Advances in Animal and Veterinary Sciences
Research Article
Efficacy of Different Doses of Cloprostenol in the Treatment of the Persistently Elevated Progesteronemia in Infertile Dromedary Camel
Hany A Zaher1,2, Abdullah F Al-Fares2, Majdi E Badawi3, Eid S Almansoori2, Ayman A Swelum1,4*
1Department of Theriogenology, Faculty of Veterinary Medicine, Zagazig University, Sharkia 44519, Egypt; 2Research and Development Division, Abu Dhabi Agriculture and Food Safety Authority, P.O. Box 52150, Abu Dhabi, United Arab Emirate; 3College of Veterinary Medicine, Sudan University of Science and Technology, P. O. Box 204, Hilat Kuku, Khartoum North, Sudan; 4Department of Animal production, College of Food and Agriculture Sciences, King Saud University, P. O. Box 2460, Riyadh 11451, Saudi Arabia.
Abstract | This study was performed using twenty dromedary she-camels, to compare the efficacy of different doses of cloprostenol in treatment of the persistently elevated progesteronemia in non-pregnant dromedary camel. The she-camels having persistently elevated progesteronemia were randomly and equally assigned to two groups during the breeding season. The first group received intramuscular injection of 0.75 mg cloprostenol (PG0.75 group). Second group was received intramuscular injection of 1.25 mg cloprostenol (PG1.25 group). Trans-rectal ultrasound examination and sexual receptivity assessment were performed for all on the day of the admission every 10 days (days 0, 10, 20, 30 and 40). Additionally, blood samples were collected on the day of the admission and every 10 days (days 0, 10, 20, 30 and 40) for measuring the progesterone level. Uterine swabs were collected from all camels for bacterial culture and antibiotic sensitivity testing. The results revealed that all camels (100%) showed abstinence (erection and curving of her tail, raising head and refusing the male) at day 0 and day 10 with present of luteinized structure in ovaries (2.45±0.15 cm) and high serum progesterone levels (2.45±0.30 and 2.67±0.28 ng/mL) in PG0.75 and PG1.25 groups. The most isolated bacteria were Escherichia coli (70%) followed by Staphylococcus haemolyticus (25%). While, Pasteurella pneumotropica and Brevundimonas diminuta was isolated only one time (5% for each one). Clostridium perfringens was isolated only two times (10%). Escherichia coli and Staphylococcus haemolyticus were sensitive to gentamycin (100%). Pasteurella pneumotropica, Brevundimonas diminuta and Clostridium perfringens were sensitive to Ampicillin (100%). No camel was responded to first dose of cloprostenol treatment in both groups. Seventy percentages of camels in PG1.25 group were responded after second dose. While, 80% of camels in PG0.75 group were responded after third dose which significantly (p-value is 0.00729) higher than in PG1.25 group (20%). Some camels were not responded to three doses of cloprostenol treatment in PG0.75 group and PG1.25 group (20 and 10 %, respectively). The pregnancy rate was higher (p= 0.068) in PG1.25 group than PG0.75 group (60 and 20 %, respectively). In conclusion, one dose of intramuscular injection of 0.75 mg or 1.25 mg cloprostenol was inefficient for inducing lysis of luteinized structure and treating the persistently elevated progesteronemia. She-camels needed at least three doses of 0.75 mg cloprostenol or two doses of 1.25 mg cloprostenol to be recovered. The most isolated bacteria were Escherichia coli followed by Staphylococcus haemolyticus and both were highly sensitive to gentamycin. Further studies are needs to evaluate higher doses of cloprostenol for treatment of the persistently elevated progesteronemia.
Keywords | Progesteronemia; Cloprostenol; Endometritis; Fertility
Received | September 27, 2020; Accepted | October 20, 2020; Published | December 10, 2020
*Correspondence | Ayman A Swelum, Department of Theriogenology, Faculty of Veterinary Medicine, Zagazig University, Sharkia 44519, Egypt; Email: aymanswelum@zu.edu.eg
Citation | Zaher HA, Al-Fares AF, Badawi ME, Almansoori ES, Swelum AA (2021). Efficacy of different doses of cloprostenol in the treatment of the persistently elevated progesteronemia in infertile dromedary camel. Adv. Anim. Vet. Sci. 9(1): 82-93.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.1.82.93
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Swelum et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
The low fertility rates in camels constitute an obstacle in camel reproduction and hence in camel production. In order to increase offtake rate in any population of camels, one has to improve the fertility rate in that population. Such an improvement may be necessary to convince camel owners to trade young camels which might then be conditioned for better meat quality (Musa, et al., 1990).
Despite the high benefits of camel meat and milk, camel production is still not undertaken on a commercial scale. The reproductive efficiency of dromedary camels is generally considered to be low. Birthing rates rarely exceed 40% in nomadic herds or 70% in more intensive herds (Tibary and Anouassi, 1997a; Tibary et al., 2005; Kaufmann, 2005). The relatively short breeding season limits reproductive performance and is considered one of the most constrains facing camel’s breeding (Swelum et al. 2018a; Swelum et al., 2018b; Swelum et al., 2019). Therefore, any delay in the treatment of infertility camels leads to missing of conceiving in the current breeding season and waiting the subsequent breeding season with prolongation to inter-calving interval.
