Advances in Animal and Veterinary Sciences
Research Article
Synergistic Effect of Nanocurcumin and Platelet Rich Plasma on Resolving Experimentally Induced Osteoarthritis in Albino Rats
Abdelmoneim A. Ali1*, Al-Sayed R. Al-Attar1, Nahla Abdel Ghafar Ahmed Refat1, Mohamed Gomaa2, Zienab E.Eldine3, Aya Samy1
1Pathology Department, Faculty of Veterinary medicine, Zagazig University; 2Surgery, Anesthesiology and Radiology Department, Faculty of Veterinary Medicine, Zagazig University,44511, Egypt; 3Faculty of Postgraduate Studies for Advanced Sciences (PSAS), Beni-suef University, 62511, Beni-suef, Egypt.
Abstract | An efficient therapeutic approach for osteoarthritis (OA) is yet to be discovered. In the current study, we evaluated coadministration of nanocurcumin (NCur) and platelet rich plasma (PRP) as a potential therapeutic strategy for treating experimentally induced osteoarthritis. Thirty male albino rats weighing 180± 20 gm were divided into 5 groups, 6 rats each. The first group was injected with saline (Control group). To induce osteoarthritis in the other four groups, rats were injected with 2 mg of monosodium-iodoacetate (MIA) in a total volume of 50 ul of saline. All injections were done in the right knee joint through the infra-patellar ligament to induce osteoarthritis. Two weeks after injection of MIA, one group was left untreated (Osteoarthritic group) and another group received 200 mg/kg NCur through gastric intubation for 14 consecutive days. A fourth group was intra-articularly injected with 150 ul activated PRP. The final group was concurrently treated by both PRP injection and NCur gavage. Fifteen days post treatment, radiography and Mankin scoring of joint lesions beside serum inflammatory cytokines were analyzed. Lowest Mankin scores with normal radiographic picture of joint structure and normal serum levels of IL6, IL1β and TNFα were observed in rats concomitantly treated with PRP and NCur. It can be concluded that concurrently use of nanocurcumin and platelet rich plasma is a potential efficient therapy for osteoarthritis and can greatly restore joint structure and function.
Keywords | Osteoarthritis, Nanocurcumin, platelet rich plasma, Interleukin-6, Interleukin-1β, Tumor necrosis factor-α
Received | September 05, 2020; Accepted | September 16, 2020; Published | December 05, 2020
*Correspondence | Abdelmoneim Ahmed Ali, Pathology Department, Faculty of Veterinary medicine, Zagazig University, Egypt; Email: abdelmoneim.ahmedali@yahoo.com
Citation | Ali AA, Al-Attar AS, Refat NAGA, Gomaa M, Eldine ZE, Samy A (2021). Synergistic effect of nanocurcumin and platelet rich plasma on resolving experimentally induced osteoarthritis in albino rats. Adv. Anim. Vet. Sci. 9(1): 26-34.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.1.26.34
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Ali et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Osteoarthritis (OA) is a degenerative disease caused by joint instability and is common worldwide (Gamble et al., 2000). It is a complex disease that involves the entire synovial joint, including the articular cartilage, synovium and subchondral bone (Kuyinu et al., 2016). Hip and knee joints are the most commonly affected, but other skeletal joints in hands, feet and spine may be affected (D’Ambrosia, 2005). OA is characterized by degeneration of articular cartilage, subchondral bone sclerosis and osteophyte formation. In addition changes could be detected in the menisci, synovium, ligaments and peri-articular muscle. Gradual loss of function occurs due to pain and stiffness as the disease progresses (Holyoak et al., 2016).
