Advances in Animal and Veterinary Sciences
Research Article
Advances in Animal and Veterinary Sciences 2 (2): 111 – 115Apoptotic Gene Expression in Endosulfan Exposed IBD Infected Birds
Deepak Kumar1*, Atul Prakash2, Kuldeep Dhama3, Swati Saxena1, Satish Kumar4, Sanjeev Kumar Shukla5, J. K. Malik6
- Division of Veterinary Biotechnology, Indian Veterinary Research Institute, Izatnagar, Bareilly
- Department of Pharmacology, College of Veterinary Science and Animal Husbandry, Pandit Deen Dayal Upadhyay Veterinary and Animal Sciences University, Mathura, India
- Avian Disease Section, Indian Veterinary Research Institute, Izatnagar, Bareilly
- Central Instrumentation Facility, Indian Veterinary Research Institute, Izatnagar, Bareilly
- Division of Animal Genetics, Indian Veterinary Research Institute, Izatnagar, Bareilly
- Division of Pharmacology and Toxicology, Indian Veterinary Research Institute, Izatnagar, Bareilly
*Corresponding author: deep_biotek@yahoo.com
ARTICLE CITATION:
Kumar D, Prakash A, Dhama K, Saxena S, Kumar S, Shukla SK, Malik JK (2014). Apoptotic gene expression in endosulfan exposed IBD infected birds. Adv. Anim. Vet. Sci. 1 (2): 111 – 115.
Received: 2013–12–04, Revised: 2014–01–05, Accepted: 2014–01–07
The electronic version of this article is the complete one and can be found online at
(http://dx.doi.org/10.14737/journal.aavs/2014/2.2.111.115)
which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Chlorinated insecticide endosulfan is a common environmental contaminant. Humans, animals, and birds are equally exposed through water and food. The side effects of the endosulfan are comparatively well documented; the toxicological impact of endosulfan in infectious bursal disease (IBD)–infected birds is still unpredictable and unknown. The present study was undertaken to assess whether the endosulfan exposure in IBD infected birds induces significant alterations in apoptotic, heat stress and genes of immunological functions. The chicks were divided into three groups (Group–1: Control, Group–2: IBD infected birds and Group 3: endosulfan intoxicated IBD infected birds). All the chicks from group–2 and –3 were infected with IBD IM+ strain in the laboratory on day 7 and chicks of Group–3 were exposed to 30 ppm of endosulfan–mixed feed for up to 28 days. After the exposure period of 21 days, chicks were sacrificed. Heparinized blood and the liver were collected aseptically and processed under standard protocol of RNeasy® Minikit of Qiagen for the gene expression study with single step real–time PCR and DNA content analysis using Propedium Iodide dye using FACS (Fluorescence–activated cell sorting) technique. The FACS analysis showed increased percentage of apoptotic cells in Group–3 birds from that of Group–2 and Group–1 birds. The mRNA level expression of apoptogenic genes (Caspases–3 and NF–κB) was found positively regulated in the Group–3 and Group–2 birds as compared to Group–1. However the expression of Caspase–3 and NF–κB was significantly higher in Group–3 from Group–2 birds. In Group–2 and Group–3 the genes of cell protection machinery such as hemeoxigenase 1, Cyclin–D1, HSP–70, and antiapoptotic mitochondrial pathway genes expression BCL–Xl, BCL–2, MCL–1, apoptotic pathway genes CARD, RIPIC, FAS–L, Caspase–10 were negatively regulated as compared to Group–1 birds, but their expression was more profound in Group–2 birds. The results of the study imply that the experimental exposure of birds with Endosulphan follows Caspase–3, NF– κB and mitochondrial pathways of apoptosis in the liver of IBDV infected birds. This is the preliminary report describing the mechanism of apoptosis in liver following exposure to endosulfan at environmentally realistic concentrations in IBD infected birds. The study suggests that in presence of toxicants in feed/water, the pathogenic disease may produce differential and rather more pathogenic consequences.
