Advances in Animal and Veterinary Sciences
Review Article
Endemic Status and Zoonotic Potential of Avian Influenza Viruses in Egypt, 2006-2019
Mohamed E.M. Mohamed1, Heba A. Ahmed1*, Ahmed M. Erfan2, Lubna Abdelkarim1,3, Maysa A.I. Awadallah1
1Department of Zoonoses, Faculty of Veterinary Medicine, Zagazig University, 44511, Sharkia Governorate, Egypt; 2National Laboratory for Veterinary Quality Control on Poultry Production, Animal Health Research Institute, Giza, Egypt; 3Aga District Veterinary Hospital, Dakahlia Governorate, Egypt.
Abstract | Endemicity of highly pathogenic avian influenza (HPAI) H5N1 and LPAI H9N2 viruses in Egypt represents a threat to animals and humans. Moreover, introduction of HPAI H5N8 virus into Egypt through migratory birds during late 2016 increased the challenge. A novel non distributing H5N2 reassortant was recently detected in domestic ducks in winter 2018. The co-circulation of these subtypes and their zoonotic importance make Egypt a hotspot for the generation of new subtypes of the virus. Occasionally, humans are infected with H5N1 subtype leading to high mortalities; however, no human to human transmission has been reported. Here we review the status of AIV in Egypt from 2006 to 2019 and the mode of transmission of the virus in relation to its zoonotic implications.
Keywords | Egypt, HPAI, LPAI, Zoonoses
Received | September 19, 2019; Accepted | October 26, 2019; Published | December 12, 2019
*Correspondence | Heba A. Ahmed, Department of Zoonoses, Faculty of Veterinary Medicine, Zagazig University, 44511, Sharkia Governorate, Egypt; Email: heba_ahmed@zu.edu.eg
Citation | Mohamed MEM, Ahmed HA, Erfan AM, Abdelkarim L, Awadallah MAI (2019). Endemic status and zoonotic potential of avian influenza viruses in Egypt, 2006-2019. Adv. Anim. Vet. Sci. 7(s2): 154-162.
DOI | http://dx.doi.org/10.17582/journal.aavs/2019/7.s2.154.162
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2019 Mohamed et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Avian flu is described as one of the most common contagious respiratory diseases, affecting millions of birds every year, leading to huge losses in poultry production sector and threats human life (Spackman, 2008). It is caused by Influenza A viruses, negative–sense, single stranded RNA viruses belonging to family Orthomyxoviridae. Their classification is based on their main surface glycoproteins; haemagglutinin (HA) and neuraminidase (NA) into 18 HA and 11 NA subtypes (Wang et al., 2017). According to their pathogenicity, low pathogenic avian influenza viruses (LPAIVs) and highly pathogenic avian influenza viruses (HPAIVs) are the two main types of avian influenza (Swayne and Suarez, 2000). In February 2006, HPAI H5N1 was introduced to Egypt from China by migratory birds, then the virus spread all over the country, which was then considered endemic (Abdelwhab et al., 2016). HPAIVs cause infection and significantly increased mortalities in chickens. Since 2011, the LPAI H9N2 has been detected in domestic quails, and then became endemic in domestic poultry (El-Zoghby et al., 2012). Recently in 2016, H5N8 has also been introduced into Egypt through the common coot (Fulica atra) (Selim et al., 2017). A novel HPAI reassortant of H5N2 subtype was detected in a domestic duck farm in Dakahlia Governorate that seemed to be the first natural reassortant of AIVs in Egypt (Hagag et al., 2019). Hence, with this brief background, Egypt may be considered a hotspot for evolution of new subtypes owing to the co-circulation of three endemic subtypes and the zoonotic importance of H5N1 virus, thus increasing concerns for public and animal health (Abdelwhab and Abdel-Moneim, 2015; Naguib and Harder, 2018). Here we summarized the status and zoonotic potential of avian influenza viruses circulating in Egypt from 2006 until 2019.