The definitive etiology of OA remains unknown. Previous studies explain that inflammation is the main process in OA. Inflammatory cytokines, such as interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) play critical role in the progression of OA (Feng et al., 2017).There are number of risk factors for the development of osteoarthritis (OA) such as prior joint injury, obesity, genetics, sex, and anatomical abnormalities and excessive load; however, the most obvious risk factor is increasing age (Johnson and Hunter, 2014). Despite the high prevalence of OA, there is no effective treatment that halts or reverses disease progression. The current pharmacologic treatments such as analgesics and non-steroidal anti-inflammatory drugs (NSAIDs) provide symptomatic relief, without effect on disease prevention or modification. Prolonged use of such drug has been associated with considerable gastrointestinal, renal, and cardiovascular side effects. There is a clear and urgent need for new therapeutic strategies that are effective, safe in stopping and/or reversing disease progression (Zhang et al., 2016).
Over the last two decades, platelet rich plasma (PRP) therapy has accumulated considerable attention due to its potential ability in regenerative medicine (Pavlovic et al., 2016). Platelet-rich plasma (PRP) is an autologous blood product produced by the centrifugation of whole blood resulting in a concentration of platelets that is higher than baseline values (Smyth et al., 2013). PRP treatment rationale is due to presence of pools of growth factors like platelet-derived growth factors (PDGF), transforming growth factor beta (TGF-β), vascular endothelial growth factor (VEGF), epithelial growth factor (EGF) which are stored in platelet α-granules and are believed to be involved in the repair and regeneration of articular cartilage (Chen et al., 2017a; Khoshbin et al., 2013).
Biomedical applications of nanoparticles have been widely used due to their unique properties such as large surface-to-mass ratio, quantum properties, and ability to load and deliver small-sized biomolecules (proteins and drugs) (Dashnyam et al., 2018). Curcuma longa is one of the most studied plants and its main component curcumin (Cur) bears beneficial effects such as anti-inflammatory, antioxidant, anticancer, anti-microbial, hepatoprotective and anti-hyperlipidemic (Niazvand et al., 2017b). Curcumin-loaded nanoparticles (NCur), advantages are high permeability and retention that can increase curcumin’s bioactivity (Chen et al., 2017b). Curcumin has chondroprotective effects once it is able to inhibit the production of MMP-1, MMP-3, MMP-9, MMP-13 by inhibiting the Activator protein1 (AP-1) pathway, the NF-kB and Jun N-terminal kinase (Yeh et al., 2015).
This study was designed to evaluate the therapeutic effect of both NCur and PRP on experimental induced OA via radiography and histopathological scoring of knee joint and sera inflammatory cytokines.
MATERIAL AND METHODS
Animals
Thirty male albino rats weighing 180± 20 gm at the beginning of the experiment were housed in cages at 25±0.5°C, under a 12:12 light/dark cycle, with free access to feed and water. Rats of all groups were kept under the same environmental conditions of temperature, illumination, acoustic noise, and ventilation, and received the determined feed during the course of the experiment. Feed and water were kept in special open containers fixed in the walls of the cages. Cleaning and changing water and food were done for all rats twice daily. The experimental protocols were approved by the Faculty of Vet. Medicine Zagazig University, Egypt, ZU-IACUC/2/F/24/2019.
Preparation of NCur
An aqueous solution (100 ml) NaOH (1.52 g, 0.003 mol) and Cur (1.03 g, 0.003 mol) was added drop wise to a solution (250 ml) containing Mg (NO3)2·6H2O (1.53 g, 0.006 mol) and Al(NO3)3·9H2O (0.75 g,0.002 mol) (initial Mg/Al=3.0) under nitrogen atmosphere with vigorous stirring until the final pH of the solution =10. The resulting was caged at 25 °C for 48 h. Then the resultant was filtered, washed with de-ionized water until pH of the solution =7 and finally dried in vacuum at room temperature for 12 h. The product was denoted as Cur-LDHs (cop). Ion-exchange under nitrogen atmosphere, 0.5 g of NO3-LDH was added to 50 mL of a 0.06 M aqueous drug solution. The pH of mixture solution was held constant at 10 by simultaneous addition of 2 mol·L−1NaOH solution. The solution was stirred vigorously for 24 h at room temperature. The precipitate was washed by de-ionized water, and finally air-dried. The product was denoted as Cur-LDH (Mohottalalage & Kottegoda, 2015).