Elevated progesteronemia in absence of pregnancy is from persistent luteal function or presence of luteinized hemorrhagic follicles (Rodriguez et al., 2014). The persistent luteal structure in female camels causes reproductive disturbances and infertility, in addition of causing the female camels to show false signs of pregnancy (Tibary and Anouassi, 1997). The presence of active luteal tissue on the ovary leads to the anovulatory state where growing follicles of consecutive waves proceed to dominance but fail to ovulate. This is due to the negative feedback of progesterone on LH release (William, 2007). Persistent corpus luteum (CL) is mostly associated with pathological conditions of uterus such as pyometra, mummification and maceration. If these previous pathological conditions exist for months or even longer, the CL becomes centrally located in the ovary and difficult to be palpated and treated (Noakes et al., 1991). In camel, the CL is usually palpated at pregnancy; however, some camels show corpora lutea in a non-pregnancy state following embryonic death and pyometra (Waheed, 2012). Persistent corpora lutea can be treated by injection luteolytic dose of prostaglandin F2α (PGF2α) (Tibary and Anouassi, 1997).
Uterine inflammation results in diminished fertility and is a considerable barrier to camel production, often resulting in significant economic loss. Uterine inflammation has been described as the most commonly encountered form of infertility in dromedary camels (Tibary and Anouassi 2000). Inadequate clinical trials comparing the efficacy of different treatments for endometritis have been performed in the camel. Additionally, the efficacy of antibiotics should be evaluated periodically because resistant strains of bacteria can arise owing to the indiscriminate use of antibiotics (Vekateswaran and Rajeswar, 1991). Most veterinarians treat camels with treatments that are traditionally used for the treatment of bovine or equine endometritis, including uterine flushing, intrauterine antibiotic infusion, systemic antibiotic treatment or a combination of the above methods. For antibiotic to be successful, it should 1) be effective against the pathogens present, 2) not inhibit the uterine defense mechanisms, 3) be effective in a pyogenic environment, 4) leave no residues in milk or meat, 5) be administered at an adequate concentration and with an adequate number of treatments and 6) be cost-effective.
It is hypothesized that determination of best dose of cloprostenol and the best antibiotic which can kill the most causative bacteria for endometritis can improve the reproductive efficiency of camels via effective treatment of the most common reproductive disorders. Therefore, the present study was aimed to 1) Evaluate the efficiency of using of 0.75 or 1.25 mg cloprostenol for treatment of persistent CL. 2) Determine the number of doses of cloprostenol (PGF2α) required to regress the persistent CL. 3) Determine the most causative bacteria for endometritis in camels and the best antibiotic can be used for treatment of endometritis in camels.
Material and methods
Animals and Management
The study was conducting during the period during the breeding season on Twenty dromedary camels which were brought to the Artificial insemination and Embryo Transfer section which follows to Animals production Division belong to Animal Wealth Sector of Abu Dhabi Food Control Authority (ADFCA), in the emirate of Abu Dhabi, UAE; (Latitude and longitude coordinates are: 24.466667, 54.366669 Zone 40R, Elevation (m) 7 m) for treatment for infertility. All camels were Arabian camels dromedary characterized by golden-brown color, long legs and big feet, a dropped-down nose and lower lip, big, thick eyelashes, a fit body and soft dark hair on its hump; that originally came from southern Oman. The animals were housed in open yard (8 * 8 meters) shaded by slopped shades of 5-8 m high, this shad was oriented east -west and was made by reed mats. The camels were fed Alfalfa mixture with Rhodes grass (7 kg / head twice daily) to meet their requirement according to Farid (1995). Drinking water was provided adlib to all camels.
Owners Complain and Case History
The owners complain was absence of conceiving for last 1-3 years in spite of showing typical signs of pregnancy like tail lifting, anestrus, the posture of standing with the head high and dribbling of urine in the presence of male camels. History regarding the camels were collected from the camel owners and recorded. Age of the camel was recorded according the owner data and dentition. Number of parities, age at first calving and time of last calving were recorded according the owner data. Body condition score was assigned for all camels based on the nutritional status and physical appearance of the camel (Faye et al., 2001). Health condition of the camel is recorded based on the external condition of skin and any other signs of disease like nasal discharge, mange, lameness, signs of nutrition deficiency etc. A file was reported for each animal to record the animal details, history, treatment details and observations. Blood samples were collected via the jugular vein from all received animals to be tested for brucellosis using the Rose Bengal test. All camels showed no agglutination in the rapid blood test and were free from brucellosis.
Gynecological Examination
Camels were restrained inside steel chutes in standing position. The hind limbs were tied together using a nylon rope and the tail was restrained by tying to the chute in upward direction. Camels were sedated by 2.5 ml xylazine 2% (Xyl-M2, VMD Livestock pharma, Arendonk, Belgium). Dung from the rectum was removed using a gloved lubricated hand. Vagina of the camel was examined by inspection of the vaginal wall mucus using speculum and palpation the vaginal wall by passing lubricated gloved hand per vagina.
Trans-rectal ultrasound examination was performed for all camels in the standing position using a linear-array 5 MHz transducer (UST-5820-5C, SSD ProSound 2, ALOKA, Co., Japan). The reproductive organs of all camels were checked on the day of the admission every 10 days (days 0, 10, 20, 30 and 40). The camels were examined per rectum after applying adequate lubricant to the probe. Cervix, uterus, two ovaries and two fallopian tubes were examined ultrasonography by placing the rectal probe above these organs per rectum. Conditions of the reproductive organs were recorded. The presence of abnormal uterine content, increased uterine size and increased thickness of the uterine wall were noted. The CL and follicle diameters were measured by electronic calipers.
