Responses of Strawberry Plant to Pre-Harvest Application of Salicylic Acid in Drought Conditions
Research Article
Responses of Strawberry Plant to Pre-Harvest Application of Salicylic Acid in Drought Conditions
Nargis Bano and Khalid Mahmood Qureshi*
Department of Horticultural Research and Development (DHRD), National Agricultural Research Center (NARC), Park Road, Chak Shahzad, Islamabad, Pakistan.
Abstract | Salicylic acid is classified as a compound that effectively reduces the sensitivity and environmental stress of crops as it alleviates the adverse effects of numerous stress causing factors. Numerous SA levels are found to protect several species of plants against environmental stresses by initiating different processes that are involved in the mechanism of stress tolerance. SA is part of an extremely complex signal transduction network’s part and it works differently in different systems. Drought stress is a major restraint for crop production in arid and semi arid states such as Pakistan. In this study experiments were conducted against the responses of strawberry plant to both pre-harvest application of salicylic acid and drought treatments. The design of the experiment was a factorial randomized complete block design (RCBD) with five water regimes (150ml, 175ml, 200ml, 225ml, 250ml) and five levels of SA at (0.0mML-1, 0.5mML-1 1mML-1, 2mML-1, and 3mML-1) as the main factors and four replications. The experiments were conducted in the research area of AAUR Pakistan. Statistical analysis showed drought stress significantly reduced vegetative and reproductive growth, along with various and biochemical attributes, but augmented the leaf proline. SA application significantly increased plant fresh and dry weight, leaf area, proline and decreased root length, number of roots and water use efficiency. SA treatment therefore decreased adverse effect of stress on strawberry plants. Thus the result suggests that SA at a concentration of 3mML-1 concentration could be used commercially to improve yield of strawberry.
Received | July 28, 2017; Accepted | September 11, 2017; Published | September 28, 2017
*Correspondence | Khalid Mahmood Qureshi, National Agricultural Research Center (NARC), Park Road, Islamabad; Email: kmq_2008@hotmail.co.uk
Citation | Bano, N. and K.M. Qureshi. Responses of strawberry plant to pre-harvest application of salicylic acid in drought conditions. Pakistan Journal of Agricultural Research, 30(3): 272-286.
DOI | http://dx.doi.org/10.17582/journal.pjar/2017.30.3.272.286
Keywords | Fragaria ananassa, Salicylic acid, Drought tolerance, Morphological responces
Introduction
Salicylic acid is known to be an endogenous monitoring agent (Zavala et al., 2004). Pre harvest use of SA initiates tolerance against pathogens in fruits such as pear (Jiankang et al., 2006) also helps to reduce the risks of development of disease (Yao and Tian, 2005). 2mM concentration of SA has efficiently increased antioxidant activity, ascorbic acid content and TSS of strawberry, and it also prevents fungal infection (Asghar, 2006). SA alters the effect and treatment of SA in different stages of growth such as fruit development, vegetative, and pre harvesting stage. It also helps to prevent of softening of fruits such as banana and kiwi fruit (Srivastava and Dwivedi, 2000; Zhang et al., 2003). Salicylic Acid produces free radical ions and is an electron contributor preventing respiration (Wolicka et al., 2005). Salicylic acid reduces rate of respiration and loss in fruit weight by inducing the closing of stomata (Manthe et al., 1992; Zheng and Zhang, 2004). SA is a simple abundant plant phenolic used to monitor and control different processes in plants which includes production of heat, resistance against disease, germination of seed, ethylene production and sex polarization (Raskin, 1992; Zhang et al., 2003). Currently, the scientific literature accumulates more and more information about the indispensible role of phytoharmones in particular of salicylic acid, in response to stress factors, action from the external environment. It is considered that an important compound of the mechanism of SA’s protective action is to prevent the stress induced disturbance of the phytoharmone balance (Raskin, 1992; Shakirova et al., 2003).
Salicylic acid being phenolic in nature, acts as a natural inductor of thermogenses in Aram lily, which initiates flowering and helps in the uptake of ions and conductance through stomata (Raskin, 1992). During the past two decades SA has gained attention among scientists as it has a strong ability to initiate systematic acquired resistance (SAR) towards different pathogens in plants which is exhibited only in pathogenesis related proteins (PR) and SA acts to stimulate such genes (Metraux, 2001). Salicylic acid plays a vital role in environmental stress resistance and has gained importance because of its plant protective properties (Muhammad et al., 2013). SA is a strong indicator molecule and is used in producing responses towards environmental and biotic stresses (Kranter et al., 2006). SA is a plant hormone performing as a significant indicator molecule adds to acceptance against a biotic stresses. It plays vital role in uptake of ions during transpiration. SA is also involved in activating plant defensive mechanisms against different pathogens (Khan et al., 2003) and also participates in plant water relation (Barkosky and Einhelling, 1993) photosynthesis stomata opening and closing parameters (Khan et al., 2003; Arfan et al., 2007) in stress environments.