Mode of transmission of AIV
The ability of AIV subtypes H1N1, H3N8, H3N2, H6N2, H6N1, H9N2, H4N8, and H10N7 for replication in human cells was proved by experimental infection of 81 healthy human volunteers (Beare and Webster, 1991). The principal modes of AIV transmission include the uncontrolled transportation of infected birds, contaminated fecal material on inanimate and animate vectors including humans (farm workers, veterinarians), vehicles used to transport poultry, and equipment used on poultry farms (Webster et al., 1992). Interspecies transmission occurs as a result of rearing different species together (Swayne et al., 2000). Migratory birds played a vital role in the circulation of AIVs allover Egyptian Governorates. Egypt is a junction ring for the land bridge between Eurasia and Africa, which is included in the migratory flyways of wild birds (Ibrahim, 2013; BirdLife, 2018). The essential areas for migration of waterfowl in Egypt are the wetlands along the Mediterranean coast and the river Nile delta and valley (BirdLife, 2018). The most frequent AIV carriers are wild birds particularly dabbling ducks, gulls and shorebirds; in the northern part of earth (Krauss et al., 2004). They arrive to Egypt in winter each year and infect resident wild waterfowl, in turn, infected waterfowl transmit the viruses to local domestic birds (Naguib et al., 2019). Fortunately, to date; no human cases have been reported due to human to human transmission of H5N1 viruses, thus indicating lack of these viruses to sufficient alterations for inter-human transmission (Kim, 2018).
Reservoirs of avian influenza
Wide range of hosts can be affected by AIV, such as humans, birds and other mammalian species including pigs, horses, dogs, mink, cats, whales and seals. Chickens, turkeys, upland game birds, caged pet birds and wild birds represent most of the major families of birds throughout the world. Waterfowl and other aquatic birds play a vital role as reservoirs for influenza viruses (Alexander, 2007). Porcine cells have both avian and human influenza viruses’ receptors which makes it possible to establish themselves in pig (Ito et al., 1998). Thus, pigs are considered as potential intermediate hosts for the generation of pandemic influenza virus through genetic mutation (Ludwig et al., 1995). Moreover, racing dogs’ infection by equine H3N8 viruses occurred in United States as a result of feeding horse meat (Crawford et al., 2005).
Avian influenza in Egypt
On 17th February 2006, the first wave of AI occurred in Giza and El Menia Governorates in Egypt. Since then, the risk of human exposure to AIV has been increased due to persistence of the virus in the environment (Balish et al., 2010). Poultry production in Egypt is present in many diverse forms, from large- and small-scale commercial sectors, to ‘traditional’ household poultry (HP) keeping where people and birds are in close contact (Aly et al., 2008; El Masry et al., 2014). Biosecurity measures are mostly enforced in farms, while no measures can be successfully applied in backyard sector. Birds reared in the backyard have a potential role in propagation of the virus and infection to whomever in close contact with slaughtered and diseased backyard birds (Hafez et al., 2010).
In January, 2006, the prevalence of H5N1 from migratory birds was investigated in Egypt by collection of 1034 cloacal swab samples from birds caught in live bird markets or trapped in cages in Port Said, Damietta, Fayoum, Arish, and Sharm el Sheikh Governorates (Saad et al., 2007). Two hundred and three samples (15.6%) were positive for Influenza A virus when tested by reverse transcription (RT) PCR and only two (1.9%) were positive for H5 gene. Sequence analysis of the H5 gene from the two migratory birds indicated that both were clustered with HPAI (H5N1) from humans and chickens in Egypt. Figure 1 shows a map with the previously reported AIV serotypes in different Egyptian Governorates.
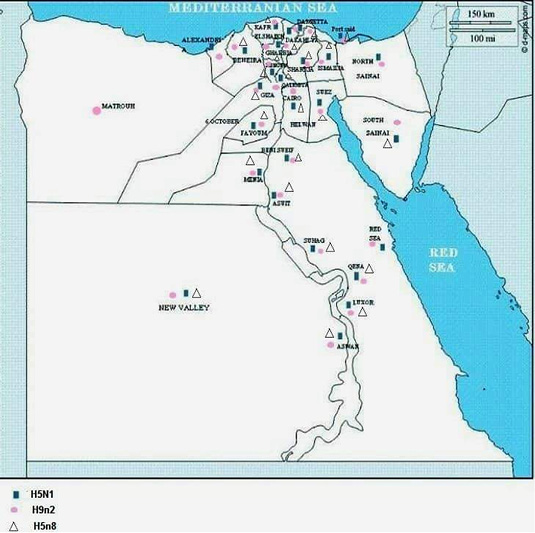
Figure 1: Shows a map with the previously reported AIV serotypes in different Egyptian Governorates.
