Vermicompost and Mycorrhiza Effect on Yield and Phosphorus Uptake of Wheat Crop
Research Article
Vermicompost and Mycorrhiza Effect on Yield and Phosphorus Uptake of Wheat Crop
Sadiq Hussain1*, Muhammad Sharif1, Sarmad Khan1, Fazli Wahid1, Hina Nihar3, Wiqar Ahmad1, Imran Khan1, Nadeem Haider2 and Tabassum Yaseen3
1Department of Soil and Environmental Sciences; 2Department of Horticulture, The University of Agriculture, Peshawar, Khyber Pakhtunkhwa, Pakistan; 3Department of Botany, Bacha Khan University, Charsadda, Khyber Pakhtunkhwa, Pakistan.
Abstract | A pot experiment was conducted to assess the effect of mycorrhiza inoculation with vermicompost on yield and P uptake of wheat. The experiment was carried out in Completely Randomized Design replicated four times during season 2014-15. Phosphorus (P) was applied at recommended level of 90 kg ha-1. Vermicompost was used as a source of P on the basis of its P concentration. The recommended dose of N @ 120 kg ha-1 was applied in the form of vermicompost and urea, while K was applied in the form of sulphate of potash. Spores of mycorrhiza were isolated from fresh growing crop of berseem at the University research farm and used as inoculums in this experiment. Results showed the maximum grain yield of 15.09 and 14.7 g pot-1, highest total dry matter yield of 36.3 g and 36 g pot-1, maximum roots dry weight of 5.6 and 5.41g pot-1, hundred grains weight of 4.64 g and 4.6 g were observed for the mycorrhiza inoculation with half and full dose of vermicompost treatments, respectively. Highest straw yield of 21.5 g and 21.2 g pot-1, maximum plant N uptake of 0.71 g and 0.68 g pot-1 were obtained by mycorrhiza inoculated with half and full doses of vermicompost treatments. Plants P uptake of 0.09 g and 0.08 g pot-1 were found in mycorrhizal inoculation along with full and half doses of vermicompost, respectively. Maximum concentration of Zn (0.7 mg kg-1), Cu (0.164 mg kg-1), Fe (1.0 mg kg-1) and Mn (1.63 mg kg-1) were noted in mycorrhiza inoculated treatments with recommended dose of vermicompost and these were statistically at par with treatments receiving mycorrhizal inoculation along with half and full dose of vermicompost without inoculation. Maximum spores density of 58 and 46 were recorded by mycorrhiza inoculation with half and full level of vermicompost, while root colonization of 57.8% and 46% were maximum by the treatment of mycorrhiza inculcation with vermicompost. Results suggested that inoculation of mycorrhiza with vermicompost at half as well as full dose has potential to improve yield, yield component and nutrients uptake of wheat under prevailing soil and environmental conditions.
Received | August 26, 2016; Accepted | October 28, 2016; Published | November 13, 2016
*Correspondence | Sadiq Hussain, Department of Soil and Environmental Sciences, The University of Agriculture, Peshawar, Khyber Pakhtunkhwa, Pakistan; Email: fahad_hussainagri@yahoo.com
Citation | Hussain, S., M. Sharif, S. Khan, F. Wahid, H. Nihar, W. Ahmad, I. Khan, N. Haider and T. Yaseen. 2016. Vermicompost and mycorrhiza effect on yield and phosphorus uptake of wheat crop. Sarhad Journal of Agriculture, 32(4): 372-381.
DOI | http://dx.doi.org/10.17582/journal.sja/2016.32.4.372.381
Keywords | Vermicompost, Mycorrhiza, Phosphorus, Wheat, Yield
Introduction
Phosphorus (P) is the most important nutrient for best growth and yield of plants. Phosphorus makes plant dry matter of about 0.2 %. Our soils contain P but 95-99% is fixed in soil, which is unavailable to plant (Bhattacharya et al., 2013). Phosphorus metabolism is due to its essentiality (Abel et al., 2002). Enzymatic reactions and metabolic pathway are regulated by phosphorus (Theodorou and Plaxton, 1993). Organic and mineral phosphorus are two pools of the soil P present in soil. Of the total soil P approximately 20 to 80% is in organic form that mainly consists of phytic acid (Richardson, 1994).