Characterization of NCur: Characterization of particle size distribution and encapsulation efficiency were determined. The encapsulation efficiency (EE) of nanocomposite was determined analyzing the supernatant of the final emulsion once the NCur were removed from it by centrifugation at 10000 RPM for 10 minutes, for estimation of Curcumin present in supernatant. The absorbance was measured spectrophotometrically at 427 nm and the amount of drug present was calculated from calibration curve of concentration versus absorbance with known standard of the drug. EE and curcumin loading were calculated using the following formula: (Mohottalalage & Kottegoda, 2015).
Encapsulation efficiency (%) =
Cur loading (%) =
Where,
Amount of Cur in the nanoparticles = Total amount of Cur – free Cur
Transmission Electron microscopy (TEM) method was used to determine the size and morphology of the synthesized NCur.
Preparation and Activation of PRP
The PRP preparation was carried out by adapting the protocol of the double centrifugation tube method (Pazzini et al., 2017). In brief, rats were anesthetized with ether and 3 ml of blood were collected under aseptic conditions from the retro-orbital plexus using a capillary tube initially dipped in 3.2% sodium citrate and collected into tubes containing 0.3 mL anticoagulant. The blood was subjected to two consecutive centrifugations; initial 1600 revolutions per minute (rpm) for 10 minutes to collect plasma followed by 2000 rpm for 10 minutes to separate platelet-poor plasma (PPP) on the top from the platelet- rich plasma (PRP) in the bottom. The platelet count in the collected PRP was confirmed to be more than 1000000/ul. Before injections, PRP was activated by adding 10% calcium chloride solution and 300 IU bovine thrombin. One hundred and fifty microliters of PRP was mixed with 25 µL of thrombin (300 IU) together with 25 µL of 10% CaCl2 (van den Dolder et al., 2006).
Experimental Design
The animals were randomly divided into five groups. All injections were done in the right knee joint through the infra-patellar ligament. In the control group, only saline was injected. In the other four groups 2 mg of monosodium-iodoacetate (MIA) (BioUltra, ≥98%, Sigma- Aldrich, United kingdom) in a total volume of 50 ul of saline was injected to induce osteoarthritis. Two weeks after injection of MIA, one group was left untreated and another group received 200 mg/kg NCur through gastric intubation for 14 consecutive days. A fourth group once was injected with 150 ul activated PRP. The final group was treated with both PRP injection and NCur gavage.
Radiographical examination: At the indicated time points, the animals were sedated by injection of a xylazine HCl - ketamine HCl mix and antero-posterior and lateromedial views of the right knees were x-rayed.
Determination of Serum Markers
To measure serum levels of inflammatory cytokines, 2 ml blood were withdrawn from the retro-orbital veins of the rat at the indicted time points. The blood was left to clot for 20 min at room temperature, then centrifuged for 15 mins at 8000 rpm. The collected sera were stored at -80°C until the measurements were done. Interleukin-6(IL-6), Interleukin -1 beta (IL1β) and tumor necrosis factor α (TNF-α) were measured in the sera using ELISA kits (Ray Biotech Inc., DRG International Inc., USA, and BD Biosciences respectively) according the manufacturers’ instructions.
Histopathology and Scoring
Fifteen days after treatment, the rats were sacrificed and the right knee joints were removed and fixed in 10% formalin for 48 hours. The fixed joints were then decalcified in 10% EDTA pH 7.0 for 14 days. After which they were dehydrated, and paraffin embedded. Paraffin sections (4-5 m) were stained with either hematoxylin and eosin (H&E) or safranin O and examined microscopically (Zhou et al., 2016).