The follicular wave was divided into two phases: the breeding (ovulatory) phase and the non-breeding (non-ovulatory) phase (Swelum and Alowaimer, 2015; Swelum et al., 2018c). The breeding (ovulatory) phase is defined as the period of fertile ovulation in which the ovaries have mature ovulatory follicles (12-18 mm) with/without the presence of other sized follicles. The non-breeding (non-ovulatory) phase is defined as the period in which the ovaries have no mature ovulatory follicle and may have follicles ≤11 mm or ≥19 mm. According to the ultrasound examination results, the percentages of camels belonging to the breeding phase and the non-breeding phase were calculated at each examination.
Sexual Receptivity Assessment
The behavior signs of she camel in presence of camel-bull were assessed. The sexual receptivity of the females was evaluated without allowing mating day after day during the course of the experiment until natural mating day using a camel-bull. The females’ sexual receptivity was graded: abstinence, incompletely receptive, and completely receptive (Swelum and Alowaimer 2015; Swelum et al., 2018c).
Progesterone Assay
Blood samples were collected on the day of the admission and 10 days later for measuring the progesterone level. Then 10 days after PGF2α injection blood is collected again from all camels. The blood was collected from jugular vein in a plastic tube without anticoagulant (Vacuette tube for serum 9 ml). The blood samples were centrifuged for 3 minutes at 3000 rpm to separate serum. The progesterone level (ng/ml) was measured using commercial ELISA kits (eProTest camel serum, Labstock Microservices, Ireland) and automatic ELISA test eProCheck® 2.0, Labstock Microservices, Ireland).
Bacterial Culture and Antibiotic Susceptibility (Sensitivity) Testing
Uterine swabs were collected from all camels by double guarded culture swab for bacterial culture and antibiotic sensitivity testing. The swab having uterine sample was preserved in Amies clear medium (transporting medium) and transported cooled to the laboratory within 3 hours of collection. A sterile swab was dipped into the transporting medium; the swab was gently squeezed against the tube wall in order to remove excess fluid. The swab was used to streak the agar plate for a lawn of growth. Sheep blood agar, MacConkey agar and Sabouraud agar were used. After the streaking was completed, the plate was allowed to be dried for 5 minutes. Then, antibiotic discs were placed and gently pressed into the surface of the agar using flame sterilized forceps or inoculation loop and the discs. The antibiotic discs which used in the current study were Ampicillin, Gentamycin, Penicillin, Amoxycillin, Oxacillin, Neomycin, Oxytetracycline, Chlortetracycline, Chloramphenicol, Polymixin, Streptomycin, Furazolidin, Trimethoprim-sulphamethoxazole, Enrofloxacin, Tetracyclin and Cortimoxazol (Mast Diagnostic, UK). The inoculated plates were carefully inverted and incubated for 24 hours at 37° C. After incubation, a metric ruler was used to measure the diameter of the zone of inhibition for each antibiotic used. The obtained measurement from the individual antibiotics was compared with the standard table to determine the sensitivity zone (whether the tested bacterial species was sensitive or resistant to the tested antibiotic).
Experimental Design and Animal Treatment Regime
The camels which had high level of progesterone (>1.5 ng/ml) and had the same CL on the same ovary in the days of admission and 10 days later were included in our study and considered to be suffered from persistent CL. These animals divided into two groups according to the dose of cloprostenol (a synthetic functional analogue of prostaglandin PGF2α). First group (n=10) was received 0.75 mg cloprostenol I/M (3 ml Oestrophan 0.25 mg/ml injection solution) and called PG 0.75 group. Second group (n=10) was received 1.25 mg Cloprostenol I/M (5 ml Oestrophan 0.25 mg/ml injection solution, Bioveta, Czech Republic) and called PG1.25 group.
After collecting the uterine swab, uterine douching was performed using local 120 ml of antiseptic and disinfectant product called Lotagen (1.728 g Policresulenum as Polycondensate of m-cresolsulfonic acid and formaldehyde at the mass ratio of 14:1) (Bioveta, Czech) and the Bovivet disposable uterine catheter (Kruuse, Denmark) with the use of a 60 ml disposable syringe. For uterine douching, recto vaginal method was used. Excess dung from the rectum is removed using gloved lubricated hand and vulva and perineal region of the camel is cleaned with water and the area is wiped and dried using a clean tissue paper. Gloved lubricated hand is passed into the rectum and the uterine catheter was passed into the uterus through the vagina and cervix. The uterine douching by Lotagen was repeated day after day in three doses.
Beside local treatment and based on the antibiotic sensitivity test results, the recommended doses of GENTAJECT 10% Gentamycin (as sulphate) 100 mg/ml (FATRO/Italy) or Ampidexalone (Ampicillin trihydrate 87,00 mg per 1 ml and Dexamethasone 25mg per 1 ml) (Maravet/ France) were started parenterally.
Assessment of Recovery Response
At least ten days after the treatments, all camels were re-examined ultrasonographically to evaluate their recovery from persistent CL and/or endometritis. Only recovered camels were allowed to mate naturally when the follicle was mature (12–18 mm) and ovulation was induced using 0.021 mg of buserelin acetate (5 ml Receptal®, MSD). Pregnancy was diagnosed by ultrasonography on day 20 post-mating and confirmed on day 40.