Mycorrhizal fungi are soil beneficial microorganism and have a symbiotic association with roots of the crop plants (Sharma, 2003). Yield and growth of chickpea plants in P deficient soil have been improved where mycorrhiza fungi were inoculated (Zaidi et al., 2003). Mycorrhiza fungi increase the uptake of P in many plants but its availability gets decreased by application of P fertilizers (Grant et al., 2005). Mycorrhiza fungi play important role in microbial activity, nutrients dynamics and plant ecology. These fungi are like other beneficial microorganisms such as P solubulizing bacteria (Panhwar et al., 2009) and N fixing bacteria (Naher et al., 2009). Wheat grain yield, plant biomass and higher P concentration were recorded where mycorrhiza were applied (Al-Karaki et al., 2004). Mycorrhiza fungi form a network in plant roots for enhancement of nutrients movement and enable plants more tolerance to environmental stresses (Minaxi et al., 2013). Mycorrhizal fungi are currently well recognized as bio-fertilizer, for the control of root pathogens, bioremediation and increase in plant biomass and nutrient content (Kumar et al., 2009). The mechanism of root infection is different for both types of mycorrhiza as the ectomycorrhiza infect the roots of forest trees while endomycorrhiza infect roots of agriculture crops (Tisdale et al., 1995).
Decomposition of many organic materials by earthworms to vermicompost has been known as a cheaper and environment friendly process (Mathivanan et al., 2013). It is a rich source of different essential nutrients which improve overall soil condition and promote yield and growth of plant (Pezeshkpour et al., 2014). Vermicompost finally converts to highly decompose organic materials which improve soil physical and chemical properties (Atiyeh et al., 2002; Arancon et al., 2004). Small amounts of vermicompost are required for better crop growth and yield than conventional compost because of their good quality (Atiyeh et al., 2000). Vermicompost contain different types of soil beneficial microbes that can improve plant growth through vitamins, hormones and antibodies (Lourduraj, 2006). Vermicompost containing different enzyme which are responsible for degrading of large organic molecule for enhancement of further microbial activity (Gupta, 2003). Vermicompost making is an easy method to improve soil properties and crop yield (Reddy and Reddi, 2002).
Wheat (Triticum aestivum L.) serves as a staple food for the world (Yongqing et al., 2005). In Pakistan it is grown by about 80% of the farmers, approximately equal to 40% of the total cultivated area (Coleman and Faruquee, 1996). It is grown in almost all provinces of Pakistan. In Pakistan it is sown on an area of 9151 thousand hectares producing 25980 thousand tons of wheat grains with an average yield of 4054 kg ha-1 (Pakistan Bureau of Statistic, 2013-2014). In Khyber Pakhtunkhwa, an area of 777 thousand hectares was planted to wheat crop with a total production of 1362 thousand tons (Crop Statistic of Khyber Pakhtunkhwa, 2013-2014). Keeping in view the significant role of mycorrhiza and vermicompost in improving soil properties and crop production, an experiment was conducted to investigate the effect of mycorrhiza inoculation with vermicompost on yield and P uptake of wheat crop in alkaline calcareous soil in Khyber Pakhtunkhwa.
Materials and Methods
A pot experiment was conducted in the Institute of Biotechnology and Genetic Engineering, The University of Agriculture Peshawar under natural conditions to evaluate the effect of mycorrhiza inoculation with and without vermicompost on P uptake and yield of wheat. Five kg of soil was taken in each pot. There were eight treatments with four replications. Total of eight seeds of wheat variety Siran were sown initially and were thinned to four plants pot-1 after germination. Pots were rearranged on weekly basis to maintain uniformity among pots. Vermicompost was used as source of P on basis of its P concentration at recommended level of 90 kg P ha-1 (98 g vermicompost pot-1), sulphate of potash was applied as a potassium source @ 60 kg ha-1 (0.3 g sulphate of potash pot-1). Urea and vermicompost were applied as nitrogen sources @ 120 kg ha-1. Three split doses were used to apply nitrogen. A wooden box having size of three feet length, two feet width and depth respectively was filled with soil, straw and animal dung at ratio of 1:2:1. At least 5000 of earthworms two species Eisenia fetida and Pheretima posthuma were added in the box for vermicomposting. The prepared compost was analyzed for nutrients concentration (Table 1). The following treatments combinations were used during the experiment. Treatment 1) control (no fertilizer), Treatment 2) N & K as basal dose, Treatment 3) N, P & K 120,90 & 60 kg ha-1, Treatment 4) Mycorrhiza alone treatment (M), Treatment 5) Vermicompost @ half of recommended dose (VC-I), Treatment 6) Mycorrhiza+Vermicompost @ half of recommended dose (M+VC-I), Treatment 7) Vermicompost @ full recommended dose (VC-II), Treatment 8) Mycorrhiza +Vermicompost @ full recommended dose (M+VC-II). The properties of vermicompost used for the experiment shown in Table 1.