Introduction
Endosulfan is a member of the cyclodiene group of organochlorine pesticides and is used worldwide in agriculture. The cyclodiene insecticides are among the most toxic and environmentally persistent insecticides known so far. Endosulfan is a highly toxic pesticide, and it belongs to toxicity class 1 in the Environmental Protection Agency (EPA) (ExToxNet, 2000). Endosulfan is also a known environmental endocrine disrupter (Rose et al., 1999). Organochlorine insecticides are now harshly regulated in the United States but are still in production and are used extensively in developing countries because of their effectiveness and inexpensiveness. Along with picrotoxin–binding, cyclodienes inhibit Na+, K+–ATPase, but more importantly inhibit Ca2+, Mg2+–ATPase. This leads to an accumulation of intracellular free calcium ions, which promotes the release of neurotransmitters and subsequent depolarization of adjacent neurons.
Endosulfan is stable in the environment and, because of its lipophilicy, can be stored in fat tissue and released later during sickness or starvation. Endosulfan has genotoxic effects on HeoG2 cells (Yuquan et al., 2000) and has been found to inhibit testicular function in pubertal rats (Chitra et al., 1999). Along with these negative effects, endosulfan is immunotoxic, inhibiting the metabolic activity of peripheral blood lymphocytes and splenocytes in chickens (Aggarwal et al., 2008), induces oxidative stress (Sohn et al., 2004, Ayub et al., 2003, El–Shenawy, 2010, Pradhana et al., 2012) and apoptosis (Aggarwal et al., 2003, Kannan et al., 2000).
IBDV is a double stranded RNA virus belonging to the Avibirnavirus genus in the family Birnaviridae causes an acute disease known as Gumboro disease. This disease is characterized by high mortality and morbidity. Chickens infected with IBDV at 3 weeks of age usually have little or no clinical signs but they are immunosuppressed. IBDV can induce apoptosis in chicken peripheral blood lymphocytes (Vasconcelos and Lam 1994, Jain et al., 2013), indicating that it may play a crucial role in the depletion of lymphoid cells, in apoptosis and immunosuppression. Previous studies have shown chicken embryo fibroblasts exhibited morphological and biochemical features of apoptosis when infected in vitro with IBDV (Tham et al., 1995, Kumar 2009). Infection of chicken embryos and young chickens with IBDV induced contagious disease of young chickens (Vasconcelos and Lam 1995).
There are several types of viruses and pesticides contribute to oxidative stress and apoptosis. Reports also confirm the ability of endosulfan and IBDV to induce apotosis independently. But no report confirms the toxic effects of endosulfan inducing apoptosis, heat stress and other related genes of apoptotic functions in IBDV infection. The main purpose of the present research was to investigate the side effects of sub acute exposure of endosulfan in environmentally realistic conditions on IBD infected chickens, and secondarily to analyze the potential pathways involved in endosulfan–induced apoptosis in IBDV infected birds.
MATERIALS AND METHODS
Animals
Day old chicks were procured from Central Avian Research Institute, Izatnagar, India. The chicks were acclimatized for seven days and maintained under controlled conditions of temperature and light (12/12–h light/dark cycle). Food and water were provided ad–libitum. After the period of acclimatization the chicks were divided into three groups (Control (Group–1), IBD infected birds (Group–2) and endosulfan intoxicated IBD infected birds (Group–3) comprising seven birds in each group.
Endosulfan was purchased from Sigma Aldrich Ltd. and was mixed in feed with a rate of 30 ppm (30 mg/kg feed). The endosulfan–mixed feed were prepared just before use and fed to chicks ad–libitum.
Treatments
All the chicks were infected with the IBD IM+ strain by ocular route with 103.0 TCID50 of virus on day 7. Same day chicks were exposed to 30 ppm of endosulfan–mixed feed for up to 28 days. The dose of the endosulfan in the present study was chosen based on the study of Aggarwal et al., (2003).
Sample Collection
After the exposure period of 21 days, chicks were sacrificed. Heparinized blood, bursa and the liver were collected aseptically and processed under standard protocol of RNeasy® Minikit of Qiagen for the gene expression study with single step real–time PCR and DNA content analysis using PI dye using FACS (Fluorescence–activated cell sorting) technique.