Salicylic acid is basically an ortho-hydroxy benzoic acid that is helpful in performing different biochemical and morphological roles in plants and also regulates their productivity and growth of plants (Hayat et al., 2010). Improved seedling growth and germination was noted in wheat crop, when the grains were treated with SA before sowing (Shakirova, 2007). Fariduddin et al. (2003) described the accumulation of dry matter being amplified in Brassica juncea, when sprayed with low concentrations of SAE. Khodary, (2004) observed an important increase in pigment contents growth characteristics and photosynthetic role in maize crops treated with SA. In tomato and cucumber, total production of the fruit improved considerably when treated with low concentration of SA (Larque-saavadra and Martin-Max, 2007). SA treatment on leaves increase pod formation and flowering in soybean (Kumar et al., 1999). SA is categorized as of python hormones that control numerous processes in plants such as photosynthesis, closing of stomata, protein and chlorophyll synthesis, inhabitation of ethylene, transpiration, biosynthesis, uptake of nutrient (Raskin, 1992; Khan et al., 2003). Other applications of SA include osmo regulation maintenance in plants during water deficit stress (Shakirova et al., 2000; El-Tayeb, 2005). Furthermore salicylic acid is a natural hormone and an indicator molecule that helps in the triggering of plant’s defense mechanism (Klessing and Malamy, 1994).
Materials and Methods
Present research study was conducted at the Research Area of Horticulture Department, Pir Mehr Ali Shah Arid Agriculture University Rawalpindi during 2010-2012 in Rawalpindi. The agro-climate of region comprises humid sub-tropical climate and fall under semi-arid zone having hot and long summers followed by mild and short winter. Annual rainfall is about 1044 mm, most of which occur during monsoon season (July – September). The runners of strawberry cv “Chandler” used in the research were collected from Swat, KPK province and were planted in clay pots (25 cm diameter x 25 cm height) individually. The experimented pots were filled with homogenous mixture of sand, silt and FYM (1:1:1). Hundred plants were studied as one replicate and each treatment comprised of four replicates. Presence of small holes at the base of each pot was made sure for water percolation. The experiment was conducted in October 2010 to April 2012. Water regimes were calculated according to field capacity.
Stress was applied to each plant when they produced three to four true leaves, according to the respective treatment. A foliar spray of salicylic acid was applied at 30 days after sowing (DAS). Total two applications of salicylic acid sprays were carried to the foliage (entire aerial plant parts) during the research. The experiment was conducted according to a Randomized Complete Block Design (RCBD) and sampling for various parameters was carried out accordingly.
Climatic conditions of rawalpindi during strawberry growing period 2010-2012
The data for temperature (minimum and maximum), rainfall of entire cropping periods were obtained from Metrological department of Rawalpindi. Average monthly temperature was lowest in December 2010 and January 2011 while it was highest in September 2010 and May 2011. In contrast, total monthly rainfall was highest in February 2010 and March 2011 but lowest in November and December as indicated in Table 1. Minimum temperature was recorded during january 2011 with average rainfall during cropping season as evident from table.
Soil analysis
Physical and chemical properties of soil were presented in Table 2. The soil analysis showed that soil was
Table 1: Climatic conditions of Rawalpindi during 2010-2012
| Months | Rainfall (mm) |
Max. Temp (°C) |
Min. Temp (°C) |
Average Temp (°C) |
|
2010-2011 |
||||
| SEP | 92 | 33 | 21 | 27 |
| OCT | 23 | 30 | 14 | 22 |
| NOV | 16 | 25 | 7.5 | 16.25 |
| DEC | 36 | 21.1 | 3.3 | 12.2 |
| JAN | 17.5 | 21.5 | 2.9 | 12.2 |
| FEB | 105.6 | 20.1 | 7 | 13.55 |
| MAR | 43.8 | 29 | 12.6 | 20.8 |
| APR | 22.4 | 33.9 | 16.9 | 25.4 |
| MAY | 35.6 | 36.4 | 20.5 |
28.45 |
|
2011-2012 |
||||
| SEP | 104.5 | 30.86 | 18.03 | 24.445 |
| OCT | 22 | 26.1 | 10.16 | 18.13 |
| NOV | 0 | 20.64 | 3.77 | 12.205 |
| DEC | 22 | 20.9 | 1.7 | 11.3 |
| JAN | 7 | 17.7 | 1.5 | 9.6 |
| FEB | 118.3 | 18.6 | 5.8 | 12.2 |
| MAR | 231.5 | 26.1 | 10.6 | 18.35 |
| APR | 32 | 29.1 | 14.1 | 21.6 |
| MAY | 70 | 37.7 | 21.1 | 29.4 |
relatively rich in plant nutrients. Soil was well drained sandy loam with pH 8, Ec. 0.45dsm-1, available phosphorus 74.0mg kg-1, available potassium 140mg kg-1mg kg ,organic matter 2.50% while saturation was 26. Whereas in year 2011-2012 the analysis of soil revealed that soil was sandy loam in texture with the same pH 8, Ec. 0.45dsm-1dsm, available phosphorus 75.0mg kg-1, available potassium 145mg kg-1mg kg and organic matter 2.70% while saturation was 30.
