Advances in Animal and Veterinary Sciences
Research Article
Advances in Animal and Veterinary Sciences. 2 (1): 31 – 36In vitro Effect of Radiofrequency on hsp70 Gene Expression and Immune–effector Cells of Birds
Pradip Kumar Das1, Chandrakanta Jana, Thulasiraman Parkunan, Probal Ranjan Ghosh, Siddhartha Narayan Joardar2*, Guru Desika Vishaga Pandiyan3, Joydip Mukherjee, Sagar Sanyal
Department of Veterinary Physiology, West Bengal University of Animal and Fishery Sciences (WBUAFS), 37, K. B. Sarani, Kolkata– 700037, West Bengal, India
1. KVK Burdwan, CRIJAF (ICAR), BudBud, Burdwan–713403, West Bengal, India
2. Department of Veterinary Microbiology, WBUAFS, West Bengal, India
3. Presently attached to College of Veterinary Science, TANUVAS, Tamil Nadu, India
*Corresponding author: [email protected]
ARTICLE CITATION:Das PK, Jana C, Thulasiraman P, Ghosh PR, Joardar SN, G. D. V. Pandiyan, Mukherjee J and Sanyal S (2014). In vitro effect of radiofrequency on hsp70 gene expression and immune–effector cells of birds. Adv. Anim. Vet. Sci. 2 (1): 31 – 36.
Received:2013–10–28, Revised:2013–12–04, Accepted: 2013–12–05
The electronic version of this article is the complete one and can be found online at (http://dx.doi.org/10.14737/journal.aavs/2014.2.1.31.36) which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Abstract
Poultry birds under free range system of management are a good biological indicator for low–intensity Electro–Magnetic Frequency radiations (EMF–r) for their vulnerability to EMF–r. The effect of EMF–r on different biological systems was extensively studied but no consistent effects had been inferred in this regard. With the aim of assessing the in vitro effect of radio frequency (RF) exposure on cellular stress and on functional activities of immune–effectors cells of poultry, this study was conducted. Two rural breeds [one best adapted exotic breed–Rhode Island Red (RIR) and one indigenous breed–Haringhata Black (HB)] were used. Blood samples, collected from six healthy birds of each species, were used for neutrophil functional assay by nitro blue terazolium (NBT) reduction test. Peripheral blood mononuclear cells (PBMC), obtained from blood were used for lymphoproliferation assay by 3 – [4, 5 – dimethlythiazol–2yl] – 2, 5 – di phenyltetrazolium bromide (MTT dye) and expression of hsp70 gene by reverse transcription– polymerase chain reaction (RT–PCR) analysis. Prior to the experiment, PBMC were cultured into tissue culture plates and blood for NBT assay was taken in separate micro–centrifuge tubes from the sample of each bird in duplicates under bio–safety cabinet for radiofrequency exposure. The plates and tubes were exposed to 850 and 1200 MHz radiofrequency field from a standard mobile phone separately along with a power analyzer (SAR 1.7 W/Kg) for 5 minutes, 15 minutes, 25 minutes, 40 minutes and 60 minutes. The common trend of the hsp70 gene expression in RIR and HB breed of birds were observed upon 850 RF exposures from 25 minutes onward. The results showed that upon 1200 MHz RF post exposure, hsp70 gene expression occurred at 25 minutes onward in HB breed birds and 60 minutes post exposure in case of RIR bird. A typical trend of initial significant (P ≤ 0.05) decrease (5 minutes through 15 minutes post exposure) followed by significant (P ≤ 0.05) increase in lymphoproliferation from 25 minutes to 60 minutes, was observed in both the breeds and magnitude of exposures. All the test groups no significant variation in superoxide production by neutrophil. It may be concluded that, lower radiofrequency (850 MHz) emitted by mobile phone poses more stressful effect than the higher radiofrequency (1200 MHz) on in vitro exposed cells in both the breeds as indicated by expression of hsp70 gene and initial suppression of proliferation ability.