A modified Mankin grading system was used to assess the pathologic changes in cartilage during OA (Mendoza et al., 2013) as follow: The cartilage structure was scored from 0 – 6; where 0 : normal, 1 :surface irregularities 2 : pannus, 3 : absence of superficial cartilage layers, 4 : slight disorganization evidenced by (an absent cellular row and some small superficial clusters), 5 : fissures into the calcified cartilage layer and 6 : disorganization (chaotic structure, clusters, and osteoclasts activity). Additionally, cellular abnormalities were scored on a scale of 0 to 3; where 0: normal, 1: hypercellularity, including small superficial clusters, 2 : clusters and 3 : hypocellularity. The matrix staining was also scored on a scale of 0 to 4; where 0: normal/slight reduction of staining, 1: staining reduced in the radial layer, 2: staining reduced in the interterritorial matrix, 3: staining present only in the pericellular matrix, and 4: staining absent. Finally tidemark was scored on a scale of 0 to 1; where 0: intact and 1: destroyed.
Statistical Analysis
All statistical analyses were performed using Prism 6, Graph Pad, CA, USA, Program. Results were presented as mean ± SD. Statistical difference were determined using Student’s t-test and p-values <0.05 were considered significant.
Results
Characterization of NCur
The particle size distribution of the NCur particles ranged from 5 nm to 100 nm. The encapsulation efficiency of Cur-loaded LDH nanospheres was 89.00 ± 2%. Transmission electron microscope (TEM) assessments revealed the size and morphology of the synthesized particles. The nano-composite showed spherical, curcumin morphology as shown in Figure (1).
Radiographical Examination
To observe the changes in the knee joints, X-ray examination was performed on osteoarthritic rats treated with PRP, NCur or the combination of the two therapies. As shown in Figure 2 anteroposterior and lateromedial X-ray views of the control joints showed normal radiopacities, smooth articular surfaces with clear radiolucent joint space and distinct sub-patellar opacity. While the untreated osteoarthritic joints showed disappearance of joint space with sclerosis, osteophyte formation and roughness of articular surfaces both at 15 and 30 days after MIA injection. The knee joints of the osteoarthritic rats treated with NCur showed improvement in the form of widening of the joint space, with little osteophyte formation and irregular articular surfaces. The radiographical picture of the PRP treated joints was almost the same as the NCur treated joints. However, the knee joints of the rats treated with both NCur gavage and PRP injection showed significant improvement of the radiographical picture with normal joint space, little osteophyte formation and smooth articular surfaces.
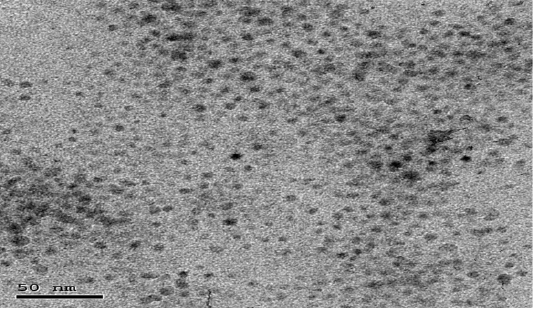
Figure 1: Showing TEM image of Nanoparticles showed distinct spherical particles in size range between 5 and 100 nm.
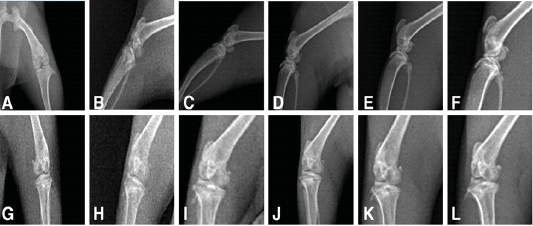
Figure 2: X-ray of the knee joints treated and untreated rats (A to F) showing lateromedial views, (G to L) anteroposterior views showing.
A, G: radiography of normal rat, showing normal radiopacities, smooth articular surfaces with clear radiolucent joint space and distinct sub-patellar opacity.
B, H: radiography of untreated osteoarthritic rat after15 days from MIA injection, showing disappearance of joint space with sclerosis, osteophyte formation and roughness of articular surfaces.
C, I: radiography of untreated osteoarthritic rat after 30 days from MIA injection, showing disappearance of joint space with sclerosis, osteophyte formation and roughness of articular surfaces.