During February to December of 2006, H5N1 subtype was molecularly detected in 1024 commercial flocks and rural cases from chickens, turkeys, ducks, geese and quails. The isolated subtypes were belonged to subclade 2.2 of the H5N1 virus of Eurasian origin that entered Africa in 2006 by comparing the full sequence of hemagglutinin (HA) gene with previous strains recorded in Africa, Russia and the Middle East (Aly et al., 2008). Moreover, subtype H5N1 virus was detected in 0.97% (35/3,610) and 0.31% (27/8682) of the examined vaccinated commercial poultry farms and 30% (246/816) and 5.2% (89/1723) of backyard birds sampled during an active surveillance in different Egyptian Governorates in 2007 and 2008 (Hafez et al., 2010). The authors interpreted the persistence of infection in Egypt due to the inability of the vaccine to stop the virus circulation especially when the backyard birds play an essential role in its circulation. In addition, during 2005-2007, Mady et al. (2010) reported AIV infection in 13/16 chickens (81%), 1/5 duck (20%) and 1 quail (100%) flocks with a total prevalence of 68% out of the total examined flocks (n=22) using Rapid antigen test. Seven of chicken isolates (53.8%) were subtyped as H5 strains. However, El-Zoghby et al. (2011) could not isolate AI viruses from 400 tracheal and cloacal swabs (200 samples, each) from 64 ducks, 66 chickens, 12 pigeons, 11 turkey and 47 geese during an active surveillance around the border of El-Abassa Lake after the detection of H7 AIV in migratory ducks. However, they detected antibodies in 4.9% serum samples (4/84) against H5 using a commercial generic influenza A indirect enzyme-linked immunosorbent assay (ELISA, Laboratories Inc., Montpellier, France).
Between January and April 2009, H5N1 subtype was molecularly detected in 12.4% (71/ 573) of live bird markets distributed in 24 Governorates in Egypt (Abdelwhab et al., 2010). Moreover, Soliman et al. (2012) detected influenza A matrix gene in 9.4% of cloacal swabs (754/7894) out of migratory birds captured or shot by hunters in different localities (Sinai Peninsula, Nile Delta, Suez Canal, Ismailia, Middle and Upper Egypt) during an active surveillance in Egypt, 2003-2009. From PCR positive samples, only 39 AI viruses were isolated, of which 17 subtypes were identified; H6N2, H5N2, H1N2, H10N1, H1N1, H7N1, H10N7, H11N9, H7N7, H4N6, H9N9, H5N1, H7N9, H7N3, H10N9, H2N8 and H10N4. New AIVs subtypes were identified during this surveillance that have not been previously reported in Egypt. The results confirm the role of wild and migratory birds as mixing vessels for most serotypes of avian influenza.
An argument about the virus distribution and increased pathogenicity occurred in Sharkia Governorate, in March 2011 due to the first description and characterization of HPAIV in naturally infected pigeons in Egypt (Mansour et al., 2014). With this, there began a new era of new clinically affected hosts like pigeons which became more susceptible to H5N1 HPAIVs and therefore could be a source of infection to other birds and humans.