Statistical Analysis
Data were analyzed to determine the effect of different doses of PGF2α (Cloprostenol) treatment on the recovery and pregnancy rate of dromedary camels. The differences in the percentages achieved in each treatment group were evaluated by the Chi-square test. The level of significance was tested at a 0.05 level of probability. The progesterone hormonal levels were analyzed using analysis of variance (ANOVA) and presented as mean± standard deviation. The SPSS statistical program was used to perform the statistical analyses (SPSS 2007).
Results
Cases History
All camels were anestrus and showing pregnancy signs (tail lifting). These camels were multiparous (7 heads delivered twice, 5 heads delivered 3 times, 8 heads delivered 4 times). The age in the first birth ranges from 5-7 years. The times of last birth were as following: twelve heads gave last birth before two years, five heads gave last birth before three years, and three heads gave before four years. All camels were apparently healthy with medium body condition score (3-4).
Sexual Receptivity Results
Before treatment (at day 0 and day 10), all camels (100%) showed abstinence (erection and curving of her tail, raising head and refusing the male) (Figure 1). Ten days after treatment by first dose of PG (Day 20), 50% and 80% of she-camels showed incomplete receptivity in PG0.75 and PG1.25 groups, respectively. Ten days after treatment by second dose of PG (Day 30), 80% and 40% of she-camels showed incomplete receptivity and 0% and 50% of she-camels showed complete receptivity in PG0.75 and PG1.25 groups, respectively. Therefore, most of she-camels in PG1.5 were mated at day 30. Ten days after treatment by third dose of PG (Day 40), 50% and 66.6% of she-camels showed incomplete receptivity and 30% and 0% of she-camels showed complete receptivity in PG0.75 and PG1.25 groups, respectively.
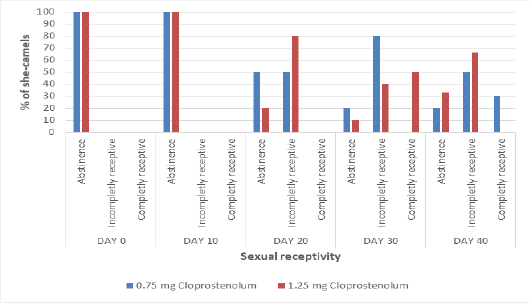
Figure 1: The percentage of she-camels showing different types of sexual receptivity before and after treatment by different dose of cloprostenol (PGF2α).)
Results of Ovarian Monitoring
The diameters of CL before and after treatment by different dose of PG are presented in Figure 2A. The diameters of CL were almost the same (2.45±0.15 and 2.47±0.29) at Day 0 and (2.43±0.15 and 2.43±0.30) at Day 10 in PG0.75 and PG1.25 groups, respectively. Ten days after treatment by first dose of PG (Day 20), the diameters of CL were significantly (p≤ 0.05) decreased in both groups and reached 1.74±0.09 and 1.35±0.29 in PG0.75 and PG1.25 groups, respectively. Ten days after treatment by second dose of PG (Day 30), the diameters of CL were significantly (p≤ 0.05) decreased in both groups and reached 1.19±0.07 and 0.74±0.24 in PG0.75 and PG1.25 groups, respectively. Ten days after treatment by third dose of PG (Day 40), the diameters of CL were significantly (p≤ 0.05) decreased in PG0.75 group and reached 0.83±0.05; while no significant decrease was observed in PG1.25 groups (0.68±0.06). The diameters of CL were significantly (p≤ 0.05) higher in PG0.75 group than in PG1.25 group at Day 20 and 30.
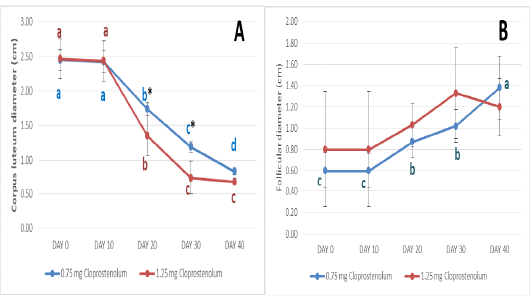
Figure 2: The diameters of corpus luteum (A) and follicles (B) before and after treatment by different dose of cloprostenol (PGF2α).
The diameters of dominant follicle before and after treatment by different dose of PG are presented in Figure 2B. The diameters of dominant follicle were almost the same (0.60±0.17 and 0.80±0.54) at Day 0 and (0.60±0.16 and 0.80±0.54) at Day 10 in PG0.75 and PG1.25 groups, respectively. After treatment by first and second dose of PG (Day 20 and 30), the diameter of dominant follicle was significantly (p≤ 0.05) increased in PG0.75 group and reached 0.87±0.15 and 1.02±0.15, respectively. Ten days after treatment by third dose of PG (Day 40), the diameter of dominant follicle was significantly (p≤ 0.05) increased in PG0.75 group and reached 1.38±0.30. No significant increase in the diameter of dominant follicle was observed in PG1.25 groups after treatment by different doses. Additionally, no significant difference in the diameter of dominant follicle was observed between PG0.75 and PG1.25 groups after treatment by different doses.
The percentage of camels in breeding phase and non-breeding phase before and after treatment by different dose of PG are presented in Figure 3. At Day 0 and 10, all camels were in non-breeding phase in both groups. Ten days after treatment by first dose of PG (Day 20), all or most camels were in non-breeding phase in both groups. Ten days after treatment by second dose of PG (Day 30), the percentage camel in the breeding phase was significantly (p≤ 0.05) higher in PG1.25 than PG0.75 group. Ten days after treatment by third dose of PG (Day 40), the percentage camel in the breeding phase was significantly (p≤ 0.05) higher in PG0.75 than PG1.25 group.