D, J: radiography of treated rat by NCur showing widening of the joint space, with little osteophyte formation and irregular articular surfaces.
E, K: radiography of treated rat by PRP showing widening of the joint space, with little osteophyte formation and irregular articular surfaces.
F, L: radiography of treated rat by PRP and NCur showing normal joint space, little osteophyte formation and smooth articular surfaces.
Serum inflammatory Cytokines
IL6, IL1β and TNFα levels were measured in the sera of the different groups to monitor the change of the inflammatory cytokines as an indication of the healing process. The level of these inflammatory cytokines in the treated rats significantly decreased compared to the osteoarthritic group with the lowest values were observed in the NCur + PRP group (Figure 3 and Table 1).
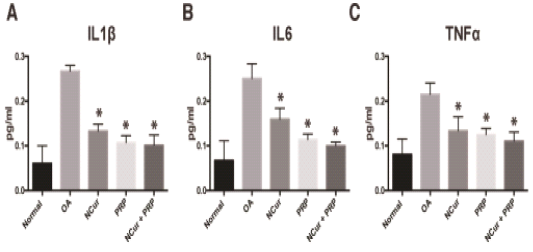
Figure 3: Serum levels of IL1, IL6 and TNFα measured 15 days after treatment with NCur, PRP or NCur + PRP. Each bar represents the average levels from 6 rats (pg/ml) ± SD and (*) indicates P-value <0.05 when compared with untreated rats, Student’s t-test.
Table 1: Serum levels of IL1, IL6 and TNFα measured 15 days after treatment among experimental groups.
| IL1 | Treatment | Mean ± SD a | P-value b |
| Normal | 0.06 ± 0.03 | ||
| MIA (Untreated) | 0.26 ± 0.02 | ||
| NCur | 0.13 ± 0.02 | <0.0001 | |
| PRP | 0.12 ± 0.02 | <0.0001 | |
| NCur +PRP | 0.10 ± 0.02 | <0.0001 | |
| IL6 | |||
| Normal |
0.08 ± 0.04 |
||
| MIA (Untreated) | 0.25 ± 0.03 | ||
| NCur | 0.16 ± 0.02 | 0.0003 | |
| PRP | 0.11 ± 0.01 | <0.0001 | |
| NCur +PRP | 0.09 ± 0.01 | <0.0001 | |
|
TNFα |
|||
| Normal | 0.06 ± 0.04 | ||
| MIA (Untreated) | 0.22 ± 0.03 | ||
| NCur | 0.13 ± 0.03 | 0.0006 | |
| PRP | 0.13 ± 0.01 | <0.0001 | |
| NCur +PRP | 0.11 ± 0.02 |
<0.0001 |
a Measured as pg/ml.
b P-value when compared to untreated MIA group with student’s t-test.
Histopathology
The joints of the control group showed smooth articular surfaces with relatively flattened chondrocytes in the superficial zone and the cells sparsely and randomly distributed in the transitional zone while chondrocytes radially oriented in the radial zone of articular cartilage with intact tidemark. Intercellular matrix was deeply and uniformly stained with Safranin O (Figures 4A & 5A).
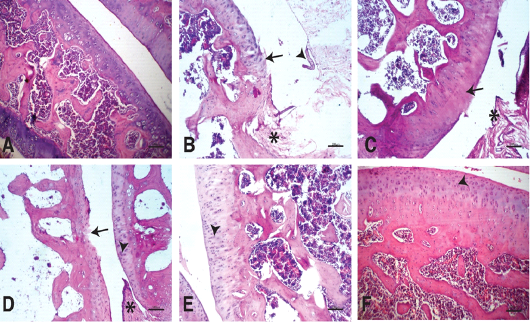
Figure 4: Photomicrographs of knee joints (H & E stain): A) Normal knee joint. Chondrocytes are viable across all regions of the joint articular surface. B) Untreated knee joint 15 days after MIA injection. Fissures and clefts into the transitional and radial zones together with fibrillation(arrow),synovial hypertrophy (arrowhead), pannaus formation (star). C) Untreated knee joint 30 days after MIA injection. Hypocellularity and thinning of the articular cartilage (arrow), pannaus formation (star). D) Knee joint treated by NCur. Hypercellularity with moderately reactive chondrocytes (arrowhead). Pyknotic chondrocytes, articular surface fibrillations and clefts (arrow), Pannus formation (star). E) Knee joint treated by PRP. Hypercellularity of the articular cartilage with highly reactive chondrocytes. The articular cartilage thickness returned to normal (arrowhead). F) knee joint treated by NCur+PRP. Restoration of all zones of the articular cartilage to similar to normal (arrowhead).