Table 1: Characteristics of vermicompost under investigations
| Properties | Unit | Values |
| pH (1:5) | - | 7.4 |
| ECe (1:5) | (d S m-1) | 2.6 |
| Total Nitrogen | % | 0.39 |
| AB-DTPA extractable P | mg kg-1 | 2310 |
| AB-DTPA extractable Zn | mg kg-1 | 4.66 |
| AB-DTPA extractable Cu | mg kg-1 | 1.64 |
| AB-DTPA extractable Fe | mg kg-1 | 7.07 |
| AB-DTPA extractable Mn | mg kg-1 | 17.24 |
Spores of mycorrhiza were isolated from ongoing crop of berseem and were used as inoculums in this experiment. Total of 100 mycorrhizal spores were inoculated with seeds at sowing time uniformly in each pot of mycorrhizal inoculation.
Wheat Yield and Nitrogen, Phosphorus Uptake
Grain yield pot-1 of wheat was noted in each treatment after threshing. Hundred grains weight of wheat was noted by randomly counting grains from each treatment and weighted with the help of sensitive electronic balance. Plant N and P uptake by wheat were calculated from plant concentration of N and P by using the following formula:
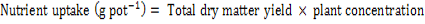
Isolation of Mycorrhiza Fungi
Mycorrhizal spore’s density of soil was determined by wet sieving and decanting method of Gerdemann and Nicolson (1963). Wheat rhizosphere soil of 100 g was added to a beaker along with 200 ml of distilled water. The flask was shaken for 30 minutes and kept it in undisturbed condition for 24 hours. After that heavier particles were allowed to settle down. After that decanted suspension through 250 µm, 75 µm and 45 µm sieves consequently. Whole residues were collected on 45 µm sieves. Spores were mounted on a slide and were observed under the microscope and photographed. Needle was used to pick up spores and a drop of Canada balsam was added to the slide. Cover slip was used to cover these specimen Hall and Fish (1979) and Schenck and Perez (1990). Standard method of Stahl and Christensen (1982) was used for calculating spores density.
Estimation of Mycorrhiza Fungal Infection Intensity in Wheat Roots
Roots of plants under investigation were cut, collected, preserved in the formalin acetic acid solution. The procedure given by Phillips and Hayman (1970) was followed for staining fungal structures with some modifications for non-pigmented roots. Root preserved in 70% alcohol was carefully washed with tap water. Cytoplasm and most of the host nuclei were eliminated by heating the roots for 5 to 10 minutes in 10% KOH solution. The roots were again washed with water and dried on filter paper. The roots were stained in 0.025% Methylene blue for 3 to 5 minutes. For evaluation of root infection intensity the procedure of Giovannetti and Mosse (1980) was followed.
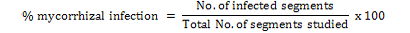
Microphotographs of the best selected slides were preserved for record.
Statistical Analysis
The data was statistically analyzed through analysis of variance (ANOVA). The variation among the treatments were compared using LSD-test of significance (p≤0.05) (Steel and Torrie, 1980).
Results and Discussion
Wheat Grain Yield
Statistical analysis of the data showed that grain yield was affected significantly (P ≤0.05) by inoculation of soil with mycorrhiza along with vermicompost to the soil in pot. Mean values for the grains yield showed that the highest grain yield of 15 g and 14.7 g pot-1 with the increase of 91% and 86%, respectively (Figure 1), were recorded by soil inoculation with mycorrhiza along with vermicompost to the soil in pots applied at half and full recommended levels followed by treatments of mycorrhiza inoculation and N, P & K treatments. In control treatment the minimum grain yield of 7.88 g pot-1 followed by N & K treatment which was 10.56 g pot-1. Pezeshkpour et al. (2014) presented similar findings who stated that inoculation of mycorrhiza fungi showed more significant effect on grain yield followed by vermicompost and bacteria treatment. Similarly, Shishehbor et al. (2013) showed that grain yield was significantly affected by interaction of Azotobactor and vermicompost. Highest grain yield was recorded in the treatments where vermicompost was applied along with azotobactor.
Total Dry Matter Yield
Data for total dry matter yield (Table 2) was affected significantly (P ≤ 0.05) by soil inoculation with mycorrhiza along with vermicompost application. Results showed that maximum root dry matter yield of 36.28 g and 36.20 g pot-1 were recorded by soil inoculation with mycorrhiza along with vermicompost at half and full recommended doses of vermicompost. These treatments increased total dry matter yield by 104% and 103 % (Figure 2) followed by NPK, and vermicompost @ full recommended, respectively. The lowest total dry matter yield of 17.83 g pot-1 was noted in control treatment followed by N & K treatment which was 23.37 g pot-1. These results are in accordance with the findings of Shishehbor et al. (2013) who noted that total dry matter were affected significantly by mycorrhiza inoculation with vermicompost and azotobactor. Inoculation with G. intraradices significantly increased total dry matter yield of maize. Similarly, Mahesweri et al. (2006) stated that inoculation of mycorrhiza along with vermicompost or rhizobium showed highest yield than non-inoculated treatment with mycorrhiza and control treatments.