Drought stress /Water treatment
In October all plants were watered to excess 1 day before root damage commenced and weighed after water stopped dripping from the pot to determine weight at field capacity. Thereafter, drought-stressed plants were weighed daily and then watered to bring them up to 85% of this weight. After 1 week, this maximum level of hydration was increased to 87% due to severe wilting in some plants within 24h of watering. Several plants continued to wilt on sunny days and these were given an additional 5 ml daily. Non stressed plants were watered just until water appeared below the pot to avoid excessive irrigation. A tensiometer reading, which measures the soil matric potential, was taken on selected plants prior to daily watering to confirm drought stress. Values depend on soil characteristics but in this experiment they were typically 28–40 kPa for stressed plants and 0-12 kPa for well-watered plants on a scale from 0 kPa (saturated soil) to 40 kPa. On the final day before harvesting started, the 47 drought-stressed plants averaged 82.2% (70.9% sd) of weight at field capacity 24 h after last watering, whereas measurements taken on a subset of non stressed plants averaged 89.8%(72.1% sd, n=15) of field capacity (Victoria, 2010).
Treatments of water regimes and salicylic acid concentration
| Treatments | Drought stress | Salicylic acid |
| T1 | 150ml/plant |
0mML-1 |
| T2 | 175ml/plant |
0.5mML-1 |
| T3 | 200ml/plant |
1mML-1 |
| T4 | 225ml/plant |
2mML-1 |
| T5 | 250ml/plant |
3mML-1 |
Table 2: Soil analysis of potting media
| Year | E.C. dSm-1 | Ph | Organic matter (%) | Available Phosphorus (mg kgˉ¹) | Available potassium (mg kgˉ¹) | Saturation (%) | Texture |
| 2010-2011 | 0.45 | 8 | 2.5 | 74 | 140 | 26 | Sandy loam |
| 2011-2012 | 0.45 | 8 | 2.7 | 75 | 145 | 30 | Sandy loam |
Methodology
The length of every plant from each treatment per replication was measured by scale and the average figures were determined individually. The numbers of leaves of arbitrarily chosen plants from each treatment were counted and their average was calculated. A measurement of leaf area was obtained with an ADC area meter AM 100. According to readings, twenty leaves per treatment per replication were gathered arbitrarily to build the contrast. Average number of trusses was calculated for every plant separately. The Average number of flowers was calculated from the plants per treatment per replication from 1st flower open to fruit setting. At ripeness the overall number of fruit produced by every plant was counted individually to see the result of each treatment on fruit yield. The percent amount of fruit set from flower from period of one to last picking was measured by following formula:
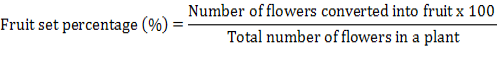
The readings of fruit size were obtained by measuring the length and width of ten randomly chosen fruits per treatment per replication with the assistance of vernier caliper at extreme ripeness. The weight of fruit of ten selected plants from all treatments per replications was recorded in grams by using calibrated weighing balance (SHIMADZU EL-6000SA). Root length was measured with a scale at the end of experiment. Shoot length was also measured with a scale at the end of experiment. The root: shoot ratio of the plant was calculated by using following formula. Root shoot ratio= root length/shoot length.
The diameter of plant crown was also increased independently in centimeters by using vernier caliper, after the harvesting and the average was calculated. The Canopy extend for N – S and E – W direction was obtained in centimeters through graduated scale and average was calculated. Biomass was measured by taking fresh and dry weight of the plants. The total runners formed by the plants were calculated. Weight of twenty fresh and dried leaves at 700C in an oven was determined by balance (SHIMADZU EL-6000SA) and their mean values were obtained to calculate the difference between treatments. PH (Hydrogen ion concentration) of strawberry fruit was measured with the pH meter from homogenized juice. Leaf chlorophyll contents were tested using chlorophyll meter spad-502 (Minolta camera Co. Ltd) Osaka Japan. To record the reading shows in pre-calibrated spad units, a leaf was inserted half way from the leaf tip and collar, and on middle point between the leaf midrib and leaf margin. The area of leaf has a range 2x3 cm at flowering phase the juvenile completely stretched leaf which had an uncovered collar was selected for measuring readings (Noguchi and Hansen, 1999).The soluble solid contents of fruit from each treatment were measured by means of digital refractometer (Atago-palette 101; Atago Co; Itabashimku, Tokyo, Japan). Wedge form pieces of uniform dimension from fine fruits were chosen at random from every treatment for every replication. A compound sample was prepared using a homogenizing by juicer. TSS values were then measured from the prepared homogenized juice.