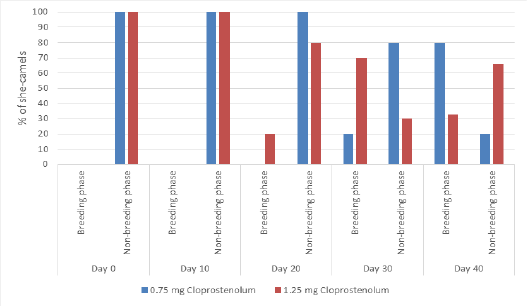
Figure 3: The percentage of camels in breeding phase and non-breeding phase before and after treatment by different dose of cloprostenol (PGF2α).
The serum progesterone level results and the correlation between the corpus luteum diameter and serum progesterone concentration
The serum progesterone level before and after treatment by different dose of PG are presented in Figure 4A. The serum progesterone levels were almost the same (2.45±0.30 and 2.67±0.28 ng/mL) at Day 0 and 10 in PG0.75 and PG1.25 groups, respectively. Ten days after treatment by first dose of PG (Day 20), the serum progesterone levels were significantly (p≤ 0.05) decreased in both groups and reached 1.73±0.21 and 1.50±0.34 ng/mL in PG0.75 and PG1.25 groups, respectively. Ten days after treatment by second dose of PG (Day 30), the serum progesterone levels were significantly (p≤ 0.05) decreased in both groups and reached 1.23±0.16 and 0.92±0.37 ng/mL in PG0.75 and PG1.25 groups, respectively. Ten days after treatment by third dose of PG (Day 40), the serum progesterone levels were significantly (p≤ 0.05) decreased in PG0.75 group and reached 0.84±0.10; while no significant decrease was observed in PG1.25 groups (0.91±0.04 ng/mL). The serum progesterone level was significantly (p≤ 0.05) higher in PG0.75 group than in PG1.25 group at Day 30. The correlation between the CL diameter and serum progesterone concentration is presented in Figure 4B. The very strong positive correlation was reported between the CL diameter and serum progesterone concentration (R= 0.96).
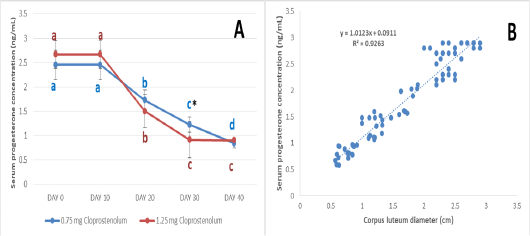
Figure 4: The serum progesterone level before and after treatment by different dose of cloprostenol (PGF2α) (A) and the correlation between the corpus luteum diameter and serum progesterone concentration (B).
The results of bacterial isolation and antibiotic sensitivity test
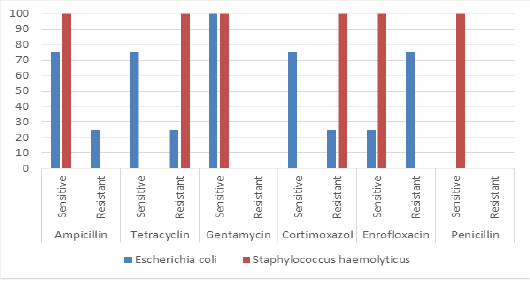
The most isolated bacteria were Escherichia coli (70%) followed by Staphylococcus haemolyticus (25%). While, Pasteurella pneumotropica and Brevundimonas diminuta was isolated only one time (5% for each one). Clostridium perfringens was isolated only two times (10%). The results antibiotic sensitivity test for Escherichia coli and Staphylococcus haemolyticus are presented in Figure 5. Escherichia coli and Staphylococcus haemolyticus were sensitive to gentamycin (100%). The results antibiotic sensitivity test for Pasteurella pneumotropica, Brevundimonas diminuta and Clostridium perfringens are presented in Figure 6. Pasteurella pneumotropica, Brevundimonas diminuta and Clostridium perfringens were sensitive to Ampicillin (100%).
The response of camels for treatment and the pregnancy rate after treatment
No camel was responded to first dose of PGF2α treatment in both groups. After second dose, seventy percentages of camels in PG1.25 group were responded; while, no camel responded in PG0.75 group. After third dose, 80% of camels in PG0.75 group were responded which significantly (p-value is 0.00729) higher than in PG1.25 group (20%). Some camels were not responded to three doses of PGF2α treatment in PG0.75 group and PG1.25 group (20 and 10 %, respectively). There was no significant difference (p= 0.068) between PG0.75 group and PG1.25 group in the pregnancy rate after treatment.
Figure 6: The antibiotic sensitivity test for Pasteurella pneumotropica, Brevundimonas diminuta and Clostridium perfringens.
Discussion
Poor reproductive efficiency is a major problem in camelids. The reproductive rate in camelids varies from 25% to 80% depending on the management and level of veterinary care provided (Tibary and Anouassi, 1997a). Various uterine disorders have been described and may play an important role in the reduced fertility observed in camels (Tibary and Anouassi 1997b). Similar many domestic animal species, uterine infections are the most commonly acquired reproductive problems that result in infertility in camels (Wernery and Kumar, 1994; Tibary et al., 2001; Ali et al., 2010). The major contributing factors to this condition are over-breeding, postpartum complications and unsanitary gynecological manipulations (Tibary, 2004). A uterine infection should be suspected in any animal with a history of repeat breeding or persistent CL and can be confirmed by clinical examination; manual vaginal examination can also be used to diagnose uterine infection and is likely more practical and simpler than a complete clinical examination (Sheldon et al., 2002).