Apoptosis Detection (Flowcytometric Method Using Propidium Iodide Dye)
The experimental birds were sacrificed and bursa was collected. Bursa was minced finely with scissors, and minced tissue was washed several times in PBS. Bursal cell suspension obtained from control and IBDV infected birds were layered on Histopaque and centrifuged at 1500 r.p.m. for 10 minute. The cells at the interphase was collected, washed twice in Phosphate buffer saline and suspended in 500 µl of propidium iodide hypotonic buffer and incubated at 37 ͦ C for 15 Minuit in the dark. The PI fluorescence was measured through FL–2 filter in Becton Dickenson Flowcytometer (NJ, USA).
Real–Time Polymerase Chain Reaction (RT–PCR)
The aseptically collected liver were homogenized for single cell suspension and total RNA was isolated using RNeasy® Minikit of Qiagen under standard protocol for the gene expression study with single step real–time PCR. The primer set for NF– κB, caspase–8, FAS–L, BCL–Xl, BCL–2, cyclin–D1, Heme oxygenase (decycling)–1 (HMOX1), heat shock protein–70 (HSP–70), and glyceraldehyde phosphate dehydrogenase (GADPH) (House Keeping Gene) were the published sequences (Kumar et al., 2010). Other primer sequences used were sense: 5’–CACGGACGCATTGGTCTCAT–3’, anti–sense: 5’–CAGGTCCTCAACTCGGAAGAAG–3’ for MCL–1; sense: 5’–GTGTGGCAGCAGAAGCAATG–3’, anti–sense : 5’–TTAGCACCATCCCTGAGACAAC–3’ for Ripic, Receptor–Interacting Serine–Threonine Kinase–2, (RIPK–2); sense: 5’–CAGCGAGAAGGACATCAAC–3’, anti–sense: 5’–AAGGAGCCACAGGACAAG–3’ for Caspase Recruitment Domain Family, Member–10 (CARD); sense: 5’–TATTCTACTGCTCCAGGCTACTAC–3’, anti–sense: 5’–ACACGAGTTAAAATCTGCATGAGT–3’ for Apoptosis–Related Cysteine Peptidase (caspase–3) and Sense : 5’–CAGCGTCCTGTCTATGTTG–3’; anti–sense: 5’–AAGAGGCATATCCATCAATCG–3’ for Apoptotic Cysteine Protease (Caspase–10). We used a relative quantification method (∆∆Ct method) to calculate the gene expression values as described (User Bulletin No. 2, Applied Biosystems). In brief, the amplification plot is the plot of fluorescence versus PCR number. The threshold cycle value (Ct) is the fractional PCR cycle number at which the fluorescent signal reached the detection threshold. Therefore, the input mRNA copy number and Ct are inversely related. Ct value was automatically converted to fold change RQ value. The Fold change (RQ) =2 – (∆∆CT), where = (∆∆CT)=–(∆CTtrt–∆CT control)= –[(CtTarget–CtGADPH)trt–(CtTarget–CtGADPH)control. The RQ values from each gene were then used to compare the apoptotic gene expression across all time intervals. Statistical analysis of data was performed using SPSS 10 software. Various parameters were expressed as mean±SE. Means of various parameters in IBDV Im+ virus treated group with control group by using one way ANOVA with Tukey’s B multiple comparisons as post hoc test (Snedecor and Cocharan, 1989). A p value of p < 0.05 was considered as statistically significant.
RRESULT AND DISCUSSION
The FACS analysis using Propedium Iodide dye showed increase in the percentage of apoptotic cells in bursal cells harvested from endosulfan fed IBDV infected birds from that of IBD alone infected and control birds. In endosulfan intoxicated IBDV infected birds the proportion of cells with a lower DNA content increased considerably, than the IBDV alone infected and control birds, indicating the profound induction of apoptosis.