A randomized complete block design (RCBD) was used as experimental design with two factors each with four replicates. Data was statistically analyzed by using analysis of variance (ANOVA) techniques for the soundness of analysis and difference among treatment means was compared by using Least Significance Difference (LSD) test at 5 % probability level. Statistical analysis was carried out with the help of statistix8.1 software. The experiment was repeated in the subsequent year. Data on the following parameters were recorded for all experiments:
Result and Discussion
Effect of drought and sa on plant height (cm)
The plant height significantly varied with the different water regimes in 2010-2011 (Figure 1). The maximum plant height was (50.58cm) under 3mM SA treatment and stress level 225ml.The minimum plant height was (26.48cm) under stress level 150ml(control) and 3mM SA treatment. Analysis of variance showed highly significant differences among water regimes and salicylic acid treatment for plant height during 2011-2012. The greatest plant height was (50cm) less than 3mM SA treatment and stress level the 225ml. The lowest plant height was (26.73cm)
under stress level in the150ml (control) and 3mM SA treatment. According to the outcome the plants treated with water stress and salicylic acid had the greatest plant height. Khodary, (2004) and Niakan et al. (2010) described that pre-harvest sprays of salicylic acid increased the shoot length in plants. Our findings agree Salicylic acid when combined with sodium chloride salt, increased height compared to the control but the concentration of salinity and salicylic acid affected the plant height. Overload concentration of salt and growth regulator may negatively affect the plant height (Borsani et al., 2001).
Effect of sa and drought on number of leaves
Analysis of the number of leaves of strawberry plant during 2010-2011 were significant at p <0.05 (Figure 2). Plants treated with 3mM SA treatment and stress level 250ml produced maximum number of leaves (10.53). But the lowest number of leaves was 4.30 in the stress treatment 150ml (control) and 0mM SA treatment. While the number of leaves significantly varied with the different water regimes in the year 2011-2012. Interactions of treatment were highly significant. The maximum number of leaves was (10.53) in the 3mM SA treatment and stress level 250ml. The minimum number of leaves was (4.32) under stress level 150ml (control) and 0mM SA treatment. Salicylic acid therefore improved the numbers of leaves per plant similar results had been recorded by Eraslan et al. (2007).
Effect of drought and sa on leaf area (cm2)
In 2010-2011 a significant effect was found for leaf area (Figure 3). The highest leaf area of plant was (88.75cm2) less than 3mM SA treatment and stress level 250ml. The lowest leaf area of plant ranged was (27.5cm2) under the stress level 150ml (control) and in the 0mM SA treatment. Whereas significant differences among means for water regimes and salicylic acid interaction were also found for this parameter. The result of interaction during 2011-2012 indicated that plant grown under drought stress with application
of different concentration of SA produced maximum leaf area. The highest leaf area of plant was (40.62cm2) in the 3mM SA treatment and stress level 250ml. The least leaf area of plant ranged was (23.30cm2) under stress level 150ml (control) and 0.0mM SA treatment. Exogenous application of salicylic acid increased the leaf area in the strawberry plants. Our results are parallel to El-Tayeb, (2005) on barley; Khodary, (2004); Amin et al. (2007) on onion; Szepsi et al. (2005); Stevens et al. (2006) in tomato; Yildirim et al. (2008) on cucumber. SA has an anti-senescence persuade on plant parts and its treatment may extend the development of vegetative parts important to increased leaf area (Jamali et al., 2011). Sayyari et al. 2013 also observed that fresh and dry weight, leaf area of lettuce plants significantly were affected by drought and SA application (Bhatt et al., 2005; Zahra et al., 2010). Delavri et al. (2010) also found that SA raises the leaf area in sweet basil plants, which is in agreement of our findings. Senaratna et al. (2000) have recommended same system to be accountable for SA induced several stress tolerance in bean and tomato plants.
The effect of sa on crown size under drought
Analysis of variance for crown size during 2010-2011 (Figure 4) showed the maximum crown size was (16.45 cm) at 3mM SA treatment with stress level 250ml whereas (15.39 cm) at 2mM SA treatment on stress level 250ml. It revealed that both results are at par. Furthermore, the smallest crown size was (7.4 cm) under stress level 150ml (control) and 0.0mM SA treatment. Significant water regimes and salicylic acid interactions were found for this trait. The enlarged crown diameter referred to more vegetative development including increased number of side branches, flowering trusses, runners and leaves. These findings are similar to Eraslan et al. (2007). Many researchers have shown evidence that salicylic acid improved the fresh mass of the crown, shoot and roots of the plants (Karlidag et al., 2009 b, Amborabe et al., 2002).
Effect of sa and drought on shoot length
The effect of different levels of stress on shoot length was highly significant during 2010-2011 (Figure 5). The highest shoot length was (36.58cm) in the 3mM SA treatment and stress level 225ml. The shortest shoot length was (10.98cm) in the 150ml and 3mM SA treatment. Whereas significant differences among means for water regimes and salicylic acid interaction during 2011-2012, were also revealed for this trait. The highest shoot length was (34.65 cm) under 3mM SA treatment and stress level 225 ml. The shortest shoot length was (10.98cm) under stress level 150ml and 3mM SA treatment.