Ali et al. (2015) detected H5 subtype in 81% of freshly dead chicken (87/107) and 100% of the tested farms (21, 14 broilers and 7 layer) using HA, HI tests and real time RT-PCR. Moreover, AI subtype H9 and subtype H5, were respectively detected in 6 flocks (33.3%) and 2 flocks (11.1%) out of 18 examined during an outbreak affecting commercial poultry flocks in Giza, Sharkia and Gharbia Governorates, Egypt during 2012 (Abo-Elkhair et al., 2014). However, Fadel and Afifi (2017) recorded AIVs infection in 91 (67.4%) samples out of 135 wild birds (44 house crows, 6 house teals, 23 moorhen, 33 cattle egrets and 29 semi-captive pigeons) examined during 2010-2013 in Ismailia and Damietta Governorates. The identified subtypes were H5 (51.6%), H7 (1.1%), H9 (36.3%), H5/H9 (8.8%), H7/H9 (1.1%) and H5/H7/H9 (1.1%). In addition, AIVs subtypes H7 and H9 were respectively detected in 417 (37%) and 605 (49.4%) broilers, layers and breeders’ serum samples out of 1225 examined (Afifi et al., 2013). Meanwhile, Awad et al. (2013) molecularly detected Influenza A viruses in 38 (42.2%) commercial and native chicken and duck flocks out of 90 examined in West Delta region, Egypt. The identified subtypes were H5 (31.6%) and H9 (68.4%). Moreover, AIV was detected in 4.3% of tracheal and cloacal samples from chickens (152), ducks (119), geese (44) and turkeys (60) from live bird markets in Suez, Port Said and Ismailia Governorates. Each of H9, and H5 serotypes were detected with a percentage of 56.3% and 43.8%, respectively (Helal et al., 2017). Moreover, Kayed et al. (2016) detected Influenza A virus subtypes H5 and H9 in 4.5% of 4260 (1877 oropharyngeal and 2383 cloacal) swabs collected from backyard flocks, commercial poultry farms, abattoirs and live-bird markets in 10 Governorates (Dakahlia, Gharbia, Menofia, Qalubia,Sharkia, Fayoum, Menia, Beni-Suef, Assiut and Sohag). The prevalence rates were 3.7% in chickens, 2.7% in ducks and 0.9% in pigeons. The highest occurrence was recorded in commercial farms, household and markets (4.6−4.9%) compared to abattoirs (1.3%). Moreover, Ahmed et al. (2017) detected AIV antigen in one broiler flock (6.67%) using the chromatographic test out of 15 examined in Desouk and Qallin (Kafr El-Sheikh Governorate), however, RT-PCR revealed AIVs subtype H5 in two broiler flocks (13.3 %) and one cattle egret out of 30 examined. Meanwhile, AIVs belonged to five different hemagglutinin (H3, H5, H7, H9, and H10) and five different neuraminidases (N1, N2, N3, N6, and N9) subtypes were recorded by Kayed et al. (2019) in 18 samples (1.37%) out of 658 cloacal swabs and 658 oropharyngeal swabs from wild birds at live bird markets along the Egyptian Mediterranean coast during 2014-2016. In