Wheat Straw Yield
Statistical analysis for straw yield (Table 2) was affected significantly (P ≤ 0.05) by mycorrhiza inoculation with vermicompost. Findings revealed that the highest straw yield of 21.5 g, and 21.24 g pot-1 were recorded by inoculation of mycorrhiza inoculation with
Table 2: Vermicompost and mycorrhiza effect on wheat grains, total dry matter, straw yields and hundred grains weight
| Treatments | Grain Yield | Total dry matter | Straw Yield | Roots dry weight | 100 grains weight |
| --------------------- Yield (g pot-1) ----------------------- | ( g ) | ||||
| Control (no fertilizers) | 7.88 e* | 17.83 f* | 9.95 e* | 3.2 e* | 3.67 f* |
| N & K as basel dose | 10.56 d | 23.37 e | 12.1 d | 3.91 d | 3.92 e |
| N, P and K | 14.12 b | 34.38 b | 20.26 ab | 4.86 bc | 4.49 b |
| Mycorrhiza (M) | 14.13 b | 32.43 c | 18.30 c | 4.84 bc | 4.55 ab |
| Vermicompost @ half of recommended dose (VC-I) | 12.82 c | 30.84 d | 18.02 c | 4.97 bc | 4.19 d |
| M + VC-I | 15.03 a | 36.28 a | 21.24 a | 5.61 a | 4.64 a |
| Vermicompost @ full recommended dose (VC-II) | 13.84 b | 33.18 bc | 19.34 bc | 4.34 cd | 4.35 c |
| M + VC-II | 14.70 a | 36.20 a | 21.50 a | 5.41 ab | 4.61 a |
| LSD ( P≤0.05 ) | 0.357 | 1.47 | 1.46 | 0.606 | 0.1043 |
* Means with different letter(s) in columns are significantly different at P ≤ 0.05
Table 3: Plant N and P uptake and mycorrhiza spores density and root infection intensity as influenced by mycorrhiza inoculation with vermicompost
|
Treatments
|
N Uptake | P Uptake | Soil spores density | Root infection intensity |
| --------- (g pot-1)---------- | per 10 g soil | % | ||
| Control (no fertilizers) | 0.167 d | 0.018 e | 19.5 g | 11.5 f |
| N & K as basel dose | 0.27 cd | 0.025 e | 26.0 fg | 13.25 f |
| N, P and K | 0.498 b | 0.062 bc | 27.5 ef | 17.5 e |
| Mycorrhiza (M) | 0.511 b | 0.073 ab | 42.75 bc | 34.75 c |
| Vermicompost @ half of recommended dose (VC-I) | 0.384 bc | 0.034 de | 33.5 de | 16.75 e |
| M + VC-I | 0.712 a | 0.088 a | 58 a | 46 b |
| Vermicompost @ full recommended dose (VC-II) | 0.447 b | 0.051 cd | 36 cd | 24.25 d |
| M + VC-II | 0.681 a | 0.093 a | 45.75 b | 57.75 a |
| LSD ( P≤0.05 ) | 0.1297 | 0.0216 | 7.25 | 3.07 |
* Means followed by different letter (s) are significantly different at P≤0.05
full and half doses of vermicompost, followed by N, P, K and vermicompost @ full recommended dose treatments, respectively over control treatment, while the lowest straw yield of 9.95 g pot-1 was noted in control followed by N & K treatment which was 12.1 g pot-1. Vermicompost is a type of product obtained by rapid biological degradation of organic material by microorganism and earthworms. These organic wastes are disintegrated and utilized by earthworms and microorganism for their survival. Vermicompost contains more quantity of nutrients, which are slowly released to plants. Mycorrhiza hyphae make an access to these nutrients and solubilize them by creating favorable environment through releasing of organic acids. Mycorrhiza hyphae have a relatively small diameter for nutrients absorbance to increased straw yield of wheat. Jan et al. (2014) reported similar result, which showed that straw yield was significantly increased over control treatment by inoculation of mycorrhiza fungi with rock phosphate enriched compost which were statistically similar.
Roots Dry Weight
Statistical analysis of the data showed that roots dry weight were affected significantly (P ≤ 0.05) by inoculation of mycorrhiza with vermicompost. Result showed that the highest roots dry weight of 5.61 g pot-1 was noted in mycorrhiza with half dose of vermicompost treatment, followed by mycorrhiza with full dose of vermicompost treatment, while the lowest roots dry weight of 3.2 g pot-1 was noted in control, followed by N & K. Akhzari et al. (2015) reported same results who stated that mycorrhiza inoculation with vermicompost have highest roots dry weight as compare with non-inoculated mycorrhiza, vermicompost treatment and control treatments. These results are similar with the result of Adhikary (2012) who represented that vermicompost enhanced vegetative, root and shoot growth and also change root morphology such as increased number of branches in root.