Previous studies revealed that endosulfan induces oxidative stress (Dorval et al., 2003 Sohn et al., 2004, Ayub et al., 2003, Dorts et al., 2009, Ballesteros et al., 2009, El–Shenawy 2010). Previous studies have indicated that certain chemical exposures can result in the alteration of secondary messengers, such as free radicals or ROS, and these alterations have been linked to the induction of apoptosis in immune cells (Corcoran et al., 1994). Free radicals influence gene expression, regulate cellular responses to cytokines, as well as proliferative events of a cell. All these events have also been implicated as possible triggering mechanisms of apoptosis. Oxidative stress may trigger the mitochondrial pathway. Increased permeability of the mitochondrial membrane during apoptosis triggers the release of cytochrome c from mitochondria in the apoptotic process. The release of cytochrome c into the cytoplasm is a critical step, which is followed by the activation of different caspases like caspase–9 and caspase–3 (Shen et al., 2004). In the present study there is significant down regulation of mitochondrial pathway genes like MCL–1 and BCL–XL was observed in both the endosulfan intoxicated IBDV infected birds (0.36±0.23 and 0.33±0.008 respectively) (Figure 1) and IBDV alone infected birds and (0.11±0.0012 and 0.008±0.0012 respectively) in comparison to control birds. Down regulation in of mitochondrial pathway genes were significantly more profound in the IBDV alone infected birds. This finding suggests though the roles of intrinsic pathway genes in apoptosis of hepatocytes in IBDV infected endosulfan intoxicated birds are there but it is of less magnitude in endosulfan intoxicated IBDV infected birds. This resulted in several morphological and biochemical changes leading to apoptosis of the cell (Hengartner 2000). Mitochondrial (Intrinsic) pathway was shown to get activated at different time point of virus life cycle in IBDV induced apoptosis (Kumar et al., 2010). Anti–apoptotic genes like BCL–2 and BCL–Xl acts as inhibitors of cell death and the suppression of apoptosis. BCL–2 can neutralize one another’s function, suggesting that their relative concentration in the given cell may act as a rheostat for the suicide program. BCL–2 is localized on the cytoplasmic face of the mitochondrial outer membrane, endoplasmic reticulum and nuclear envelope and counter balance the oxidative damage done to these compartments and affect their behavior (Hockenberry 1995, Antonsson et al., 1997, Yang and Korsmeyer 1999).
Mitochondrial DNA is also heavily damaged by ROS at the bases. Transcription factors such as NF–kB, p53 and AP–1 are also sensitive to redox changes in mammalian cells, primarily through redox regulation of their DNA binding regions (Morel and Barouki 1999; Ho and Bray 1999; Du et al., 1999; Williams and Smith 1993; Li et al., 2013; Li et al., 2014). NF–kB is a transcription factor that has a crucial role in regulation of cell survival, protecting cells from apoptosis under most circumstances and accelerating apoptosis in others. NF–kB is activated via degradation of the inhibitor of NF–kB (IkB. In the present study, gene expression studies by real–time PCR revealed the mRNA level expression of apoptogenic genes Caspases–3 and NF–kB were found positively regulated in the endosulfan intoxicated IBDV infected birds (1.33±0.22 and 3.99±1.2 respectively) and down regulated in IBD alone infected birds ( 0.93±0.0014 and 0.10±0.0029 respectively) in comparison to control birds.
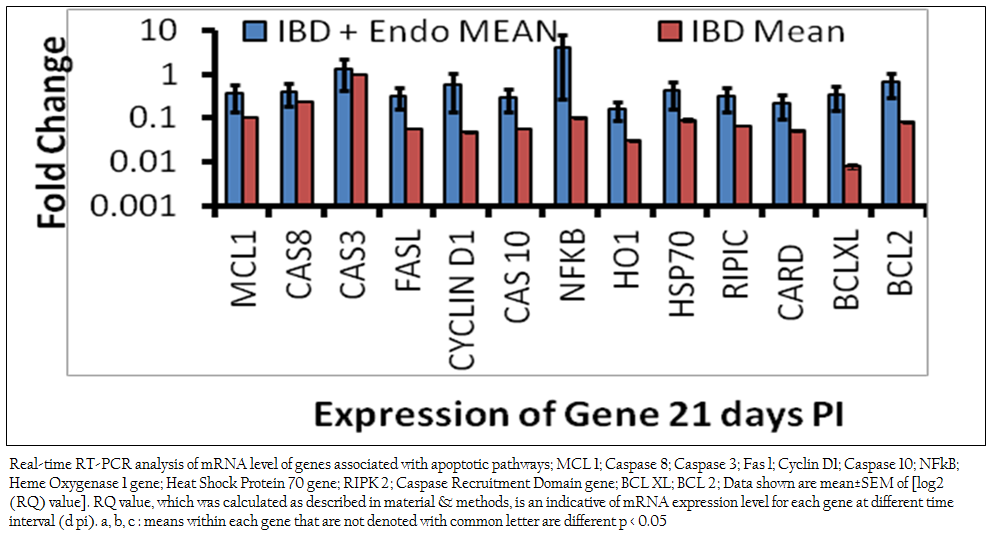
Figure 1: Real-time RT-PCR analysis of mRNA level of genes associated with apoptotic pathways; MCL 1; Caspase 8; Caspase 3; Fas l; Cyclin D1; Caspase 10; NFkB; Heme Oxygenase 1 gene; Heat Shock Protein 70 gene; RIPK 2; Caspase Recruitment Domain gene; BCL XL; BCL 2; Data shown are mean±SEM of [log2 (RQ) value]. RQ value, which was calculated as described in material & methods, is an indicative of mRNA expression level .........................