Effect of drought and sa on fresh weight of plant (mg)
The result pertaining to fresh weight of strawberry fruit for 210-2011 (Figure 6) was found to be significantly different among the treatments. The highest fresh weight of plant was (65.62mg) under 2mM SA treatment and stress level 225ml. The lowest fresh weight of plant was (22.30mg) under stress level 150ml (control) and 0.0mM SA treatment. Whereas statistical analysis of data during 2011-2012 revealed that the interactive effect of SA treatment and stress levels on the fresh weight of strawberry plant was significant. The highest fresh weight of plant was (88.75mg) under 2mM SA treatment and stress level 225ml. The least fresh weight of plant was (27.50mg) under stress level 150ml (control) and 0.0mM SA treatment. Salicylic acid is a growth hormone which increases the vegetative characteristics of plants when used exogenously, thus escalating the growth and maturity of the plants and many research workers (Hayat et al., 2010; Hao et al., 2010; Rivas-San et a., 2011) have found similar results. Stevens et al. (2006) and Szepesi et al. (2005) showed increased shoot fresh mass in tomato and Yildirim et al. (2008) in cucumber, when exogenous salicylic acid
was applied. However, an unfavorable effect of high absorption of salicylic acid is reported by Babalar et al. (2007) which agree with our results. The statistical analysis showed that drought stress and application of SA had significant effects on the morphological, physiological and biochemical parameters of the strawberry plants. Water stress is characterized by stomata closing, wilting and decreases in enlargement and growth of cells due to decrease in water content, turgor and total water potential. Enlargement, differentiation and cell division are main processes that determine the senescence of adult leaves, premature abscission, hence decrease the photosynthesis area (Bhatt et al., 2005; Zahra et al., 2010).
Effects of sa and drought on chlorophyll contents
Leaf chlorophyll is one of main component of the photosynthetic system governing the dry matter production. Its production increased significantly with SA application under water stress as compared to the control without SA. On chlorophyll concentration, the effect of different levels of stress was highly significant. It was observed that the interactive effect of treatment and stress levels on the concentration of chlorophyll was significant (Figure 7). The highest concentration of chlorophyll was (47.6) and (47.56) less than 2mM SA treatment and stress level 225ml. Furthermore, the lowest chlorophyll concentration level was (34.4) and (35.45) under stress level 250ml and 0.0mM SA treatment. In the present study, chlorophyll were increased in stressed plants due to SA application as a foliar spray, thus effect of SA on chlorophyll agree with earlier studies in which it was found that SA increased the pigment content in soya bean (Zhao et al., 1995) maize (Singh et al., 2008; Khodary, 2004) and wheat (Singh and Usha, 2003) grown under normal or stressed conditions.
Moreover, SA significantly increased chlorophyll,
Figure 7: Effect of drought and salicylic acid on chlorophyll contents in strawberry plants during years 2011-2012.
recording maximum value in the 2mM. In contrast, the content of such pigments were reversely distorted using higher concentration of SA. Gharib (2006) also found the same results that in marjoram and sweet basil whereby SA at 10-5mM stimulates synthesis of chlorophyll whereas 10-3mM has a reverse effect. Iqbal et al. (2006) and Shakirova et al. (2003) on wheat plants and Abdel-Wahid et al. (2006) on maize plants showed that SA caused a significant raise in chlorophyll content. It is usually found that photosynthetic effectiveness depends on pigments like chlorophyll a and b, playing an important role in photochemical response of photosynthesis (Taiz and Ziegar, 2002).
In the present study chlorophyll increased in stressed plants after SA application as a foliar spray, thus effect of SA on chlorophyll can be clarify in view of some earlier studies in which it was found that SA augmented the pigments content in soybean (Zhao et al., 1995) maize (Singh et al., 2008; Khodary, 2004) and wheat (Singh and Usha, 2003) grown under normal or stressed condition. In view of some earlier studies , it can be explained that exogenous applied SA might have affected different factors governing metabolism and uptake of carbon fixation that includes RuBisCo enzyme activity and concentration (Pancheva et al., 1996) and often metabolic limiting factors such as photosynthetic carbon reduction (PCR) cycle ( Lawler and Carnic, 2002; Athar and Ashraf, 2005).
Effect of drought and sa on total soluble solids
A significant effect of drought treatment was observed on TSS of strawberry fruit for year 2010-2011 (Figure 8). The highest TSS was (8.46) in the 3mM SA treatment at stress level 250ml. Furthermore, the lowest TSS was (4.32) under stress level 150ml (control) and 0.0mM SA treatment.
The results showed that salicylic acid joint with drought stress can progress the TSS of strawberry fruits. During year 2011-2012 also significant effect of drought was observed on TSS of strawberry fruit. The highest TSS was (8.50) at 3mM SA treatment at stress level 250ml. Furthermore, the least TSS was (4.31) under the stress level 150ml (control) and 0.0mM SA treatment. The results illustrated that salicylic acid collective with drought stress can get improved the TSS of strawberry fruits. Our findings agree with the results reported by Karlidag et al. (2009 b) and Asghar (2006) that foliar application of salicylic acid at 1mM and 2mM enhanced the superiority characteristics such as total soluble solids in strawberry fruits.