Hundred Grains Weight
Data in Table 3 showed that the hundred grains weight was affected significantly (P ≤ 0.05) by mycorrhiza inoculation with vermicompost. Results showed that maximum 100 grains weight of 4.64 g and 4.61 g were recorded by mycorrhiza inoculation with vermicompost treatments, respectively followed by mycorrhiza inoculation and N, P and K treatments, respectively. The lowest 100 grains weight of 3.67 g was noted in control treatment. Vermicompost contains nutrients which were slowly available to plant, so mycorrhiza fungi help in their availability for plant growth and yield. So, because of application of vermicompost along with mycorrhiza increased grains weight. Shishehbor et al. (2013) reported that hundred grains weight was significantly enhanced over control treatment by inoculation of mycorrhiza with vermicompost and azotobactor.
Data regarding plant N and P uptake was significantly (P ≤ 0.05) affected by inoculation of mycorrhiza with vermicompost are shown in Table 3. Results revealed that maximum plant N uptake of 0.172 g and 0.681 g pot-1 were noted by inoculation of mycorrhiza with vermicompost at half and full recommended doses of vermicompost treatments, respectively. Uptakes of N were 324.27% and 305.94% (Figure 3) over control
Plant Nitrogen and Phosphorous Uptake
treatment, followed by mycorrhiza fungi inoculation, N, P & K and vermicompost @ full recommended dose, respectively. The lowest plant nitrogen uptake of 0.167 g pot-1 was recorded in control treatment followed by N & K treatment. Adhikary (2012) reported same results that uptake of macronutrients such as N and P in plants improved considerably by application of vermicompost with fertilizers, so there was significant increase in nitrogen uptake. Arbuscular mycorrhizal fungi hyphae are mainly responsible for uptake and assimilation of ammonium (NH+4) (Johansen et al., 1996; Turk et al., 2003). Mycorrhiza enhanced the uptake of nitrogen and micronutrients. Mycorrhiza fungi extend their hphae to plant roots in soil and explore to larger soil volume. Mycorrhizal association improves the plant growth as it increases translocation between root and shoot of infected plant. Maximum plant P uptake of 0.093 g and 0.088 g pot-1 were noted in mycorrhiza fungi with half and full dose of vermicompost treatments, respectively which was 402.9% and 375.67% (Figure 4) increase in phosphorous uptake over control treatment, respectively, followed by mycorrhiza treatment. The lowest plant p uptake of 0.018 g and 0.025 g pot-1 were recorded in control and N & K treatment, respectively followed by vermicompost @ half of recommended dose treatment. Similar results were recorded by Adhikary (2012) who stated that plant N, P, K and Mg uptake by rice (Oryza sativa) was enhanced through the combine application of vermicompost with fertilizer. Inoue et al. (2009) also suggested that mycorrhiza combine application with phosphatic fertilizer showed more effective growth of corn over rock phosphate alone or control treatment.
Mycorrhiza Spores Density
Mycorrhiza spores density in soil was affected significantly (P ≤ 0.05) by mycorrhiza inoculation with vermicompost. Maximum spores density of 58 mycorrhiza fungal spores was recorded in mycorrhiza with half amount of vermicompost followed by mycorrhiza with full dose of vermicompost treatments, respectively. The lowest spore’s density of 19.5 was found in control treatment followed by N & K treatment. Carrenho et al. (2007) were also showed similar results, who stated that highest root infection or root colonization percentage was observed in treatments where arbucular mycorrhizal fungi (AMF) was applied to maize crops. Carrenho et al. (2002) also use this maize crop to trap the microorganisms (AMF) in field. Adelman and Morton (1986) were also showed that root infection percentage and numbers of spores were maximum in those treatments where mycorrhiza inoculums were applied from the same location. Jan et al. (2014) reported similar results that reveal the maximum spores density and root colonization was recorded by mycorrhiza inoculation with full amount of compost and N & K treatment respectively. Similarly, Habashy et al. (2008) noted that root colonization or root infection percentage and mycorrhiza fungal spores density gradually enhanced by inoculation of mycorrhiza fungi and compost over control treatment. Sivakumar (2013) reported same results that showed mycorrhizal root colonization and spores density have a positive correlation with each other.