Introduction
Mobile telephony is one of the fastest growing industries in the world and expected to exceed one billion subscribers in India by 2013 (TRAI, 2012). This may create biological hazards as a major source of non–ionizing form of Electro–Magnetic Frequency radiations (EMF–r). Although the non–ionizing radiations are considered to be less harmful than ionizing radiations having relatively less power, can produce bio–effects and adverse health effects including heat stress upon prolonged exposure at low intensity below existing exposure standards (FCC, 1999; Kohli et al., 2012; Seletun Scientific Statement, 2011). Poultry birds under free range system of management are vulnerable to such EMF–r exposures and also a good biological indicator for low–intensity EMF–r due to thin skulls and feathers that act as dielectric receptors of microwave radiation (Balmori, 2005). Heat stress and other kind of stressors (anoxia, heavy metal ion and radiation) rapidly induce enhanced synthesis of heat shock proteins (hsp), important for various intra–cellular processes, like protein–protein interactions, including folding and assisting in establishing of proper protein conformation, and prevention of inappropriate protein aggregation (Givisiez et al., 2011). One of the important heat shock proteins, hsp70 families, is a set of highly conserved protein which acts as molecular chaperone by protecting proteins from denaturation including folding, transport and post–translational modification of protein (Al–Katanani and Hansen, 2002). Peripheral blood mononuclear cells are the major secretor cells of circulating hsp70 (Hunter–Lavin et al., 2004). Earlier studies on expression of hsp and its related genes on thermal stress have been conducted at molecular level in broilers and layers (Gabriel et al., 1996; Yu and Bao, 2008). Further, exposure of radiofrequency (RF) on human cell cultures and peripheral mononuclear cell and neutrophil had been studied (Capri et al., 2004; Kwee et al., 2001). Cleary et al. (1996) investigated the effect of isothermal radiofrequency radiation on human cytolytic T lymphocytes and revealed that exposure to 2450 MHz RIF radiation at specific absorption rates of greater than 25 W/kg induced a consistent, statistically significant reduction in CTLL–2 proliferation, especially at low IL–2 concentrations. Although several workers had been studied the effect of RF on different biological systems, no consistent effects had been inferred in this regard (WHO, 1993). It is apparent that hsp70 being one of the representative stress proteins, might be an indicator of stress analysis in the context of exposure of radiofrequency on poultry birds. However, the expression study of hsp70 on exposure of radiofrequency (RF) emitted by mobile phone and its tower is not documented so far in rural poultry, more susceptible to RF exposure during scavenging and grazing. In this outset, the present study was conducted on two rural poultry breeds for assessing the in vitro effect of RF field exposure on hsp70 gene expression and on functional activities of immune–effector cells.
MATERIALS AND METHODS
The study was conducted on two rural poultry breeds, reared under free–range system in West Bengal, India (latitude: 22.5⁰ – 27.2⁰ N and longitude: 86.1⁰ – 89.8⁰ E). One was best–adapted exotic breed (Rhode Island Red) and the other one was indigenous breed (Haringhata Black, native to West Bengal, India) (Pan et al., 2011; SAPPLPP, 2013). Six healthy birds of 22 weeks old hen from each breed were subjected for the study.
Sample Preparation
Blood samples (4 ml) were collected from wing vein of each bird in an EDTA containing vial in morning. Blood (100 µl) was taken in a sterilized microcentrifuge tube for neutrophil functional assay (NFA). Peripheral blood mononuclear cells (PBMC) were separated from the rest amount of each blood sample following standard protocol (Boyum, 1968) with some modification using HistopaqueR 1.084 (Sigma) in the ratio of 1:1. Then PBMC were dispensed @ 1X106 per well into 96 well flat bottom tissue culture plates (Nunc, Thermo–Scientific, USA) having 100 μl of RPMI growth medium (Sigma) with 10 % fetal calf serum. The PBMC were cultured into tissue culture plates (n= 11) and blood for NBT assay was taken in eleven separate microcentrifuge tubes from the sample of each bird in duplicates under bio–safety cabinet (Esco Micro Pvt. Ltd., Model No. AC2–4L1) for radiofrequency exposure.