Fifteen days after induction of osteoarthritis via MIA intra-articular injection, the knee joints showed disorganization and severe degeneration of the articular cartilage. The articular surfaces showed fissures and clefts into the transitional and radial zones together with fibrillation and pannus formation. Apoptotic chondrocytes were common, and the intracellular matrix showed severe reduction in the intensity of Safranin O staining indicating significant loss of proteoglycans (Figures 4B & 5B). At the end of the experiment (30 days after MIA injection), the untreated group still showed similar lesions in addition to marked hypocellularity and thinning of the articular cartilage (Figures 4C & 5C).
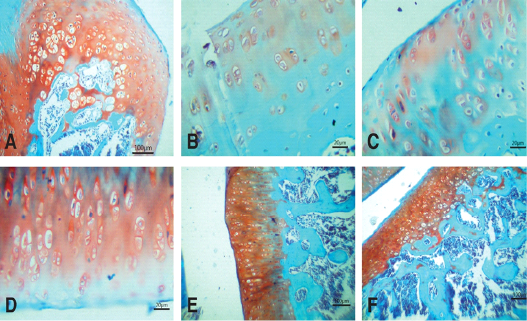
Figure 5: Photomicrographs of knee joints (Safranin O stain). A) Normal joint. Strong staining of matrix can be seen. B) Untreated joint 15 days after MIA injection. Very weak staining of matrix is observed. C) Untreated joint 30 days after MIA injection. Negative staining of matrix is observed. D) NCur treated joint. Weak staining of matrix is observed. E) PRP treated joint. Moderate staining of matrix is observed. F) NCur + PRP treated joint. Strong, uniform matrix staining similar to normal joint
Fifteen days after beginning treatment, the joints of the NCur treated group showed slight hypercellularity with moderately reactive chondrocytes. Pyknotic chondrocytes, articular surface fibrillations and clefts were still evident. The intercellular matrix showed weak staining intensity with Safranin O (Figures 4D & 5D).
The PRP treated group showed hypercellularity of the articular cartilage with highly reactive chondrocytes. The articular cartilage thickness returned to normal and the intensity of the Safranin O staining was moderate (Figures 4E & 5E).
The joints of the group where the rats were treated with both PRP injection and NCur gavage showed significant improvement of the articular surface with restoration of all zones of the articular cartilage to similar to normal. The cartilage showed uniform and intensely Safranin O stained intercellular matrix (Figures 4F & 5F).
The overall Mankin score of the histopathological lesions showed a significant improvement in the treated groups compared to the untreated group with the joints of the rats treated by PRP + NCur showing the lowest scores among the treated groups (P < 0.0001) as shown in Figure 6 and Table 2.
Table 2: Mankin scoring of knee joints 15 days post treatment among experimental groups.