Effect of sa and drought on fruit yield
The effect of the drought on fruit yield of strawberry for 2010-2011 (Figure 9) was significant at the p< 0.05. The greatest fruit yield was (55.250) in the 3mM SA treatment and stress level 250ml. The smallest fruit yield was (10.300) under stress in the 150ml (control) and 0mM SA treatment. Statistical analysis of variance showed highly significant differences among water regimes and salicylic acid treatments for fruit yield during 2011-2012. The greatest fruit yield was (54.14) in the 3mM SA treatment and stress level 250ml. The smallest fruit yield was (11.61) under stress in the 150ml (control) and 0mM SA treatment. Results demonstrated that foliar application of SA, mainly in the 3mM resulted in the highest increase in yield. Foliar application of salicylic acid has also shown to considerably amplified yield and its components in maize (Shehata et al., 2001 and Abdel-Wahed et al., 2006) and wheat plants (Shakirova et al., 2003; Iqbal and Ashraf, 2006).
Conclusions
The results described here show that SA is an important compound for reducing environmental stresses and sensitivity of crop, because under certain conditions it has been found to mitigate the damaging
effects of various stress factors in plants. Several SA levels have been shown to protect various plant species against environmental stress factors through a number of different processes such as stress tolerance mechanism. It is clear that SA also plays an important function in network of signal transduction and its according differs in different systems. From the proceeding outcome and conversation it can be concluded that leaf application of strawberry plants with SA and water regimes, independently or their relations, stimulate the growth of strawberry plants by means of increasing of the biosynthesis of pigments in photosynthesis, enhancing yield of strawberry thus SA enhanced value and nutritional value. That SA can be utilized to reduce crop losses during stress may have important practical use.
Author’s Contribution
Nargis Bano collected and analysed the data and wrote the manuscript. Khalid Mahmood conceive the idea, did overall management of the article and provided technical input at every step.
References
Abdel-Wahed, M.S.A., A.A. Amin and S.M. El-Rashad, 2006.Physiological effects of some bioregulators on vegetative growth, yield and chemical constituents of yellow maizeplants. World. J. Agic. Sci. 2: 149-155.
Arfan, M., H.R. Athar and M. Ashraf, 2007. Does exogenous application of salicylic acid through the rooting medium modulate growth and photosynthetic capacity in two differently adapted spring wheat cultivars under salt stress. J. Plant. Physiol. 164: 685-694. https://doi.org/10.1016/j.jplph.2006.05.010
Athar, H. and M. Ashraf, 2005. Photosynthesis under drought stress. – In: Pessarakli, M. (ed.): Photosynthesis, 2nd Ed. 795-810. CRC Press, New York
Amborabe, B.E., P.F. Lessard, J.F. Chollet, G. Roblin, 2002. Antifungal effect ofsalicylic acid and other benzoic acid derivatives towards Eutypalata: Structure-activity relationship. Plant Physiol.Biochem. 40: 1051-1060. https://doi.org/10.1016/S0981-9428(02)01470-5
Babalar, M., M. Asghari, A. Talaei and A. Khosroshahi, 2007. Effect of pre and postharvest salicylic acid treatment on ethylene production, fungal decay and overall quality of Selva strawberry fruit. Food Chemi. 105: 449-453. https://doi.org/10.1016/j.foodchem.2007.03.021
Barkosky, R.R., and F.A. Einhellig, 1993. Effects of salicylic acid on plant water relationship. J. Chem. Ecol. 1: 237–247. https://doi.org/10.1007/BF00993692
Borsani, O., V. Valpuesta and M.A. Botella, 2001. Evidence for a role of salicylic acid in the oxidative damage generated by NaCl and osmotic stress in Arabidopsis seedlings. J. Plant Physiol. 126: 1024 – 1030. https://doi.org/10.1104/pp.126.3.1024
Delavari, P.M., A. Baghizadeh, S.H. Enteshari, K.H.M. Kalantari, A. Yazdanpanah and E.A. Mousavi, 2010. The Effects of salicylic acid on some of biochemical and morphological characteristic of Ocimum basilicucm under salinity stress. Australian J. Basic and Applied Sci. 4(10):4832-4845.
Gharib, F.A.L. 2006. Effect of salicylic acid on the growth, metabolic activities and oil content of basil and marjoram. Int J Agric Biol 9:294–301.
El-Tayeb, M.A., 2005. Response of barley grains to the interactive effect of salinity and salicylic acid. Plant Growth. Regul. 45: 215–224. https://doi.org/10.1007/s10725-005-4928-1
Eraslan, F., A. Inal, A. Gunes, M. Alpaslan, 2007. Impact of exogenous salicylic acid on growth, antioxidant activity and physiology of carrot plants subjected to combined salinity and boron toxicity. Sci. Hort. 113: 120-128. https://doi.org/10.1016/j.scienta.2007.03.012
Fairduddin, Q., S. Hayat, and A. Ahmad, 2003. Salicylic acidinfluences net photosynthetic rate, carboxylation, efficiencyof nitrate reductase activity and seed in Brassica juncea L. Photosynthetica. 41: 281-284. https://doi.org/10.1023/B:PHOT.0000011962.05991.6c
Hao, F., S. Zhao, H. Dong, H. Zhang, L. Sun and C. Miao, 2010. Nia1 and Nia2 are involved in exogenous salicylic acid-induced nitric oxide generation and stomatal closure in Arabidopsis. J. Integr. Plant Biol. 52(3): 298-302.