Experimental Design and Exposure to Radiofrequency Field
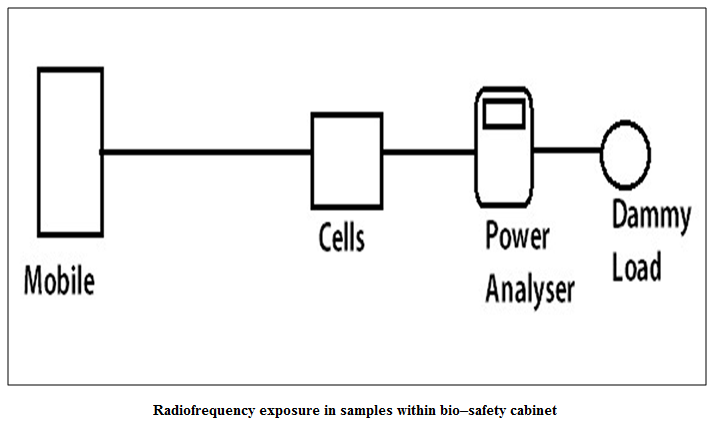
One plate and one tube from the sample of each bird, in duplicate, were exposed to 850 MHz radiofrequency field from a standard mobile phone (Spice, GSM & CDMA Duel Mode, D 88) along with a handheld power analyzer, previously standardized by Radio Test Set (Marconi, Model – 2955) (Figure 1 and Figure 2) (SAR 1.7 W / Kg) for 5 minutes. Similarly, further four plates and tubes of each bird, in duplicate, were exposed for 15 minutes, 25 minutes, 40 minutes and 60 minutes, respectively. In the same way, another five plates and five tubes from the sample of each bird, in duplicate, were exposed to 1200 MHz–radiofrequency source from the same instruments and condition for 5, 15, 25, 40 and 60 minutes, respectively. The rest plate and tube were used as control. The exposed tubes were immediately processed for Neutrophil functional assay (NFA) and the plates were incubated in a CO2 incubator (New Brunswick, Galaxy 48S) at 370C with 5% CO2 tension for further study of stress gene analysis and MTT assay.
Stress Gene Analysis
After 3 hours of incubation, lymphocytes were harvested and washed using centrifugation at 8000 rpm for 8 minutes at 40C. The cell pellets were washed with DEPC treated water and immediately mRNA was extracted using trizol reagent (Qaigen) and subjected to reverse transcription–polymerase chain reaction (RT–PCR). Initially extracted mRNA was converted to cDNA (Sambrook and Russel, 2001). Specific primers set (hsp–F 5’– GGCTGGTATGCGTGACAT–3’and hsp–R 5’– GGCTGGTATGCGTGACAT–3’) were designed from published sequence of avian hsp70 gene (accession no NM_001006685) for amplification of hsp70 from cDNA. The reaction was performed in 50 μl volume containing approximately 75 ng of cDNA, 20 pmol /μl each of forward (hsp–F) and reverse primer (hsp– R), 1.5 mM MgCl2, 200 μM each dNTPs, 10 X PCR buffer and 2 U of Taq DNA polymerase. PCR was done in a thermal cycler (Mastercycler, Eppendorf, Hamburg, Germany) with initial denaturation at 94⁰C for 4 minute, followed by 30 cycles with denaturation at 94⁰C for 1 minute, annealing at 55⁰C for 50 sec and extension at 72⁰C for 2 minute. The final extension was done at 72⁰C for 7 min. The amplified PCR products were separated by agarose gel electrophoresis using 1.5% agarose in 0.5 X TBE along with 0.5 μg/ml ethidium bromide at 100 volt for 1 hour with specific DNA Ladder. The amplified DNA fragments were observed under Gel Documentation System (UVP, UK) and analyzed. The PCR products were subjected for sequencing after excised from the gel. The nucleotide sequences were aligned and compared using Lasergene software DNA STAR. Phylogenetic comparison was performed using MEGA 4.0.2 software. Searches of homology of genes were performed by BLASTN programme in NCBI website.
Lymphoproliferation Assay (LPA)
After exposure, 100l of ConA (Sigma, USA) at a concentration of 10 g/ml was added to 100l of PBMC suspension into each test well of 96–well tissue culture plate (Nune, Denmark) for non–specific simulation. Similarly, 100l of growth medium was added to 100l of PBMC suspension into each control well. Side by side similar experiments were carried out with the cells in the unexposed (control) plate. The plates were incubated for 48 h at 370C containing 5% CO2 tension. The proliferation of PBMC was determined by the colorimetric 3 – [4, 5 – dimethlythiazol–2yl] – 2, 5 – di phenyltetrazolium bromide (MTT) assay (Daly et al., 1995). After 48 h cultures, 20 l of MTT (Sigma, USA), 5mg/ml in PBS, was added to each well and incubated for 4 h at 370C. The tissue culture plates were centrifuged at 500 X g for 10 min and then the supernatant was removed carefully without disturbing the cell plates or formazan precipitate. The formazan crystals were dissolved by the addition of 150 l of dimethylsulphoxide (DMSO, Sigma, USA) followed by 25 l of glycine buffer (0.1 M glycine, 0.1 M NaCl, pH 10.5) and mixed thoroughly with micropipette and incubated at room temperature for 10 min. The formazan development was read at 595 nm using a plate reader (ECIL, India). Lymphocyte proliferation was measured using stimulation index (S.I.) as per the following formula– mean optical density.