Mycorrhiza Roots Infection Intensity
It was evident from the data that root infection intensity were affected significantly (P ≤ 0.05) by mycorrhiza inoculation with vermicompost. Maximum root infection intensity of 57.75% was noted in mycorrhiza inoculation with full quantity of vermicompost followed by mycorrhiza with half dose of vermicompost and only mycorrhiza inoculated treatments, respectively. Minimum roots infection of 11.5% and 13.25% were recorded in control and N & K treatments, respectively followed by N, P, K and vermicompost @ half of recommended dose treatments, respectively. Shishehbor et al. (2013) presented similar findings that root infection or colonization was significantly increased by inoculation of mycorrhiza fungi with vermicompost, also statistically similar with mycorrhiza and Azotobacoter. Root infection of G. intraradices and G. mosseae showed different and maximum results when inoculated with vermicompost. Carrenho et al. (2002) presented similar results which showed higher root infection percentage in treatments of mycorrhiza inoculums. Marshner and Bell (1996) noted that as concentration of available phosphorous increases it may adversely affects to root colonization of VAM fungi. Jan et al. (2014) reported similar results which showed significantly (P ≤ 0.01) highest root infection percentage and spores density in treatments where mycorrhiza was inoculated with compost than those of non-inoculated with AMF mycorrhiza and control treatments. Habashy et al. (2008) represented that there was positive correlation of organic waste and compost with AMF on spores density and root infection intensity, which were significantly higher over control treatment. Khakpour and Khara (2012) and Sivakumar (2013) noted similar results which highlighted that root infection intensity increases as spores density for AMF increases. So there is synergistic effect of spores on root infection intensity.
Plant Micronutrients Uptake
Plant Zn, Cu, Fe and Mn uptakes by wheat as affected by mycorrhiza inoculation with vermicompost are given in Table 4. Statistical analysis of the data presented in Table 4 revealed that plant micronutrients (Zn, Cu, Fe, Mn) uptakes were significantly (P ≤ 0.05) affected by the inoculation of mycorrhiza with vermicompost. Data showed that maximum plant Zn uptake of 0.7 mg kg-1, Cu as 0.164 mg kg-1, Fe as 1.0 mg kg-1 and Mn as 1.63 mg kg-1 were observed in treatment of mycorrhiza with full dose of vermicompost which were statistically at par with mycorrhiza with half dose of vermicompost, mycorrhiza inoculation and vermicompost @ full recommended dose treatments. The lowest Zn uptake of (0.13 mg kg-1), (Cu 0.025 mg kg-1), (Fe 0.15 mg kg-1) and (Mn 0.23 mg kg-1) were recorded in control followed by N & K treatment. The immobile nutrients are not able to move from one place in to other place in soil, so mycorrhiza fungi extend their hyphae and help in their uptake. Similarly, Al-Karaki et al. (2004) reported that mycorrhiza inoculated plants showed maximum uptake of immobile nutrients like phosphorus, zinc and copper.
Table 4: Plant micronutrients uptake as influenced by mycorrhiza inoculation with vermicompost
| Treatments | Zn | Cu | Fe | Mn |
| uptake (mg kg-1) | ||||
| Control (no fertilizers) | 0.13 e* | 0.025 f* | 0.15 e* | 0.23 f* |
| N & K as basel dose | 0.24 d | 0.049 e | 0.344 d | 0.47 e |
| N, P and K | 0.56 b | 0.114 c | 0.77 b | 1.04 c |
| Mycorrhiza (M) | 0.53 b | 0.11 c | 0.76 b | 1.38 b |
| Vermicompost @ half of recommended dose (VC-I) | 0.42 c | 0.091 d | 0.64 c | 0.83 d |
| M + VC-I | 0.70 a | 0.14 b | 1.0 a | 1.37 b |
| Vermicompost @ full recommended dose (VC-II) | 0.54 b | 0.106 c | 0.754 b | 1.46 b |
| M + VC-II | 0.69 a | 0.164 a | 1.0 a | 1.63 a |
| LSD ( P≤0.05 ) | 0.062 | 0.0148 | 0.0746 | 0.1403 |
* Means with different letter (s) in columns are significantly different at P≤0.05
Conclusion
From this study it was concluded that wheat yield and yield components, plants N, P, Zn, Cu, Fe and Mn uptake were significantly improved by inoculation with mycorrhiza along with half and full recommended dose of vermicompost. Significantly improved mycorrhizal spores density in soil and roots infection intensity in wheat was observed with the inoculation of mycorrhiza and vermicompost. Further research work is suggested to investigate the impact of mycorrhiza inoculation with vermicompost on yield and nutrients uptake of different crops under various agro ecological conditions.
Authors’ Contribution
Muhammad Sharif designed and conducted research with Wiqar Ahmad, Hina Nihar,Fazli Wahid, Sarmad Khan, Imran Khan, Nadeem Haider,Tabassum Yaseen. They helped in conducting the experiment, taking the observation, data analysis and write up of the manuscript in the form of teamwork.