| Groups | Total Mankin | P-value | Structure | Cellularity | Matrix staining | Tidemark integrity |
| Normal | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 | 0.0±0.0 | |
| MIA (Untreated) | 10.17 ± 1.60 | 3.33 ± 0.82 | 2.83 ± 0.41 | 3.33 ± 0.82 | 0.67 ± 0.52 | |
| N(cur) | 6.67 ± 2.50 | <0.0163 | 2.5 ± 1.05 | 1.67 ± 0.82 | 2 ± 0.63 | 0.5 ± 0.54 |
| PRP | 4.17 ± 0.75 | <0.0001 | 1.5 ± 0.55 | 1.17 ± 0.75 | 1.16 ± 0.75 | 0.33 ± 0.52 |
| N(cur)+PRP | 2.17 ± 1.60 | <0.0001 | 0.83 ± 0.75 | 0.67 ± 0.52 | 0.33 ± 0.52 | 0.33 ± 0.52 |
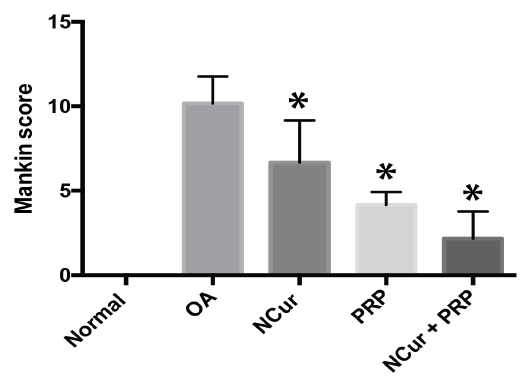
Figure 6: Mankin score of the histopathological lesions 15 days after treatment with NCur, PRP or NCur + PRP. Each bar represents the average levels from 6 rats (pg/ml) ± SD and (*) indicates P-value <0.05 when compared with untreated rats, Student’s t-test.
Discussion
By 2050, approximately 20% of the human population will be above 60 years old (Nations, 2004). The World Health Organization estimates that at least 15% of those aged above 60 will have symptomatic OA, with around 5% suffering severe disability (Kuyinu et al., 2016). Although numerous medications and devices has been made available to treat the associated pain and improve the quality of life of OA patients, a definitive treatment that can reverse the damage induced by OA is yet to be found (Zhang et al., 2016).
Curcumin-loaded nanoparticles (NCur) gavage and platelet rich plasma (PRP) intra-articular injection has both been proposed by previous studies as potential therapeutic strategies for treatment of OA (Dashnyam et al., 2018; Pavlovic et al., 2016), but they haven’t been compared side by side or used simultaneously. In the present study, we compared the ability of NCur and PRP to reverse the effects of MIA induced OA in the knee joints of rats when administered alone or concurrently. Our data showed that while both treatment strategies indeed had the potential to remodel the damaged joints, NCur and PRP can work synergistically to boost that effect. This assessment was based on histopathological examination with Mankin scoring of the lesions, radiographical examination of the joints, and measurement of serum inflammatory cytokines 15 days after MIA intra-articular injection and 15 days after treatment.
MIA is a chondrocyte metabolic inhibitor, it interferes with the activity of glyceraldehydes-3 phosphate dehydrogenase in chondrocytes resulting in inhibition of glycolysis, production of reactive oxygen species (ROS), energy depletion and eventually cell death with induction of an inflammatory reaction. The number of chondrocytes decreases progressively and subsequently, the synthesis of proteoglycan components for the cartilage matrix stops (Moilanen et al., 2015). The resemblance of the histological and biochemical changes induced by MIA in the articular cartilage to the human disease is well established and hence its wide use in experimental induction of OA and its selection for OA induction in our study (Al-Saffar et al., 2009; Naveen et al., 2014).
The observed histopathological changes induced by MIA injection in the knee joints of rats in this study including chondrocytes apoptosis, degeneration and disorganization of the articular cartilage and severe decrease in safranin-O staining intensity of proteoglycans are consistent with both the mechanism of action of MIA and with earlier studies (Naveen et al., 2014; Niazvand et al., 2017b).