Stimulation index (S.I.) = (Mean optical density of test wells having ConA/Mean optical density of control wells) –1
Neutrophil functional assay (NFA)
Radiofrequency exposed blood samples kept in microcentrifuge tubes was used for nitroblue tetrazolium (NBT) reduction test Neutrophil functional assay (NFA)
Radiofrequency exposed blood samples kept in microcentrifuge tubes was used for nitroblue tetrazolium (NBT) reduction test
to assess superoxide production by neutrophils (Siwicki et al., 1985). Briefly, equal volume (100l) of the blood sample and nitroblue tetrazolium (NBT) solution (0.2% in phosphate buffered saline, PBS) were mixed in the micro–centrifuge tubes and incubated for 30 min in room temperature. Then 50l cell suspension was added to 1ml N, N dimethylformamide (SRL, India) and centrifuged for 5 min at 3000 g. The optical density (O.D.) of the colour formed was read using spectrophotometer at 540nm.
RESULTS
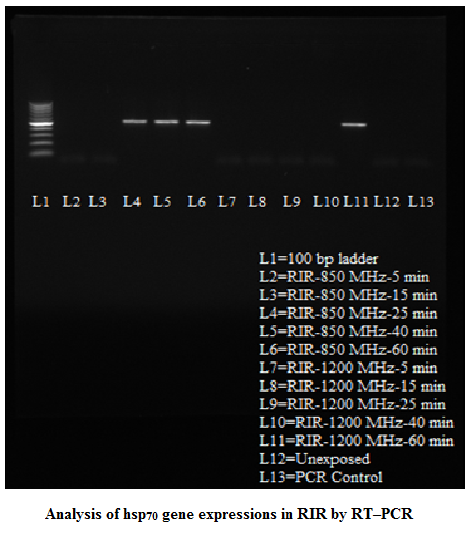
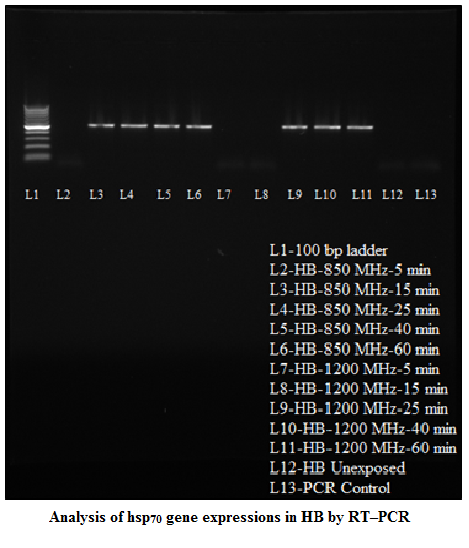
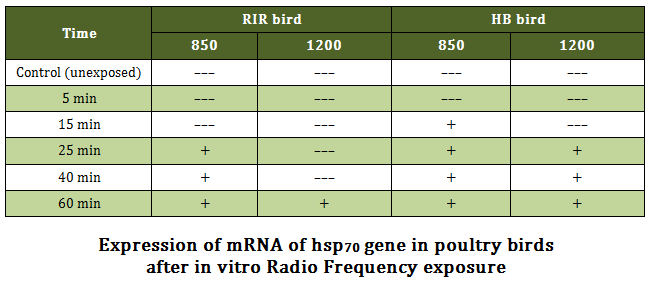
Stress Gene (hsp70) Analysis RT–PCR yielded an amplification product of 515 bp in positive samples upon 1% agarose gel electrophoresis. Nucleotide sequence of the amplicons of both breeds revealed the product of 515 bp, which was corresponding to the sequence of hsp70 gene of Gallus gallus from 688 bp to1202 bp (accession no NM_001006685). Nuclueotide sequence of hsp70 gene of HB and RIR breed had 98.8 and 98.4 % identity, respectively. There were changes in nucleotide sequence at 334 and 359 position of hsp70 gene sequence of RIR breed in compared to HB breed. Variation of expression in hsp70 gene was observed in poultry birds upon exposure to radio frequency at different time intervals. A general trend of the gene expression in RIR and HB breed of birds was observed upon 850 MHz RF exposures where from 25 minutes onward electrophoresis bands were observed in the gel in both the breeds (Figure 3 and Figure 4). However, 15 minutes post exposure also showed gene expression in HB birds upon 850 MHz RF exposure. The results showed that upon 1200 MHz RF exposure, hsp70 gene expression took place 25 minutes onward in HB breed birds. However, this only took place on 60 minutes post exposure in case of RIR bird (Table –1).