References
Abel, S., A.C. Ticconi and A.C. Delatorre. 2002. Phosphorus sensing in higher plants. Physiol. Plantarum. 115: 1–8. http://dx.doi.org/10.1034/j.1399-3054.2002.1150101.x
Adelman, M.J., and J.B Morton. 1986. Infectivity of vesiculararbuscular mycorrhizal fungi: influence of host-soil diluent combinations on MPN estimates and percentage colonization. Soil Biol. Biochem. 18: 77-83. http://dx.doi.org/10.1016/0038-0717(86)90106-9
Adhikary, S. 2012. Vermicompost, the story of organic gold: A review. Agric. Sci. 3(7): 905-917. http://dx.doi.org/10.4236/as.2012.37110
Akhzari, D., B. Attaeian, A. Arami, F. Mahmoodi and F. Aslani. 2015. Effects of vermicompost and arbuscular mycorrhizal fungi on soil properties and growth of medicago polymorpha. Compost. Sci. Utili. 23: 142–153. http://dx.doi.org/10.1080/1065657X.2015.1013585
Al-Karaki, G.N., B. Mcmichael and J. Zak. 2004. Field response of wheat to arbuscular mycorrhizal fungi and drought stress. Mycorrhiza. 14(4): 263-269. http://dx.doi.org/10.1007/s00572-003-0265-2
Arancon, N., C.A. Edwards, P. Bierman., C. Welch and J.D. Metzger. 2004. Influences of vermicomposts on field strawberries, Effects on growth and yields. Bioresour. Technol. 93: 145-153. http://dx.doi.org/10.1016/j.biortech.2003.10.014
Atiyeh, R.M., C.A. Edwards, S. Subler and J. Metzger. 2000. Earthworm-processed organic wastes as components of horticultural potting media for growing marigold and vegetable seedlings. Compost. Sci. Utili. 82: 15–223. http://dx.doi.org/10.1080/1065657x.2000.10701994
Atiyeh, R.M., N. Arancon, C.A. Edwards and J.D. Metzger. 2002. The influence of earthworm- processed pig manure on the growth and productivity of marigolds. Bioresour. Technol. 81: 103-108. http://dx.doi.org/10.1016/S0960-8524(01)00122-5
Carrenho, R., S.F.B. Trufem and V.L.R. Bononi. 2002. Effects of using different host plants on the detected biodiversity of arbuscular mycorrhizal fungi from an agroecosystem. Revista Brasileira de Botanica. 25: 93-101. http://dx.doi.org/10.1590/S0100-84042002000100012
Carrenho, R., S.F.B. Trufem, V.L.R. Bononi and E.S. Silva. 2007. The effect of different soil properties on arbuscular mycorrhizal colonization of peanuts, sorghum and maize. Acta Botanica Brasilica J. 21(3): 723-730. http://dx.doi.org/10.1590/S0102-33062007000300018
Crop statistics Khyber Pakhtunkhwa. 2013-2014. Government of Khyber Pakhtunkhwa crop reporting services, Agricultural, Livestock and Crop Department.
Gerdeman, J.W., and Nicolson. 1963. Arbuscular mycorrhiza and plant growth. Ann. Rev. Phytopathol. 6: 297-418.
Giovannetti, M., and Mosses. 1980. An evaluation of techniques for measuring VAM infection in roots. New Phytol. 84: 489-500. http://dx.doi.org/10.1111/j.1469-8137.1980.tb04556.x
Grant, C., S. Bittman, M. Montrea, C. Plenchette and C. Morel. 2005. Soil and fertilizer phosphorus: Effects on plant P supply and mycorrhizal development. Canadian J. Plant Sci. 85: 3–14. http://dx.doi.org/10.4141/P03-182
Gupta, P.K. 2003. Vermicomposting for Sustainable Agriculture. Agrobios. Jothpur. 19-50.
Habashy, N.R., A.W.A. El-Khair and R.N. Zaki. 2008. Effect of organic and bio-fertilizer on phosphorus and some micronutrients availability in a calcareous soil. Res. J. Agric. Biol. Sci. 4: 545-552.
Hall, I.R., and B.J. Fish. 1979. A key to the Endogonaceae. Trans. Br. Mycol. Soc. 73: 261-270.
Inoue, S., I. Kheoruenromne, A. Suddhiprakran and S. Thanachit. 2009. Effects of arbuscular mycorrhizal fungi on phosphorus uptake and growth of baby corn on sandy soil. Department of Soil Science, Faculty of Agriculture, Kasetsart University, Bangkok 10900.
Jan, B., A. Ali, F. Wahid, F. Shah, S. Khan and F. Khan. 2014. Effect of arbuscular mycorrhiza fungal inoculation with compost on yield and phosphorous uptake of berseem in alkaline calcareous soil. Am. J. Plant Sci. 5: 1359-1369. http://dx.doi.org/10.4236/ajps.2014.59150
Jan, B., M. Sharif, F. Khan and J. Bakht. 2014. Effect of arbuscular mycorrhiza fungal inoculation with compost on yield and P uptake of wheat in alkaline calcareous soil. Am. J. Plant Sci. 5: 1995-2004. http://dx.doi.org/10.4236/ajps.2014.59150
Johansen, A., R.D. Finlay and A.A. Olsson. 1996. Nitrogen metabolism of external hyphae of arbuscular mycorrhizal fungi Glomus intraradices. New Phytol. 133: 705-712. http://dx.doi.org/10.1111/j.1469-8137.1996.tb01939.x
Kumar, A., A. Aggarwal and S. Kaushish. 2009. Influence of arbuscular mycorrhizal fungi and Trichoderma viride on growth performance of Salvia officinalis Linn. J. Appl. Nat. Sci. 1(1): 13-17.