addition, Avian influenza H5N1 was identified respectively with percentages of 13.6% and 17%, respectively in birds from 59 farms and 100 LBMs in Sharkia Governorate, while subtype H9N2 was identified from pigeons in farms (6.5%) and LBMs (11.4%) during winter 2015 and 2016 by Tolba et al. (2018). In the same study area during 2015, Gharieb et al. (2019) identified AI subtype H5 in 18% of chicken samples (9/50).
Shehata et al. (2019) recorded single infections with H9N2, H5N1, and H5N8, respectively in 8 (20.5%), 3 (7.7%), and 1 (2.6%) out of 39 commercial broiler farms in 9 Egyptian Governorates (Sharkia, Beni-Suef, Qalubia, Damietta Giza, Behaira, Mattroh, Port Said, and Aswan)
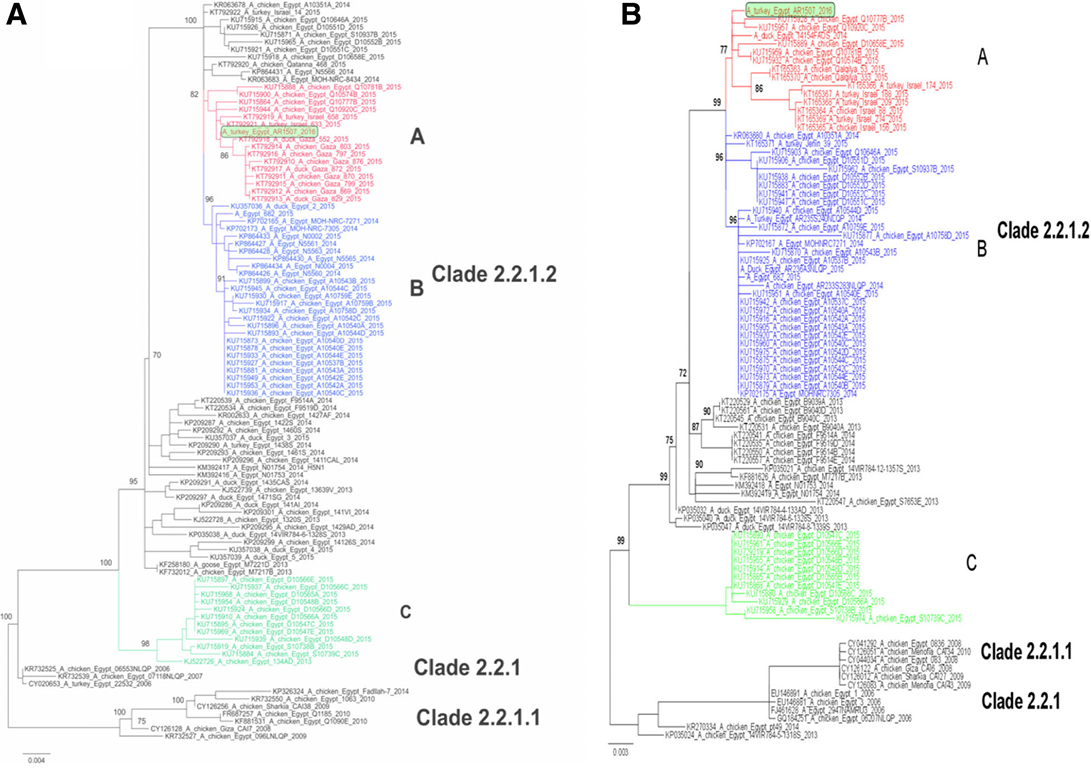
Figure 2: Phylogenetic analysis of the HA (a) and NA (b) gene segments of A/turkey/Egypt/AR1507/2016 to other related H5N1 viruses in poultry and humans. Phylogenetic analysis of the HA (a) and NA (b) genes of Egyptian viruses showing different genetic clades. Viruses in 2015 from Egypt, Gaza and Israel are clustered in clade 2.2.1.2 in three distinct subclades denoted A, B and C. A/turkey/Egypt/AR1507/2016 is located in subclade A along with recent viruses isolated in 2014 and 2015 in poultry in Egypt, Gaza and Israel. Subclade B represents viruses of human and poultry origin in 2014/2015, and subclade C contains viruses from poultry origin in Egypt in 2015 (Salaheldin et al., 2017).
during the period from January 2016 to December 2017. However, single infection with H5N1 was reported in only 2/11 (18.1%) layer farms. Wild birds examined were positive for H5N8 virus [quail (1/4), blue-bird (1/1) and Greenfinch (1/2)].
Anis et al. (2018) identified H5N8 virus in 3 dead ducks from a household flock in Qalubia Governorate, and 3 dead gooslings from a household flocks in Menofia Governorate, Egypt. The isolates were found to have the same genetic characters of H5N8 virus isolated from common coot in Egypt and strains isolated in 2016 from Asia and Europe.
Mutation of H5N1 in Egypt
The inherent tendency of influenza viruses’ RNA to regularly undergo point mutations results in continuous emergence of new viruses (Horimoto and Kawaoka, 2005). Since the introduction of clade 2.2 viruses in Egypt, multiple clades with at least two distinct genotypes have been identified; the first clade “2.2.1.1” was identified from vaccinated commercial flocks due to the ineffective use of H5 vaccines (Grund et al., 2011). The 2.2.1/C virus was isolated from household sector, small-scale commercial flocks and humans from 2008-2012 (Abdelwhab et al., 2012; Arafa et al., 2016). Since 2012, the predominant clade in commercial, household sector and humans was clade 2.2.1.2 (Arafa et al., 2015).