Lymphoproliferation assay (LPA)
The results revealed a typical trend of initial significant (P ≤ 0.05) decrease (5 minutes through 15 minutes post exposure) followed by significant (P ≤ 0.05) increase in lymphoproliferation from 25 minutes to 60 minutes post exposure. This general trend was observed in both 850 MHz and 1200 MHz RF exposure and in both the breeds (Table –2).
Neutrophil Functional Assay (NFA)
Neutrophil superoxide production was assessed by in vitro neutrophil functional assay upon RF exposure in the order of 850 MHz and 1200 MHz in both the breeds. No significant variation in superoxide production by neutrophil was observed in all the test groups. The results of lymphoproliferation and neutrophil superoxide production were shown side by side to compare the effects in each case (Table – 2).
DISCUSSION
In our study, we assessed the effect of short–term radiofrequency exposure, in vitro, on lymphocytes of two rural poultry breeds by detecting mRNA expression of hsp70 gene. Moreover, functional activities (i.e. superoxide production and proliferating ability) of immune–effector cells, viz neutrophil and lymphocyte were assessed through neutrophil functional assay (NFA) and lymphoproliferation assay (LPA), respectively. The expression of hsp70 mRNA in radiofrequency exposed groups was due to the effect of non thermal, non ionizing radiation fields as no expression of such gene was observed in unexposed groups which was incubated at 37OC. The expression of hsp70 gene was well the stress gene hsp70 upon in vitro culture of lymphocytes of both the breeds as compared to higher RF documented in human glioblastoma derived cell–lines (U87MG) in response to exposure of radiofrequency field (Chauhan et al., 2007). In the present study, lower radiofrequency exposure (850 MHz) caused early expression of exposure (1200 MHz) indicating lower RF exposure was more stressful to immune effector cells especially on peripheral lymphocytes. It was also supported by LPA. As, the production of heat shock proteins is triggered electromagnetically it needs 100 million fields remove calcium ions bound to the membranes of living cells, making them more likely to tear, develop temporary pores and leak, as the calcium ions have time to be pulled clear times less energy than when triggered by heat (Blank and Goodman, 2000). Further, weak electromagnetic of the membrane and replaced by potassium ions before the field reverses and drives them back (Diem et al., 2005). The potassium ions resonate, absorb the field’s energy and convert it to energy of motion which increases their ability to replace calcium on cell membranes (Liboff et al., 1990). Here, pulses and square waves work best because they give very rapid changes in voltage that catapult the calcium ions well away from the membrane and then allow more time for potassium to fill the vacated sites, where sine waves are smoother, spend less time at maximum voltage, and so allow less time for ion exchange (Andrew, 2007). Time difference of expression of hsp70 gene in two breeds was also observed in two different RF exposures. It might be due to polymorphism of nucleotide sequences of hsp70 genes which was earlier demonstrated by Mazzi et al. (2003) of different poultry breeds having different heat tolerance capacity.
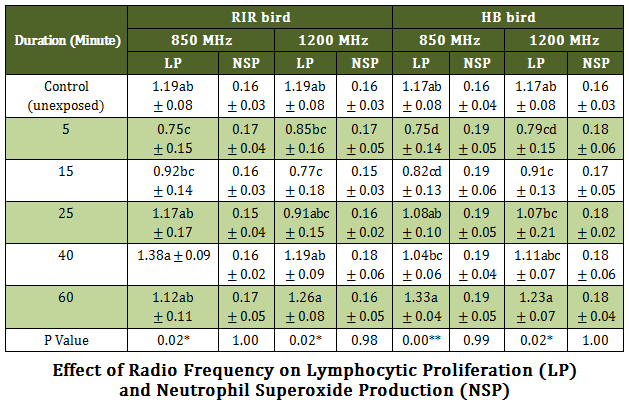
Table 2: Effect of Radio Frequency on Lymphocytic Proliferation (LP)# and Neutrophil Superoxide Production (NSP) ##
# Values are expressed as stimulation index ± SEM; ## Values are expressed as Optical density (O.D.) ± SEM
RIR = Rhode Island Red breed poultry bird, HB = Haringhata Black native poultry bird,; MHz = Mega Hurz; ** P ≤ 0.01 * P ≤ 0.05 Means having different alphabet in the same column differ significantly
A typical trend of initial significant (P ≤ 0.05) decrease (5 minutes through 15 minutes post exposure) followed by significant (P ≤ 0.05) increase in lymphoproliferation was observed from 25 minutes to 60 minutes in vitro post exposure. This trend was observed in both 850 MHz and 1200 MHz RF exposure and in both the breeds. It appears that, short duration RF exposure (at least up to 15 min) rendered considerable stress to the lymphocytes leading to inhibition in proliferation. However, initial stress could have been coped up during long depuration RF exposure (for 25 min and more) where SI of test samples showed nearly similar values to the control. This finding corroborated with the earlier opininon of Simkó and Mattsson (2004) in respect of direct activation of mononuclear cells (PBMC) by short–term exposure of electromagnetic field (EMF) that leads to proliferation and consequently, hsp70 expression. They reviewed the relevant literatures extensively and noted that EMF exposure is able to perform such activation by means of increasing the levels of free radicals.