Lourduraj, A.C. 2006. Identification of optimum quantity of vermicompost for maize under different levels of fertilizers. J. Ecobiol. 18 (1): 23-27.
Mahesweri, U., D. Stella, J.P. Sajitha and K. Haripriya. 2006. Impact of organic nutrients on yield and quality of garden bean. In: International conference on indigenous vegetables and legumes: prospectus for fighting poverty, hunger and malnutrition. pp. 485-487.
Marshner, H., and B. Bell. 1996. Nutrient uptake in Mycorrhizal symbiosis. Plant Soil. 159: 89 – 102.
Mathivanan, S., R. Kalaikandhan, A.L. Chidambaram and P. Sundramoorthy. 2013. Effect of vermicompost on the growth and nutrient status in groundnut (Arachishypogaea L.). Asian J. Plant Sci. Res. 3(2): 15-22.
Minaxi, J.S., S. Chandra and L. Nain. 2013. Synergistic effect of phosphate solubilizing rhizobacteria and arbuscular mycorrhiza on growth and yield of wheat plants. J. Soil Sci. Plant Nutr. 13: 2. http://dx.doi.org/10.4067/s0718-95162013005000040
Pakistan Bureau of Statistics. 2013-2014. Agricultural statistics. Area and production of important crops.
Panhwar, Q.A., O. Radziah, M. Sariah and I.M. Razi. 2009. Solubilization of phosphate forms by phosphate solubilizing bacteria isolated from aerobic rice. Int. J. Agric. Biol.11: 667–673.
Pezeshkpour, P., M.R. Ardakani, F. Paknejad, S. Vazan. 2014. Effects of Vermicompost, mycorrhizal symbiosis and biophosphate soulbilizing bacteria on seed yield and quality of chickpea as autumn plantation in rain fed conditions. Bull. Environ. Pharmacol. Life Sci. 3 (2): 53- 58.
Phillips, J.M., and D.S. Hayman. 1970. Improved procedure for clearing roots and staining parasitic and Vesicular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycorrhizal Soc. 55: 158-161. http://dx.doi.org/10.1016/S0007-1536(70)80110-3
Richardson, A.E. 1994. Soil microorganisms and Phosphorus availability. Soil Biota. 50–62.
Schenck, N.C., and Y. Parez. 1990. Makers for the identification of AM fungi, 3rd ed. Synergestic Publication, USA.
Sharma. O.P. 1989. Textbook of Fungi. TaTa McGraw-Hill Publishing Co., Ltd., India. pp. 312.
Shishehbor, M., H. Madani and M.R. Ardakani. 2013. Effect of vermicompost and biofertilizers on yield and yield components of common millet (Panicum miliaceum). Ann. Biol. Res. 4 (2): 174-180.
Sivakumar, N. 2013. Effect of edaphic factors and seasonal variation on spore density and root colonization of arbuscular mycorrhizal fungi in sugarcane fields. Ann. Microbiol. 63: 151-160. http://dx.doi.org/10.1007/s13213-012-0455-2
Stahl, P.D., and M. Christensen. 1982. Mycorrhizal fungi associated with Bouteloua and agropyron in Wyoming Sagebrush grass. Mycologia. 74(6): 87-885. http://dx.doi.org/10.2307/3792716
Theodorou, M.E., and W.C. Plaxton. 1993. Metabolic adaptations of plant respiration to nutritional phosphate deprivation. Plant Physiol. 101: 339–344. http://dx.doi.org/10.1104/pp.101.2.339
Turk, M. A., T.A. Assaf, K.M. Hameed and A.M. Al-Tawaha. 2006. Significance of Mycorrhizae. World J. Agric. Sci. 2 (1): 16-20.
Yongqing, M.A. 2005. Allelopathic studies of common wheat (Triticum aestivum) weed. Biol. Manage. 5 (3): 93.
Zaidi, A., M.S. Khan and M. Amil. 2003. Interactive effect of rhizotrophic microorganisms on yield and nutrient uptake of chickpea (Cicer arietinumL.). Eur. J. Agron. 19 (1): 15-21. http://dx.doi.org/10.1016/S1161-0301(02)00015-1
To share on other social networks, click on any share button. What are these?