The result suggests that the NF–kB pathway and the executioner caspase of extrinsic/intrinsic pathway, caspase–3 plays the pivotal role in endosulfan induced apoptosis in IBDV infected birds. Endosulfan induces uncoupling of oxidative phosphorylation. Oxidative stress occurs under conditions when oxygen radical production is greater than the detoxification capacity of the cell (Kowaltowski and Vercesi 1999, Halliwell and Gutteridge 1989).Oxidative stress occurs when redox homeostasis within the cell is altered. Injury to cells occurs only when the ROS overwhelm the biochemical defenses of the cell (Sies 1986, Horton and Fairhurst 1987, Sies 1985). Reactive oxygen species, (in particular, hydroxyl radicals) can react with all biological macromolecules, i.e. lipids, proteins, nucleic acids and carbohydrates. The initial reaction generates a second radical, which reacts with a second macromolecule and oxidative damage in membrane phopholipids, pose a constant threat to cellular integrity and function. The lipid radicals generated during the early encounter with an oxidant add molecular oxygen to produce lipid dioxyl radical (Jaescheke 1995).
It appears that oxidative stress acts as a pleiotropic modulator in both these pathways. Indeed, ROS have been described as second messengers for several growth factors and cytokines. It has been shown that the transcription factors such as NF–kB and AP–1, which is stimulated by ROS, could mediate apoptotic gene expression. Under conditions of mild oxidative stress, cell cycle–related genes are repressed to increase the lengthening of G1–phase. Excess ROS production causes GSH depletion and oxidative stress. Oxidative stress leads to disruption of the mitochondrial transmembrane potential and causes release of cytochrome C and other apoptosis related proteins to cytosol and finally leads to apoptosis. In the present study increased apoptotic DNA content may be due to endosulfan–induced ROS generation and oxidative damage to the cells (Kannan et al., 2000). IBDV induces apoptosis through activation of caspases (Kumar et al., 2009). Genes like hemeoxigenase–1 (0.16±0.07), Cyclin–D1 (0.58±0.44) and HSP–70 (0.42± 0.26) were negatively regulated as compared to control birds suggest that cells prosurvival machinery is not selected till this stage of intoxication. There was significant down regulation in FAS–L (0.32±0.16) gene in endosulfan exposed IBD infected birds as compared to control and IBD infected birds revealed that the activation of Caspase–3 and increased mRNA level expression might have followed the intrinsic pathway of apoptosis by activating Caspase–8. Apoptotic pathway genes CARD, RIPIC, FAS–L, Caspase–10 down regulation shows there less significance in combined apoptosis induced by IBDV infection and sub acute endosulfan intoxication.
The gene expression, in general, is modulated by both physiological and environmental stimuli. The results of the study confirm that the experimental exposure of IBDV infected birds with endosulfan affect mitochondrial pathway, Caspase–3 (extrinsic/intrinsic pathway) and NF– κB (oxidative stress–induced) pathways of apoptosis in the liver. The study shows that the infectious disease IBD in conjugation with the environmental pesticides pollutants like endosulfan, may lead to grave prognosis of the disease and may cause more economic losses.