Earlier several workers measured in vitro superoxide (reactive oxygen species, ROS) production by neutrophil upon RF exposure. Akdag et al. (2007) and Simkó (2007) demonstrated that increased electromagnetic field (EMF) exposure can modify the cellular balance by generating ROS. Physical processes at atomic level were indeed the basis of reactions between biomolecules and EMFs, as the field can magnetically affect chemical bonds between adjacent atoms and alter the energy levels and spin orientation of electrons. Overproduction of ROS can damage cellular components, mainly lipids in membranes and nucleic acids. Moreover, ROS can harm cells by depleting enzymatic and/or nonenzymatic antioxidants triggering progressive dysfunction and eventually genotoxic events (Kong and Lin, 2010). This redox–related mechanism had been mainly documented for the ELF–EMFs (Extremely Low Frequency fields, between 1 Hz up to 100 kHz – electromagnetic fields). Scaiano and co–workers (1994) first proposed that ELF–EMFs exposure can stabilize free radicals in such a way as to increase their lifetime and permit a wider dispersion rather than their return to the basal level. However, in the present study, no significant variation was observed in superoxide production of neutrophil in all test groups. This might be due to the RF exposure used in the study in the order of 850 MHz and 1200 MHz, much higher rate (8000 times) than the above cited observations, which might unable to affect the chemical bonding of the biomolecules by magnetic spinning. In this context, it may be argued that for better understanding of biological effects of EMF–r on living cells, investigation should be carried out in broader perspective considering in vivo situations, especially in relation to human health. In–vitro studies encompassing energy absorption due to mobile phones i.e. RF fields below the recommended limits (2 W/kg) did not identify reproducible effects by which cellular dysfunction in living systems could be explained. However, several studies have linked exposure to extremely low frequency electromagnetic fields (ELF–EMF) and increase the risk of childhood leukemia (Ahlbom et al., 2000; Foliart et al., 2001 and Foliart et al., 2002). International Agency for Research on Cancer (IARC), part of WHO, designated RF–EMF from cell phones as a “possible human carcinogen” Class 2B (WHO, 2011). The exposure to continuous RF–EMF radiation poses a greater risk to children, particularly due to their thinner skulls and rapid rate of growth. Also at risk are the elderly, the frail and preg¬nant women (Cherry, 2001). DNA damage via free radical formation inside cells has also been recorded (Lai and Singh, 1996). Free radicals kill cells by damaging macromolecules such as DNA, protein, and membrane are carcinogenic. In fact, EMR enhances free radical activity. This invisible health hazard pollution (IHHP) is a relatively new environmental threat.
In conclusion, lower radiofrequency emitted by mobile phone had more stressful effect on in vitro exposed cells in both the breeds of poultry as indicated by expression of hsp70 gene and initial suppression in proliferation ability of immune–effector cells.
ACKNOWLEDGEMENTS
Authors are also thankful to the Vice Chancellor and Director, Research Extension and Farms, West Bengal University of Animal & Fishery Sciences for providing necessary infrastructure to carry out the work.
REFERENCES
Ahlbom A, Day N, Feychting M, Roman E, Skinner J, Dockerty J, Linet M, McBride M, Michaelis J, Olsen JH, Tynes T and Verkasalo PK (2000). A pooled analysis of magnetic fields and childhood leukemia. Br. J. Cancer 83(5): 692–698.
http://dx.doi.org/10.1054/bjoc.2000.1376
PMid:10944614 PMCid:PMC2363518
Al–Katanani YM and Hansen PJ (2002). Induced thermotolerance in bovine two–cell embryos and the role of heat shock protein 70 in embryonic development. Mol. Reprod. Dev. 62:174–180.
http://dx.doi.org/10.1002/mrd.10122
PMid:11984827
Akdag MZ, Bilgin MH, Dasdag S and Tumer C (2007). Alteration of nitric oxide production in rats exposed to a prolonged, extremely low–frequency magnetic field. Electromagnetic Biol. Med. 26(2): 99–106.