Uterine infections were considered to be the most common cause of reproductive failure in female camels (Werny and Kumar, 1994). The major contributing factors were overbreeding, postpartum complications, and unsanitary gynecological manipulation (Tibary, 2004). Arcanobacterium pyogenes, Streptococcus pyogenes, Staphylococcus aureus, Corynebacterium, Escherichia Coli as well as Proteus were frequently isolated from female camels with uterine infections (Ali et al., 2010). The subfertility associated with uterine infections involved the effects of uterine damage as well as disruption of ovarian function (Sheldon and Dobsonb 2004). Uterine infections also suppressed hypothalamic GnRH and pituitary LH (Herth et al., 2006). Persistent luteal activity and failure of luteolysis has been attributed to lack or insufficient release of PGF2α from the endometrium. Chronic endometritis or endometrial degenerative changes may be responsible for lack of sufficient release of PGF2α. Persistent luteal activity (blood progesterone level >1.5 ng/mL) is the primary cause of persistent rejection of the male in the absence of pregnancy. Persistent luteal activity may be caused by a persistent CL or a luteinized hemorrhagic follicle. It may be observed for several weeks or months in the absence of pregnancy. Females usually show the typical behavior of pregnancy (spitting off). Diagnosis is straightforward and is based on ultrasonographic identification of the luteal tissue (CL or luteinized anovulatory follicle) or by determination of progesterone level in plasma or serum. In chronic cases, accumulation of fluid in the uterine cavity and increased echogenicity of the cervical rings may be observed. The cause of persistence of CL is not clear. Females may develop persistent corpora lutea following spontaneous ovulation (in the absence of mating), particularly during the immediate postpartum period.
The results of the present study showed that all camels (100%) showed abstinence (erection and curving of her tail, raising head and refusing the male) at day 0 and day 10. This was attributed to present of CL in ovaries (2.45±0.15 cm) which secrete high level of progesterone in serum (≥2.4 ng/mL). No camel was responded to first dose of cloprostenol treatment in PG0.75 and PG1.25 groups which indicates that these doses were no sufficient for lysis of CL. Therefore, the size of CL and the serum progesterone level after first dose was decrease without complete lysis. This made some camels show incomplete receptivity. However, the degree of decreasing in the size of CL and the serum progesterone level after first dose was higher in the group received higher dose of cloprostenol (PG1.25 group) than another group (PG0.75). Seventy percentages of camels in PG1.25 group were responded after second dose. While, 80% of camels in PG0.75 group were responded after third dose which significantly (p-value is 0.00729) higher than in PG1.25 group (20%). Some camels were not responded to three doses of cloprostenol treatment in PG0.75 group and PG1.25 group (20 and 10 %, respectively).
The decreasing in size of CL was accompanied with decreasing in serum progesterone levels which reflected on hypothalamus and pituitary gland by stimulating GNRH, FSH and LH secretion. FSH stimulated follicular growth. Therefore, the size of follicles was increased after treatment by cloprostenol. These follicles secreted estrogen which affected she-camels behavior and some females showed incomplete or complete sexual receptivity.
Camels evidencing a persistent CL should be treated with 500 µg of a commercially available cloprostenol as Estrumate (Schering Plough Animal Health, Germany) (Quzy, et al., 2013). A CL was considered persistent when it was evident on different subsequent sonographic examinations without evidence of pregnancy and tail cocking (Tibary and Anouassi, 2000). The presence of CL by sonography in 20 camels that failed to evidence tail cocking suggested previous spontaneous ovulation followed by a persistent CL or leutinization of unovulated haemorrhagic follicles (Tibary and Anouassi, 2000). Spontaneous ovulations have been recorded in camels (Nagy et al., 2005). The presence of CL on ovaries also indicated an early follicle growth and probably ovulation, luteinization of un-ovulated follicles or the persistence of a CL of the previous breeding season. Cloprostenol could easily regress the CL and mature follicles were seen on day 8 of treatment. Previous studies have shown that there is a sharp decline in progesterone after luteolysis in camel (Skidmore, 2005) and mature ovulatory sized follicles are present on the ovaries within 4 to 5 days of treatment (Ismail et al., 1998). Skidmore (2005) had mentioned that cocking of the tail can be shown by camels given progesterone therapy.
There is a lot of confusion about the definitions of uterine infections, because the same conditions may receive different names, the examinations and criteria for the diagnosis of uterine infections may differ or are often not well specified. The depth of inflammation of the uterine wall distinguishes uterine infection into metritis and endometritis (Sheldon et al., 2006). Diagnosis of uterine infections by rectal palpation was probably the basis for treatment of most cows in the field (Le Blank, 2008). Vaginal speculum examination enhances sensitivity for detecting endometritis (Dohmen et al., 1995; Le Blank et al., 2002; Barlund et al., 2008). Manual vaginal examination was probably more practical and used simple techniques (Sheldon et al., 2002).