REFERENCES
Aggarwal M, Naraharisetti SB, Tiwari AK, Degen GH, Malik JK (2008). Assessment of apoptosis in peripheral blood lymphocytes and splenocytes of chickens simultaneously exposed to arsenite in drinking water and endosulfan in feed. Toxicol. Lett. 180 (Suppl. 1): S208
http://dx.doi.org/10.1016/j.toxlet.2008.06.114
Aggarwal MK, Tiwari AK, Rao GS, Malik JK (2003) Endosulfan–induced apoptosis in chickens. Toxicol. Lett. 144 (Suppl. 1): S147.
http://dx.doi.org/10.1016/S0378-4274(03)90547-9
Ayub S, Verma J, Das N (2003) Effect of endosulfan and malathion on lipid peroxidation, nitrite and TN–α release by rat peritoneal macrophages. Intl. Immunopharmacol. 3(13 – 14): 1819 – 1828
Ballesteros ML, Wunderlin DA, Bistoni MA (2009) Oxidative stress responses in different organs of Jenynsia multidentata exposed to endosulfan. Ecotoxi. Enviro. Safety. 72(1): 199 – 205.
http://dx.doi.org/10.1016/j.ecoenv.2008.01.008
PMid:18308394
Chitra KC, Latchoumycandane C, Mathur PP (1999). Chronic Effect of endosulfan on Testicular Functions of Rat. Asian. J. Androl. 1: 203 –206.
PMid:11225895
Corcoran GB, Fix L, Jones DP, Moslen MT, Nicotera P, Oberhammer FA, Buttyan R (1994). Apoptosis: molecular control point in toxicity. Toxicol. Appl. Pharmacol. 128: 169 – 181.
http://dx.doi.org/10.1006/taap.1994.1195
PMid:7940532
Dorts J, Silvestre F, Thi TH, Tyberghein A, Phuong NT, Kestemont P (2009). Oxidative stress, protein carbonylation and heat shock proteins in the black tiger shrimp, Penaeus monodon, following exposure to endosulfan and deltamethrin. Environ. Toxicol. Pharmacol. 28(2): 302 – 310.
http://dx.doi.org/10.1016/j.etap.2009.05.006
PMid:21784020
Dorval J, Hontela A (2003). Role of glutathione redox cycle and catalase in defense against oxidative stress induced by endosulfan in adrenocortical cells of rainbow trout (Oncorhynchus mykiss). Toxicol. App. Pharmacol. 192(2): 191 – 200.
http://dx.doi.org/10.1016/S0041-008X(03)00281-3
Du X, Stockklauser FK, Rosen P (1999). Generation of reactive oxygen intermediates, activation of NF–κB and induction of aoptosis in human endothelial cells by glucose: role of nitric oxide synthase, Free Radic. Biol. Med. 27: 752 – 763.
http://dx.doi.org/10.1016/S0891-5849(99)00079-9
El–Shenawy NS (2010). Effects of insecticides fenitrothion, endosulfan and abamectin on antioxidant parameters of isolated rat hepatocytes. Toxi. Vitro. 24(4): 1148 – 1157
http://dx.doi.org/10.1016/j.tiv.2010.03.001
PMid:20214973
Hengartner MO (2000). The biochemistry of apoptosis. Nature. 407: 770 –776.
http://dx.doi.org/10.1038/35037710
PMid:11048727
Ho E, Bray TM (1999). Antioxidants, NF kappaB activation, and diabetogenesis. Proc. Soc. Exp. Biol. Med. 222: 205 – 213.
http://dx.doi.org/10.1046/j.1525-1373.1999.d01-137.x
PMid:10601879
Hockenberry DM (1995) Bcl–2, a novel regulator of cell death, BioEssays. 17: 631 – 638.
http://dx.doi.org/10.1002/bies.950170709
PMid:7646485
Jaescheke H (1995). Mechanisms of oxidant stress–induced acute tissue injury. Proc. Soc. Exp. Biol. Med. 209: 104 – 111.
http://dx.doi.org/10.3181/00379727-209-43885b
Jain P, Singh R, Saxena VK, Singh KB, Ahmed KA, Tiwari AK, Saxena M, Sundaresan NR (2013) In–vitro rapid clearance of infectious bursal disease virus in peripheral blood mononuclear cells of chicken lines divergent for antibody response might be related to the enhanced expression of proinflammatory cytokines. Res. Vet. Sci. 95: 957 – 964.
http://dx.doi.org/10.1016/j.rvsc.2013.08.018
PMid:24075224
Kannan K, Holcombe RF, Jain SK, Alvarez–Hernandez X, Chervenak R, Wolf RE, Glass J (2000) Evidence for the induction of apoptosis by endosulfan in a human T–cell leukemic line. Mol. Cell. Biochem. 205(1–2): 53 – 66.
http://dx.doi.org/10.1023/A:1007080910396
PMid:10821422
Kowaltowski A, Vercesi AE (1999). Mitochondrial damage induced by conditions of oxidative stress, Free Radic. Biol. 26: 463 – 471.
http://dx.doi.org/10.1016/S0891-5849(98)00216-0
Kumar D (2009). Elucidation of apoptotic pathway in IBDV infection using real time PCR. PhD Thesis submitted to Deemed University Indian Veterinary Research institute, Izatnagar, India.