Our data showed that NCur gavage for 14 consecutive days could moderately restore the articular damage induced by OA in the form of modest increase in the number and activity of chondrocytes with subsequent slight increase in the Safranin-O staining intensity of the extracellular matrix indicating increased production of proteoglycans by chondrocytes. However, some articular fibrillations and clefts were still evident microscopically and were reflected by irregular articular surfaces radiographically. Systemically, there was a significant decrease in serum levels of the inflammatory cytokines IL6, IL1 and TNF, indicating a fading inflammatory reaction. The anti-inflammatory, reactive oxygen species (ROS) scavenging and anti-catabolic effects of curcumin on different cell types including chondrocytes is well established (Balasubramanyam et al., 2003, Csaki et al., 2009; Henrotin et al., 2010). But, when administered systemically, curcumin has a limited bioavailability; with poor solubility, and rapid metabolism. This bioavailability could be considerably improved by encapsulation of curcumin in nanoparticles (Choi and Han, 2018; Ghalandarlaki et al., 2014). Our results are in line with literature in that respect and accordant with the findings reported by Niazvand et al showing that NCur gavage increased cartilage cellularity and matrix staining in treated animals compared to MIA control group (Niazvand et al., 2017a). This can be attributed to the decreased levels of IL6, IL1 and TNF, which are in addition to being inflammatory are also catabolic cytokines that induces the release of matrix proteases and aggrecanases resulting in degradation of the cartilaginous matrix (Martel-Pelletier, 2004). IL1 has also been shown to activate caspase-3 pathway in chondrocytes inducing its apoptosis (Shakibaei et al., 2005). The decrease in these cytokines might be credited to the ROS scavenging properties of curcumin.
PRP intra-articular injection resulted in reasonable reconstruction of the knee joints; with marked reactivity and cellularity of the articular cartilage and moderate intensity of proteoglycans staining microscopically accompanied with improved joint space and articular surface opacity radiographically. This was along with significantly decreased serum levels of IL6, IL1β and TNF. Similar findings have been reported by (Almasry et al., 2015; Gamal et al., 2019). Platelet-rich plasma (PRP) is a volume of plasma with a concentration of platelet that is higher than the average in peripheral blood (Laudy et al., 2015). Recently, PRP has gained a growing interest as an autologous blood product that can assist tissue healing in a lot of applications (Lynch and Bashir, 2016). The platelets in the PRP, when activated, release the contents of their -granules which is a pool of growth factors, cytokines and other bioactive molecules responsible for attracting macrophages and mesenchymal stem cells facilitating tissue regeneration and promoting scavenging of necrotic tissue (Anitua et al., 2014).
Huang et al. (2018), Bendinelli et al. (2010) and Wu et al. (2011) reported that platelets in PRP could release anti-inflammatory cytokines, including IL1 receptor antagonist and soluble tumor necrosis factor receptor. IL1 receptor antagonist could inhibit the bioactivity of IL-1 by blocking its receptors, while soluble tumor necrosis factor receptor could bind to free TNFα, thereby preventing signal transduction. (Huang et al., 2018) also proved that PRP counteracted the inflammatory cascade elicited by IL-1ß and tumor necrosis factor-alpha (TNF-α), showing an inhibition of IL-1ß, COX-2 and MMP-2 gene expression.
The group of rats that received the NCur gavage together with the PRP intra-articular injection showed the best repair of the articular cartilage and the joint as a whole as indicated by the lowest Mankin score and almost normal radiographical picture. While only slightly lower than the NCur and PRP groups, the average serum levels of IL6, IL1B and TNFα were significantly lower than the MIA control group. This almost efficient repair can be due to a complementary action of the two agents acting via different pathways to restore the damaged joint.
Finally, we conclude that, while both NCur and PRP has comparable ability to restore the OA damage induced by MIA in the knee joints of rats, but better results achieved when administered concurrently and thus, further investigation of the applicability of this treatment strategy might be warranted.
acknowledgements
The authors would like to thank DR Manar Ahmed Abdel-mgeed ,lecturer of pathology ,faculty of veterinary medicine,Zagazig university,for her valuable help during this work.
Conflict of Interest
The authors declare that there are no conflicts of interest.
authors contribution
All authors contribute to this research equally. They made available relevant literatures and conducted examinations as well. All authors participated in draft and revision of the manuscript. All authors read and approved the final manuscript.
REFERences