http://dx.doi.org/10.1080/15368370701357866
PMid:17613037
Andrew G. (2007). The Biological Effects of Weak Electromagnetic Fields goldsworthy_bio_weak_em_07.doc
Balmori A (2005). Possible effects of electromagnetic fields from phone masts on a population of White Stork (Ciconia ciconia). Electromag Biol. Med. 24:109–119.
http://dx.doi.org/10.1080/15368370500205472
Blank M and Goodman R (2000). Stimulation of stress response by low frequency electromagnetic fields: possibility of direct interaction with DNA. IEEE Trans. Plasma Sci. 28: 168‐172
http://dx.doi.org/10.1109/27.842894
Boyum A (1968). Isolation of Mononuclear cells and granulocytes from human blood; isolation of mononuclear cells by one centrifugation, and of granulocytes by combining centrifuse and sedimentation at 1g. Scand. J. Clin. Lab. Invest. 21 (Suppl.97): 77 – 89.
Capri M, Scarcella E, Bianchi E, Fumelli C, Mesirca P, Agostini C, Remondini D, Chuderer J, Kuster N, Franscheschi C and Bersani F (2004). 1800 MHz RF does not affect apoptosis and hsp70 level in peripherical blood mononuclear cells from young and old volunteers. Int. J. Rad. Biol. 80: 389–397.
http://dx.doi.org/10.1080/09553000410001702346
PMid:15362692
Chauhan V, Qutob SS, Lui S, Mariampillai A, Bellier PV, Yauk CL, Douglas GL, Williams A and McNamee JA (2007). Analysis of gene expression in two human–derived cell lines exposed in vitro to a 1.9 GHz pulse–modulated radiofrequency field. Proteomics 7: 3896–3905.
http://dx.doi.org/10.1002/pmic.200700215
PMid:17902192
Cherry N (2001). Evidence that electromagnetic radiation is genotoxic: the implications for the epidemiology of cancer and cardiac, neurological, and reproductive effects. http://www.neilcherry.com/documents/90_m2_EMR_Evidence_That_EMR-EMF_is_genotoxic.pdf
Cleary SF, Du Z, Cao G, Liu L and Mccrady C (1996). Effect of isothermal radiofrequency radiation on cytolytic T– Lymphocytes. FASEB J. 10: 914–919.
Daly JG, Olivier G and Moore A R (1995). A calorimetric assay for the quantification of brook trout (Salvelinus fontinalis) lymphocyte mitogenesis. Fish Shellfish Immunol. 5: 266–273.
http://dx.doi.org/10.1006/fsim.1995.0026
Diem E, Schwarz C, Aldkofer F, Jahn O and Rudiger H (2005). Non‐thermal DNA breakage by mobile phone radiation (1800 MHz) in human fibroblasts and in transformed GFSH‐R17 rat granulosa cells in vitro. Mut. Res./Gen. Toxicol. Environ. Mut. 583: 178‐183
http://dx.doi.org/10.1016/j.mrgentox.2005.03.006
PMid:15869902
Foliart DE, Iriye RN, Tarr KJ, Silva JM, Kavet R and Ebi KL (2001) Alternative magnetic field metrics: relationship to TWA, appliance use, and residential characteristic of children in a leukemia survival study. Bioelectromagnetics 22: 574–580.
http://dx.doi.org/10.1002/bem.86
PMid:11748675
Foliart DE, Iriye RN, Silva JM, Mezei G, Tarr KJ and Ebi KL (2002) Correlation of yearto–year magnetic field exposure metrics among children in a leukemia survival study. J. Expo. Anal. Environ. Epidemiol. 12: 441–447.
http://dx.doi.org/10.1038/sj.jea.7500245
PMid:12415493
FCC (1999). Questions and answers about biological effects and potential hazards of radio–frequency electromagnetic fields. OET Bulletin 56, 4th Edition. Available at: http://www.fcc.gov/encyclopedia/oet-bulletins-line
Gabriel JE, Ferro JA, Stefani RMP, Ferro MIT, Gomes SL, Macari M (1996). Effect of acute heat stress on the heat shock protein 70 messenger RNA on the heat shock protein expression in the liver of broilers. Br. Poult. Sci. 37:443–449.
http://dx.doi.org/10.1080/00071669608417875
PMid:8773853
Givisiez PEN, da Silva MM, Mazzi CM, Ferro MIT, Ferro JA, Gonzales E and Macari M (2001). Heat or cold chronic stress affects organ weights and hsp70 levels in chicken embryos. Can. J. Ani. Sci. 81: 83–87.