Kumar D, Tiwari AK, Kumar S (2010). Elucidation of Apoptotic Pathways in Infected Broiler Chickens induced by Infectious Bursal Disease Virus. World's Poult. Sci. J. 66 (Suppl.): 835
Li K, Gao L, Gao H, Qi X, Gao Y, Qin L, Wang Y, Wang X (2013). Codon optimization and woodchuck hepatitis virus posttranscriptional regulatory element enhance the immune responses of DNA vaccines against infectious bursal disease virus in chickens. Virus Res. 175: 120 – 127.
http://dx.doi.org/10.1016/j.virusres.2013.04.010
PMid:23631937
Li K, Gao L, Gao H, Qi X, Gao Y, Qin L, Wang Y, Wang X (2014). Recombinant infectious bursal disease virus expressing Newcastle disease virus (NDV) neutralizing epitope confers partial protection against virulent NDV challenge in chickens. Anti. Res. 101(1).
Morel Y, Barouki (1999). Repression of gene expression by oxidative stress, Biochem. J. 342: 481 – 496.
http://dx.doi.org/10.1042/0264-6021:3420481
PMid:10477257 PMCid:PMC1220487
Pradhana SN, Prince PR, Madhumathi J, Roy P, Narayanan RB, Antony U (2012). Protective immune responses of recombinant VP2 subunit antigen of infectious bursal disease virus in chickens. Vet. Immuno. Immunopat. 148: 293 – 301.
http://dx.doi.org/10.1016/j.vetimm.2012.06.019
PMid:22795186
Rose RL, Hodgson E, Roe RM (1999). Pesticides, in Toxicology. H Marquart, SG Schafer, RO Ecobichon DJ. 1996. Toxic Effects of Pesticides, in Casarett & Doulls Toxicology: The Basic Science of Poisons, 5th edition. CD Klaassen (ed). McGraw Hill. N.Y. 643 – 683.
Sies H (1986). Biochemistry of oxidant stress, Agrew. Chem. Int. Ed. 25: 1058 –1071.
Sies H (1985) Oxidative Stress, Academic Press, London, 1985.
PMCid:PMC1144742
Snedecor GW, Cochran WG (1989). Statistical Methods, Eighth Edition, Iowa State University Press.
Sohn H, Kwon C, Kwon G, Lee J, Kim E (2004). Induction of oxidative stress by endosulfan and protective effect of lipid–soluble antioxidants against endosulfan–induced oxidative damage. Toxicol Lett. 151(2):357 – 365.
http://dx.doi.org/10.1016/j.toxlet.2004.03.004
PMid:15183460
Vasconcelos AC, Lam KM (1995). Apoptosis in chicken embryos induced by the infectious bursal disease virus. J. Comp. Pathol. 112: 327 – 338.
http://dx.doi.org/10.1016/S0021-9975(05)80014-3
Vasconcelos AC, Lam KM (1994). Apoptosis induced by infectious bursal disease virus. J. Gen. Virol. 75: 1803 – 1806.
http://dx.doi.org/10.1099/0022-1317-75-7-1803
PMid:8021611
Williams GT, Smith CA (1993). Molecular regulation of apoptosis: genetic controls on cell death. Cell. 74: 777 – 779.
http://dx.doi.org/10.1016/0092-8674(93)90457-2
Yuquan LU, Morimoto K, Takeshita T, Takeuchi T, Saito T (2000) Genotoxic Effects of α– endosulfan and β–endosulfan on Human HepG2 Cells. Environ. Health. Perspec. 108: 559 – 561.
http://dx.doi.org/10.2307/3454619
http://dx.doi.org/10.1289/ehp.00108559