The H5N1 HA cleavage site has a multi-basic amino acid motif ``EKRRKKR/GLF’’ which is characteristic of HPAI viruses, this sequence has been identified in birds from different studies in Egypt (Mansour et al., 2014; ElBakrey et al., 2015; Arafa et al., 2016). Since the introduction of H5N1 viruses during 2006, the isolated viruses were closely related to clade 2.2.1 and this clade remained stable by 2009 (Arafa et al., 2016). In late 2007, clade 2.2.1.1, was emerged from vaccinated commercial flocks, during 2008-2011; and was subdivided into 2 clusters (2.2.1.1 and 2.2.1.1a). The predominant cluster identified in both the household and commercial poultry sectors during the period between 2009 to 2014 was cluster 2.2.1.2 with a cleavage site of “ERRRKKR”, which was described as the consensus cleavage site for clade 2.2 viruses. The classic viruses were previously mutated into a new clade 2.2.1.2 in 2008 because of HA protein genetic mutations and became the endemic 2.2.1.2 cluster and continues to circulate. After 2012, the previously mentioned pattern “EKRRKKR” disappeared, while the new pattern “ERRRKR” was closely associated with 2.2.1 cluster and it became dominant and replaced the previous pattern which was present only in 2.2.1.1a cluster since 2013. During 2012, the binding affinity to human receptors was increased due to genetic mutation at the receptor binding site and the dominant amino acid cleavage site pattern was “PQGEKRRKKR/GLF”. Different studies in Egypt reported the predominance of “EKRRKKR/GLF” pattern (Mansour et al., 2014; ElBakrey et al., 2015; Tolba et al., 2018). However, Tolba et al. (2018) observed Q322K substitution in four of H5N1 isolates compared to the reference and vaccine strains thus indicating continuous genetic evolution of the viruses.
Human infection with H5N1 subtype
Since the introduction of H5N1 AIVs in Egypt, 359 cases and 120 deaths were reported due to H5N1 by 2017 with the higher cases and mortalities in 2015 (WHO, 2019). The reported cases were mainly identified by seroprevalence to determine which human cases had the H5N1 subtype (Gomaa et al., 2015). In Assiut, 5.8% of respiratory patients were positive for H5N1 subtype by PCR (Hussien et al., 2017) while in Sharkia Governorate, 3.4% of respiratory patients in contact with poultry were positive for the subtype (Tolba et al., 2018). However, none of the humans in contact with infected birds were positive for the virus or antigens (Ghoneim et al., 2014). These findings support the low rate of H5N1 transmission from birds to humans (Njabo et al., 2016). In 2015, Kayali et al. (2016) reported 88 human cases in two months in persons exposed to backyard poultry (70%), bred domestic birds (26%), slaughtered poultry (14%), or dead birds (4%).
Zoonotic potential of H9N2 subtype
The detection of H9N2 subtype in areas where highly pathogenic H5N1 viruses are endemic raised public health implications due to increased chance of natural reassortment (Iqbal, 2009). The epidemiological status in Egypt is complicated due to the co-circulation of zoonotic H5N1 and H9N2 viruses in poultry, the two viruses enhance the transmissibility of the viruses from birds to humans (Kim, 2018).
Co-circulation of the LPAI H9N2 has been reported in Egypt with H5N1 since 2011 infecting the same hosts (Kayed et al., 2016). Subsequently, H9N2 has established an endemic status in poultry sectors. The possibility of reassortment between the two viruses could result in the emergence of new viruses infecting humans (Turner et al., 2017). The first human H9N2 case was reported during 2015 with transient flu like symptoms which subsided without sequale (Naguib et al., 2015). A seroprevalence study also reported a range of 1.2%-9% of H9N2 among examined human populations (Gomaa et al., 2015). In addition, in South Egypt; H9N2 human case was documented (OIE, 2015). Thus, Egypt is considered one of the countries (Pakistan, China, Hong Kong, Bangladesh, Egypt and Oman) that reported LPAI H9N2 in humans (Cameron et al., 2000; Peiris et al., 2001; Butt et al., 2005; Chakraborty et al., 2017; Naguib and Harder, 2018; Almayahi et al., 2020).
Human infections with both H7N9 and H10N8 viruses highlighted that H9N2 has an emerging state of new human infecting virus (RahimiRad et al., 2016). Continuous circulation of H5N1 and H9N2 viruses generate a new group of AIV mutants with single genetic segment of representative isolate of the most prevalent H5N1 and representative isolate of H9N2 lineage which is widely circulating in Egypt.