Iqbal, M. and M. Ashraf. 2006. Wheat seed priming in relationto salt tolerance, growth, yield and level of free salicylicacid and polyamines. Ann. Bot. Fennici. 43: 250-259.
Jamali, B., S. Eshghi and E. Tafazoli, 2011. Vegetative and reproductive growth of strawberry plants cv. ‘Pajaro’ affected by salicylic acid and nickel. J. Agri. Sci. Tech. 13: 895-904.
Jiankang, C., Z. Kaifang and J. Weibo, 2006. Enhancement of postharvest disease resistance in Ya Li pear (pyrus bretschneideri) fruit by salicylic acid sprays on the trees during fruit growth, European J. Plant Pathol. 114: 363-378. https://doi.org/10.1007/s10658-005-5401-8
Karlidag, H., E. Yildirim and M. Turan, 2009b. Salicylic acid ameliorates the adverse effect of salt stress on strawberry. Sci. Agric. 66 (2): 180-187. https://doi.org/10.1590/S0103-90162009000200006
Khan, W., B. Prithiviraj, and D.L. Smith, 2003. Photosynthetic responses of corn and soybean to foliar application of salicylates. J. Plant. Physiol. 160: 485– 492. https://doi.org/10.1078/0176-1617-00865
Klessig, D.F., and J. Malamy. 1994. The salicylic acid signal in plants. Plant Mol. Biol. 26: 1439–1458. https://doi.org/10.1007/BF00016484
Kumar, P., S.D. Dube and V.S. Chauhan. 1999. Effect of salicylic acid on growth, development and some biochemical aspects of soybean (Glycine max L. Merrill). Int. J. Plant Physiol. 4: 327-330.
Larque-Saavedra, A., and F. Martin-Mex. 2007. Effects of salicylic acid on the bioproductivity of the plants. In: Hayat, S., Ahmad, A. (Eds), Salicylic Acid, A Plant Hormone. Springer Publishers, Dordrecht, The Netherland. 15-23.
Lawlor, D.W. and G. Cornic, 2002. Photosynthetic carbonassimilation and associated metabolism in relation to waterdeficits in higher plants. Plant Cell Environ. 25: 275-294. https://doi.org/10.1046/j.0016-8025.2001.00814.x
Manthe, B., M. Schulz and H. Schnabl, 1992. Effects of salicylic acid on growth and stomatal movements of Vicia faba L. evidence for salicylic acid metabolization, J. Chem. Ecol. 18: 1525-1539. https://doi.org/10.1007/BF00993226
Muhammad, W.S., M. Razaq, A. Hussain, M. Yaseen, M. Afzal, and M.K. Mehmood, 2013. Yield and yield components of wheat, affected by aphid feeding and sowing time at multan, Pakistan. Pak. J. Bot. 45(6):2 011-2013.
Niakan, M., A. Jahanbani and M. Ghorbanli, 2010. Spraying effect of salicylate different concentrations on growth parameters, amount of photosynthetic pigments, anthocyanin, flavonoids and solution sugars of Coriandrum sativum L. J. Plant Sci. Researches. 18(2): 10-18.
Noguchi, N., and A.C Hensen, 1999. Nitrogen sensing for precision agriculture using cholorphyll maps. UILU. 99-7029.
Pancheva, T.V., L.P. Popova and A.N. Uzunova, 1996. Effect of salicylic acid on growth and photosynthesis in barley plants, J. Plant Physiol. 149: 57-63. https://doi.org/10.1016/S0176-1617(96)80173-8
Raskin, I. 1992. Salicylate, a new plant hormone, Plant Physiol. 99: 799-803. https://doi.org/10.1104/pp.99.3.799
Rivas-San, Vicente, M. and J. Plasencia. 2011. Salicylic acid beyond defence: its role in plant growth and development. J. Exp. Bot. 62: 3321–3338. https://doi.org/10.1093/jxb/err031
Shakirova, F.M., M.V. Bezrukova and A.R. Sakhabutdinova, 2000. Effect of salicylic acid on the yield of spring wheat and phytohormon budget in plants during ontogeny. Agrokhimiya. 5: 52-56.
Shakirova, F.M., A.R. Sakhabutdinova, M.V. Bezrukova, R.A. Fathudinova, and D.R Fathutdinova, (2003). Changes in hormonal status of wheat seedlings induced by Salicylic acid and salinity. Plant Sci. 164: 317-322. https://doi.org/10.1016/S0168-9452(02)00415-6
Shakirova, F.M. 2007. Role of hormonal system in the manisfestation of growth promoting and anti-stress action of salicylic acid. In: Hayat, S., A. Ahmad, (Eds). Salicylic Acid. A Plant Hormone. Springer. Dordrecht. Netherlands. 69-89.