For the treatment of uterine infection in female camels, the use of PGF2α has been recognized in veterinary routine therapy (Miller et al., 1980). Also, an intrauterine treatment with antiseptics or antibiotics has been found effective (Fredrikson et al.,1988). Detection of uterine infection is very important in the prevention of venereal transmission of infection to other animals. In addition, identification of the causative agents and determination of its sensitivity to different drugs allows the practitioner to choose the most efficient treatment. Various uterine disorders have been described in camelids and may play an important role in reduced fertility in these species (Tibary and Anouassi, 1997). Like so many domestic animal species, uterine infections are the most common of these disorders Fowler (1998); Johnson (1989); Tibary and Anouassi (1997); Tibary and Anouassi (2000); Wernery and Wernery (1992); Wernery and Kumar (1994), but unlike other species, little is known about their pathogenesis and evolution in camelids. Consequently, many practitioners approach the diagnosis and treatment of endometritis and metritis in female camelids in the manner described for cows and mares. The bacteria responsible for endometritis in our results were Staphylococcus aureus, Escherichia coli, Corynebacterium sp. and Salmonella sp, this agreement with the result revealed by Wernery (1991); Wernery and Wernery (1992); Wernery and Kumar (1994) where they found these bacteria in the equine and bovine species; the most common one to be isolated from camels with endometritis is Escherichia coli. Other bacteria that have been isolated are Streptococcus zooepidemicus, -haemolytic Streptococci, Enterococcus, coagulase negative Staphyloccocus, Proteus spp., Enterobacter aerogenes, Klebsiella pneumoniae and Arcanobacter pyogenes (Enany et al., 1990). Powers et al. (1990); Wernery, J. (1991); Wernery and Kumar (1994). Pseudomonas aeruginosa, Campylobacter fetus, and Trichomonas fetus have been isolated from infertile camels and may be associated with venereal transmission and should be considered in infertility or abortion outbreaks (Wernery and Wernery, 1992). The most common bacteria isolated from the uterus of camelids with endometritis were Escherichia coli, Streptococcus zooepidemicus, beta hemolytic Streptococci, Enterococcus spp., coagulase-negative Staphylococcus, Proteus spp., Enterobacter aerogenes, Klebsiella pneumoniae and Arcanobacterium pyogenes (Wernery and Kumar 1994; Enany et al. 1990; Wernery 1991; Wernery and Wernery, 1992). Because of the wide variety of bacteria that can cause uterine infections, veterinarians must either use broad-spectrum antibiotics or obtain bacterial cultures and carry out sensitivity tests to determine the most appropriate antibiotic for treating endometritis. However, most of these organisms are ubiquitous, which makes uterine culture results misleading if they are not interpreted correctly and correlated with clinical findings. Left untreated uterine infections can lead to irreversible changes and complications such as salpingitis, resulting in a total loss of fertility (Tibary and Anouassi, 2000).
In the present study, the most isolated bacteria were Escherichia coli (70%) followed by Staphylococcus haemolyticus (25%). While, Pasteurella pneumotropica and Brevundimonas diminuta was isolated only one time (5% for each one). Clostridium perfringens was isolated only two times (10%). Escherichia coli and Staphylococcus haemolyticus were sensitive to gentamycin (100%). Pasteurella pneumotropica, Brevundimonas diminuta and Clostridium perfringens were sensitive to Ampicillin (100%).
The efficacy of the endometritis treatment depends on the type of antibiotic used, the volume and frequency of infusion, the causative bacteria and the duration and degree of endometrial changes. There are little clinical trials comparing the efficacy of different treatments of endometritis in camelids. Most practitioners use treatments proposed for the bovine and equine species, which include uterine lavage or flushing, intrauterine antibiotic infusion, systemic antibiotic treatments or a combination of these. The uterus is an anaerobic environment; thus, antibiotics chosen for intrauterine use must be active in the absence of oxygen (El-Azab et al., 1988). In addition, because most antibiotics and chemicals depress the activity of uterine neutrophils and interfere with uterine defense mechanisms, the potential benefits of their use must be carefully weighed against the potential deleterious effects (Vandeplassche 1981). Organisms causing uterine infections are usually sensitive to penicillin, but bacterial contamination can produce penicillinase, which renders the drug ineffective if applied locally.
Nabih and Osman (2012) found that treatment of endometritis using of acriflavine intrauterine douching accompanied with gentamycine sulphate intramuscular injection gives the highest percentage of conception rates (85.7%). On other hand intrauterine infusion of homologous blood plasma (twice at 24-hour intervals) has also been used in llamas and alpacas (Johnson, 1989). The success of treatment of endometritis is variable and depends on its duration and on endometrial changes observed in the uterine biopsy.
In metritis cases, the isolated organisms in she camel were Corynebacterium and Proteus sp. recorded the highest percentages of isolates followed by Klebseilla sp., while Salmonella sp. was the lowest isolates (Nabih and Osman, 2012). The highest results were detected after using lotagen intrauterine douching with synulox (containing amoxycillin and clavulanic acid) intramuscular injection which revealed the highest conception rate (71.4%) (Nabih and Osman, 2012). Accordingly, the action of lotagen is not pathogen specific, but can attack a broad spectrum of pathogens (Schnyder et al., 1990). With regard to the use of antibiotics to eliminate bacterial infections, one should remember that bacterial cultures and antibiotic susceptibilities are the best way to approach the problem of an efficacious antibacterial selection. Furthermore, the basic question to be addressed is what tissues are involved in the uterine infection being treated? If the infection involves deeper layers of the uterus and other genital tissues, systemic therapy would be necessary. If, however, the infection is limited to the endometrium, then local therapy is probably warranted due to very high-sustained levels of antibiotic in the lumen and endometrium (Youngquist and Shore, 1997).
