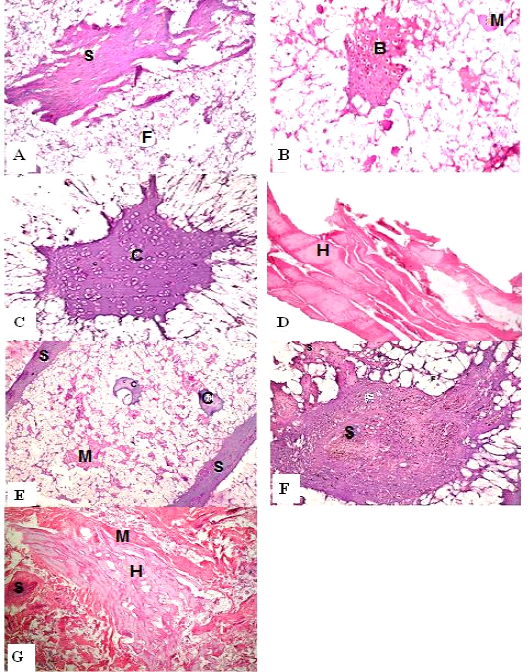
Figure 4: Periodic Acid-Schiff sections of luncheon sausages and burger patties; A (premium luncheon); B (economic luncheon); C (premium burger patties); D (economic burger patties); CHO, carbohydrates
ties with H&E (Fig. 1; 2) showed lower muscular tissues and higher amounts of fat, heart muscle, bone, skin, and cartilage were evident in all examined samples. Moreover, the staining with Trichrome green revealed that the amounts of connective tissue (green) were higher than the muscular tissue (red) in all grades for both luncheon and patties (Fig. 3). Staining of samples with Periodic Acid Schiff revealed that all examined samples contained high percentages of carbohydrates with the presence of scanty amounts of muscular tissue (Fig. 4).
The histological analysis with different stains proved the physicochemical and bacteriological results of examined luncheon and patties, where replacement of meat with other low-quality raw materials was obvious in all samples. The presence of bone and cartilage were potent indicators for addition of MRPM during the processing of these products, since these tissues are characteristic of MRPM and do not usually appear in regular meat. Furthermore, higher amounts of connective tissue that appeared in H&E (Fig. 1; 2) and Trichrome sections (Fig. 3) act as another evidence for MRPM addition (Mohamed et al., 2016; Malak et al. 2020). Periodic Acid Schiff sections clarified that adulteration of meat products is not restricted only by using different animal tissues but also by the addition of high amounts of carbohydrates instead of meat (Fig. 4).
CONCLUSION
The data obtained in the current study revealed that various grades of luncheon sausage and burger patties had lower safety and quality indices, where most examined parameters have deviated from their permissible limits. It is worth mentioned that all examined products contained higher residual nitrite and MSG contents, which constitute a public health hazard. Moreover, fatty acid analysis and histological examination proved that the various grades of both products were adulterated with low-quality raw materials as skin, MRPM, heart, and high amounts of carbohydrates, which also have a negative impact on the safety and quality of the products. Therefore, the authors appeal to the competent authorities to control and monitor all additives and materials used in the processing of meat products to ensure the production of products with high safety and quality.
Author’s Contribution
All authors contributed equally in creating the article.
Conflict of interest
The authors have declared no conflict of interest.
REFERENCES