Singh, B. and K. Usha, 2003. Salicylic acid induced physiological and biochemical in wheat seedling under water stress. Plant Growth Regul. 39: 137-141. https://doi.org/10.1023/A:1022556103536
Singh, A. and P.K. Singh. 2008. Salicylic acid induced biochemical changes in cucumber cotyledons. I. J. Agri. Biochem. 21(1-2):35-38.
Stevens, J. and Senaratna, T. 2006. Salicylic acid induces salinity tolerance in tomato (Lycopersicon esculentum cv. Roma): associated changes in gas exchange, water relations and membrane stabilisation. Plant Growth Regulation, 49:77-83.
Stevens, R., M. Buret, C.C. Garchery, Y. Carretero, M. Causse, 2006. Technique for rapid small-scale analysis of vitamin C levels in fruit and application to a tomato mutant collection. J. Agric. Food. Chem. 54: 6159–6165. https://doi.org/10.1021/jf061241e
Srivastava, M.K., and U.N Dwivedi, 2000. Delayed ripening of banana fruit by salicylic acid. Plant Sci. 158: 87-96. https://doi.org/10.1016/S0168-9452(00)00304-6
Taiz, L. and E. Zieger. 2006. Stress Physiology. In: Plant Physiology, 2nd edn. Sinauer Associates, Inc., Sunderland, M. A. 725-757.
Vale, Z. A, S.Y. Wang, C.Y. Wang and G.A.G. Aguilar. 2004. Effects of storage temperatures on antioxidant capacity and aroma compounds in strawberry fruit, Elsevier Ltd. on behalf of Swiss Society of Food Science & Technology. 101:1607-1612.
Victoria, A.B. 2010. The impact of arbuscular mycorrizal fungi on strawberry tolrence to root damage and drought stress. J. Pedobiol. 53:265-270.
Wolicka, B.A., A. Goossens and D. Inzé, 2005. Methyl jasmonate stimulates the de novo biosynthesis of vitamin C in plant cell suspensions, J. Exp. Bot., 56: 2527-2538. https://doi.org/10.1093/jxb/eri246
Yao, H. and S. Tian, 2005. Effects of pre- and post-harvest application of salicylic acid or methyl jasmonate on inducing disease resistance of sweet cherry fruit storage, Postharvest Biol. Technol. 35: 253-262. https://doi.org/10.1016/j.postharvbio.2004.09.001
Yildirim, E., M. Turan, I. Guvenc, 2008. Effect of foliar salicylic acid applications on growth, chlorophyll and mineral content of cucumber (Cucumis sativus L.) grown under salt stress. J. Plant Nutri. 31: 593-612. https://doi.org/10.1080/01904160801895118
Zavala, J.F.A., S.Y. Wang, C.Y. Wang and G.A.G. Aguilar, 2004. Effects of storage temperatures on antioxidant capacity and aroma compounds in strawberry fruit, Elsevier Ltd. on behalf of Swiss Society of Food Science & Technology.
Zhao, H.J., X.W. Lin, H.Z. Shi, and S.M. Chang, 1995. The regulating effects of phenolic compounds on the physiological characteristics and yield of soybeans. Acta Agron. Sin. 21: 351–355.
Zhang H, Philip H. Link, Jony K. Ngsee, Khai, Tran, Zheng Chi, Kerry.W.S.Ko and Zemin yao 2003. Localization of LDL receptor-related protein,( LRPI) to caveolae in 3T3-L1 adipocytes in responseto insulin treated. J. Biochem. 279: 2221-2230.
Zhang, Y., K.Chen, S. Zhang, and I. Ferguson, 2003. The role of salicylic acid in plant harvest ripening of kiwifruit, Postharvest Bio.Technol. 28: 67-74. https://doi.org/10.1016/S0925-5214(02)00172-2
Zheng, Y. and Q. Zhang, 2004. Effects of polyamines and salicylic acid postharvest storage of ‘Ponkan’ mandarin, Acta. Hort. 632: 317-320. https://doi.org/10.17660/ActaHortic.2004.63
Antioxidant Capacity and Aroma Compounds in Strawberry Fruit, Elsevier Ltd. on behalf of Swiss Society of Food Science & Technology.
Zhang H, Philip H. Link, Jony K. Ngsee, Khai, Tran, Zheng Chi, Kerry.W.S.Ko and Zemin yao 2003. Localization of LDL receptor-related protein,(LRPI) to caveolae in 3T3-L1 adipocytes in responseto insulin treated. J.Bio.chem.
Zhang, Y. Chen.K. Zhang.S. and Ferguson. I. 2003. The role of salicylic acid in plant harvest ripening of kiwifruit, Postharvest Biology and Technology, 28. 67-74.
Zheng Y. and Q. Zhang, 2004. Effects of polyamines and salicylic acid postharvest storage of ‘Ponkan’ mandarin, Acta. Hort., 632: 317-320.
To share on other social networks, click on any share button. What are these?