Emergence of H5N8 and H5N2 in Egypt
First detection of H5N8 in wild birds was during 2010 in Asia and then; the virus spread to domestic birds in China, South Korea, and Japan (Wu et al., 2014; Kang et al., 2015). In November 2016, Community Based Animal Health Outreach Program (CAHO) teams detected HPAI (H5N8) in Egypt during targeted surveillance for AI by collection of oropharyngeal and cloacal swabs samples from diseased and dead migratory birds and ducks in Damietta (Selim et al., 2017). Isolation and characterization of H5N8 by nucleotide sequencing and phylogenetic analysis explained the novel reassortants of unpredictable gene constellations of HPAIV (H5N8) strains with enzootic strains of AIV that poses a public health threat. Later in December 2016, 128 samples from 64 apparently healthy wild birds of six species were examined (Kandeil et al., 2017). Two isolates from green-winged teal were classified as H5N8 subtypes. Complication of Egyptian epidemiological situation was due to isolation of H5N8 in wild birds (Kim, 2018).
The co-circulation of the three subtypes (HPAI H5N1, H5N8 and LPAI H9N2) in Egypt raised concerns about the emergence of new subtypes that might have potential risk to humans (Naguib and Harder, 2018). In turn, it was suggested that the new reassortant H5N2 has emerged due to the reassortment between HPAI H5N8 virus of clade 2.3.4.4 (group B) and the Egyptian LPAI H9N2 virus of the G1-like lineage (Hagag et al., 2019). This indicated a higher reassortment compatibility between the Egyptian H5N8 and H9N2 compared to H5N1 and H9N2. Different amino acids substitutions have been observed in the newly detected HPAI H5N2 related to pathogenicity and host adaption (Hagag et al., 2019).
Risk factors of avian influenza in Egypt
In 2007, many specific risk factors were studied by spatial cluster analysis which ensured their role in increasing the spread of infections either in birds or to human in selected geographical clusters. High densities of domestic and wild birds raised on surface water compared with other adjacent regions was one of potential risk factors (OIE/FAO, 2005). Decreased awareness, poverty and lack of education were among the risk factors related to circulation of the virus and failure of control program of AI in Egypt (Geerlings and Heffernan, 2018). The source of chicken, season, nursery farms, rearing system, individual interview for data collection and responsibility of governmental organizations in data collection were the considerable factors affecting the prevalence of HPAI (H5N1) in Egyptian household sector (Abou El-Amaiem et al., 2013). Risk factors associated with the expected pandemic influenza in Egypt included education level of bird keepers, poverty and presence of community based animal health outreach teams and the expected pandemic influenza (H5N1) in human in Egypt (Eladl et al., 2014). Decision-makers need accurate data about poultry production sector, previous flock exposure to A (H5N1), contact of infected flocks to human, control measures for the disease, application of preventive measure, handling and disposal practices participate in AIV-H5N1 vulnerability at the community level which is called Composite Risk Index (CRI) for identification of risk factors and possible control measures (Geerlings and Heffernan, 2018).
Causes of avian influenza endemicity in Egypt
Avian influenza emerged suddenly in seven Governorates in Egypt followed by spread to 22 Governorates in few days and became endemic since July 2008 (WHO, 2009). Identification of H9N2 subtype in combination with highly pathogenic H5N1 avian influenza complicates its ability in control while threatening the public health because of such co-circulation. While increasing the evidence of establishing the virus in Egypt, H5N1 viruses have been isolated from wild, feral and zoo birds and are considerably present in donkeys and pigs (Abdelwhab and Hafez, 2011). Long standing culture of Egyptian consumers preferring fresh poultry meat of multispecies, presence of multi-age live poultry at live bird markets (LBMs) may be taken as a continuous source of AIV (Abdelwhab et al., 2010).
Conclusion
The AIV is endemic in Egypt and, owing to simultaneous existence of more than one subtype in susceptible hosts may be taken as a hotspot for the emergence of new reassortants of avian influenza viruses. Active surveillance coupled with effective vaccines and improved biosecurity is essential to decrease the dissemination of the viruses among susceptible hosts including the human.
Authors Contribution
HAA and MAI participated in the collection of the previous studies. HAA, LA and MAI participated in writing the manuscript. MEM, HAA, AME and MAI revised the final version.
Conflict of Interest
The authors declare no conflict of interest.
References