http://dx.doi.org/10.4141/A00-049
Hunter–Lavin C, Davies EL, Bacelar MM, Marshall MJ, Andrew SM and Williams JH (2004). Hsp70 release from peripheral blood mononuclear cells. Biochem. Biophys. Res. Com. 324: 511–517.
http://dx.doi.org/10.1016/j.bbrc.2004.09.075
PMid:15474457
Kohli RK, Sharma VP and Singh HP (2012). Cell–Telephony and Ecological Concerns. Env News – Newsletter of ISEB India 18 (2): 10–20
Kong Q and Lin CLG (2010). Oxidative damage to RNA: mechanisms, consequences, and diseases. Cell. Mol. Life Sci. 67(11): 1817–1829.
http://dx.doi.org/10.1007/s00018-010-0277-y
PMid:20148281 PMCid:PMC3010397
Kwee S, Raskmark P and Velizarov P (2001). Changes in cellular proteins due to environmental non–ionizing radiation. I. Heat–shock proteins. Electro– and Magnetobiol. 20: 141–152.
Lai H, Singh NP (1996). Single– and double–strand DNA breaks in rat brain cells after acute exposure to radio–frequency electromagnetic radiation. Int. J. Rad. Biol. 69: 513–521.
http://dx.doi.org/10.1080/095530096145814
PMid:8627134
Liboff AR, McLeod BR and Smith SD (1990). Ion cyclotron resonance effects of ELF fields in biological systems. In: Wilson BW, Stevens RG, Anderson LE (eds) Extremely Low Frequency Electromagnetic Fields: the Question of Cancer. Battelle Press, Columbus, Ohio, pp. 251‐289.
Mazzi CM, Ferro JA, Ferro MT, Savino VJM, Coelho AAD and Macari M (2003). Polymorphoism analysis of the hsp70 stress gene in broiler chickens (Gallus gallus) of different breeds. Gene Mol. Biol. 26:275–281.
http://dx.doi.org/10.1590/S1415-47572003000300010
Pan S, Dhawan M and Pica–Ciamarra U (2011).Towards good livestock policies: backyard poultry farming through self–help groups in West Bengal. Good Practice Note Code: SAGP11.2011: South Asia Pro Poor Livestock Policy Programme (SA PPLPP), a joint initiative of NDDB and FAO, Delhi, India.
Sambrook J and Russel DW (2001). Molecular cloning: a laboratory manual.3rd ed., Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York.
SAPPLPP (2013). South Asia Pro–Poor Livestock Policy Programme – News, NDDB House, PB 4906, New Delhi – 110029, India. Available at: http://sapplpp.org/news1
Scaiano JC, Mohtat N, Cozens FL, McLean J and Thansandote A (1994). Application of the radical pair mechanism to free radicals in organized systems: can the effects of 60Hz be predicted from studies under static fields? Bioelectromagnetics 15(6): 549–554.
http://dx.doi.org/10.1002/bem.2250150608
PMid:7880168
Seletun Scientific Statement (2011). New International EMF Alliance, Oslo, Norway. Available at: http://iemfa.org/index.php/publications/seletun-resolution
Simkó M (2007). Cell type specific redox status is responsible for diverse electromagnetic field effects. Curr. Med. Chem. 14(10): 1141–1152.
http://dx.doi.org/10.2174/092986707780362835
PMid:17456027
Simkó M and Mattsson MO (2004). Extremely low frequency electromagnetic fields as effectors of cellular responses in vitro: possible immune cell activation. J. Cell Biochem. 93(1): 83–92.
http://dx.doi.org/10.1002/jcb.20198
PMid:15352165
Siwicki A K, Studnicka M and Ryka B (1985). Phagocytic ability of neutrophils in carp (Cyprinis carpio). Bamidgeh 37: 123–128.
TRAI (2012). Indian telecom services performance indicator report for the quarter ending December. Information note to the Press. Press release No. 74/2012, New Delhi. Available at: www.trai.gov.in
WHO (World Health Organization) (1993). Environmental Health Criteria 137. Electromagnetic fields (300 Hz–300 GHz). Geneva.
WHO Press Release (2011). IARC classifies radio–frequency electromagnetic fields as possibly carcinogenic to humans. International Agency for Research on Cancer (IARC). http://www.who.int/mediacentre/factsheets/fs193/en
Yu J and Bao E (2008). Effect of acute heat stress on heat shock protein 70 and its corresponding mRNA expression in the heart, liver and kidney of broilers. Asian–Aust. J. Anim. Sci. 21: 1116–1126.