Nabih and Osman (2012) concluded that the relatively high incidence of recovery of endometritis and metritis cases in their study may reflect the importance of isolated microorganisms in inducing genital tract infections in female camels. Gentamycin I/M injection seem to be more efficient in treating female camels with endometritis in combination with aciflavine intra uterine wash. While synalox I/M has the best choice of treatment of metritis cases in combination with lotagen intra uterine douches. With regard to the use of antibiotics to eliminate bacterial infections, one should remember that bacterial cultures and antibiotic susceptibilities are the best way to approach the problem of an efficacious antibacterial selection (Nabih and Osman, 2012).
In the study of Swelum (2013), recovery was higher in cephapirin-treated animals (87.5%) than in oxytetracycline-treated animals (66.7%). Oxytetracycline is a broad-spectrum antibiotic that is active in mucopurulent and anaerobic environments. Its irritating nature causes an inflammatory response, stimulates the defensive reaction of the uterus, promotes PMN infiltration to the lumen of the uterus and stimulates the regeneration of uterine tissue. Therefore, it can be a very useful antibiotic for the treatment of endometritis, especially in cases of chronic endometritis. Oxytetracycline was the most popular agent used to treat endometritis many years ago. When antimicrobial therapy is indicated, oxytetracycline is recommended for intrauterine use when mixed bacterial populations are present (Bretzlaff, 1987). It satisfies most of the criteria for the treatment of endometritis (Noakes 1991): it is poorly absorbed in the deeper layers of the uterus (Bretzlaff et al., 1987), and it achieves higher and longer-lasting endometrial concentrations when infused than it would if administered by other parenteral routes (Masera et al., 1980). Oxytetracycline concentrations are high enough in the caruncles and the endometrium 24 hours after an intrauterine infusion of 5.5 mg/kg but insufficient in the myometrium and ovaries (Bretzlaff 1987; Sheldon and Noakes 1998). Sheldon and Noakes (1998) reported that the cure rate of postpartum endometritis using oxytetracycline, cloprostenol and estradiol were 73%, 67% and 63%, respectively. Significantly higher success rates were achieved for the treatments of cows with mild endometritis with oxytetracycline than with estradiol. The conception interval was also shorter in cows treated with oxytetracycline and PGF2α than in cows treated with estradiol.
Although oxytetracycline is widely used, Cohen et al. (1996) demonstrated that the intrauterine infusion of this drug may be inappropriate because of the lack of sensitivity of the bacteria causing the infection. Most isolates of Actinomyces pyogenes recovered from the uterus of cows were resistant to oxytetracycline, and intrauterine treatments with large doses of this antibiotic did not affect the frequency of A. pyogenes isolation. Sheldon et al. (2004) showed that oxytetracycline achieved the highest MIC values of all antibiotics frequently used to treat uterine infections, including cephapirin. Additionally, many preparations of oxytetracycline are irritating and cause chemical endometritis. Furthermore, the meat and milk residues discourage certain practitioners from using oxytetracycline. The absorption of this drug from the uterus to the peripheral blood occurs within 12 hours. Using the tetrazolium chloride assay, a single treatment was found to produce residue in milk for 1-8 days, and the period tended to be longer in cows that had received more than one dose (Tan et al., 2007).
In the study of Shawaf et al. (2019), bacteriological growth could be identified in 65% (30 swabs) of investigated samples; while, no growth was reported in 35% (16 swabs) of the investigated samples. With 9 and 8 positive swabs, Staphylococcus aureus and Escherichia coli were responsible for the majority of uterine infections within studied animals. Cytological analysis revealed that the cellular contents of studied samples were significantly different according to isolated bacterial species. In our study, five cases (11%) of studied animals were negative for both cytological and microbiological examination, whereas 29 cases (63%) were positive in bacteriology and cytology. The compatibility in the bacteriological and cytological results in the case of both positive or negative in present study appeared in 74% of animals. In 24% of studied animals there was an absence of bacterial growth on the culture, though these samples were positive for cytology. Their study confirmed the importance of combined employment of cytological and bacteriological results in the diagnosis of endometritis in dromedary camels.
In current study, the pregnancy rate was higher (p= 0.068) in PG1.25 group than PG0.75 group (60 and 20 %, respectively). Pregnancy rates after endometritis treatment vary from 30 to 60% (Powers et al., 1990; Wernery and Kumar 1994). In another study, the conception rates obtained after endometritis treatment with acriflavine 0.1%, lotagen 4% and gentamicin (300 mg/100 ml) were 58.9%, 49.3% and 42.5%, respectively (Ali et al., 2010). Treated females should always be re-examined after a period of sexual rest.
Conclusion
In conclusion, one dose of intramuscular injection of 0.75 mg or 1.25 mg cloprostenol were inefficient for inducing lysis of CL and treating persistent CL. She-camels needed at least three doses of 0.75 mg cloprostenol or two doses of 1.25 mg cloprostenol to be recovered. The most isolated bacteria were Escherichia coli followed by Staphylococcus haemolyticus and both were highly sensitive to gentamycin. Further studies are needs to evaluate higher doses of cloprostenol for treatment of persistent CL.
acknowledgements
The authors extend their appreciation to their corresponding universities and institutions.
conflict of interest
The authors have no conflict of interest.
authors contribution
H.A.Z. and A.A.S. contributed equally in the manuscript. H.A.Z., A.F.A. and A.A.S. conceived, designed, performed the experiment and wrote the draft. A.A.S. analyzed the data. M.E.B., E.S.A. and A.A.S. revised the paper. All authors have read and approved the final manuscript.
References