Repellent and Growth Inhibitory Impact of Plant Extracts and Synthetic Pyrethroids on Three Strains of Callosobruchus chinensis L.
Repellent and Growth Inhibitory Impact of Plant Extracts and Synthetic Pyrethroids on Three Strains of Callosobruchus chinensis L.
Sidra-Tul-Muntaha1, Muhammad Sagheer1*, Mansoor-ul-Hasan1 and Shahbaz Talib Sahi2
1Department of Entomology, University of Agriculture, Faisalabad, Pakistan
2Department of Plant Pathology, University of Agriculture, Faisalabad, Pakistan
ABSTRACT
The present research work was conducted to evaluate the repellent and growth inhibitory efficiency of five plant extracts (Azadirachta indica, Melia azadirach, Pegnum hermala, baryosma and Zingiber officinale) and three synthetic pyrethroids (bifenthrin, cypermethrin and deltamethrin) on three geographical populations of Callosobruchus chinensis collected during 2013 from Faisalabad, Multan and Nankana districts of Punjab, Pakistan. Three concentrations of each plant extract (5, 10, 15 and 20%) and synthetic pyrethroids (0.01, 0.02 and 0.03%) were evaluated in this study. We observed significant results with each treatment. For both repellent and growth inhibitory effects, A. indica and deltamethrin were most efficient among plant extracts and pyrethroids respectively. At highest dose rates, more than 90% repellency was recorded with both A. indica and deltamethrin. Upto 80% progeny inhibition was caused by the extract of A. indica. While more than 50% population of C. chinensis was inhibited with deltamethrin. More pronounced results were obtained at high concentrations. Plants were effective in order of Azadirachta indica > Melia azadirach > Pegnum hermala > Salsola baryosma> zingiber officinale; whereas effectiveness of pyrethroids was in order of deltamethrin > cypermethrin > bifenthrin. The findings of this research will be helpful in organic storage of pulses by integrating the reduced risk pesticides with plant extracts.
Article Information
Received 28 December 2015
Revised 05 June 2016
Accepted 08 July 2016
Available online 14 February 2017
Authors’ Contributions
MS designed the study and analyzed the data. STM executed experimental work and wrote the article. MS, MH and STS supervised the work.
Key words
Beetles, botanicals, conventional insecticides, cereals, deterrence, exposure time, growth inhibition, stored products.
* Corresponding author: sagheersharif@yahoo.com
0030-9923/2017/0002-537 $ 8.00/0
Copyright 2016 Zoological Society of Pakistan
DOI: http://dx.doi.org/10.17582/journal.pjz/2017.49.2.581.589
Introduction
Pulse beetle, Callosobruchus chinensis L. (Coleoptera: Bruchidae) also known as dhora beetle is the most destructive cosmopolitan pest of stored gram and cause both qualitative and quantitative losses in legumes (Ahmed and Din, 2009; Righi-Assia et al., 2010; Upadhyay et al., 2011). It is a pest of stored pulses in Asia and Africa (Tapondjou et al., 2002; Kiradoo and Srivastava, 2010). Callosobruchus spp. cause 12-13% loss by feeding the protein contents of grains (FAO, 1994). C. chinensis causes up to 10% damage to stored chick pea (Aslam et al., 2002), and up to 90% loss to stored gram (Qayyum and Zafar, 1978).
Synthetic pyrethroids are new class of insecticides (Kumar, 2012) which are being used since 1970s. These are neurotoxic insecticides and effect neuro-endocrine functions. Due to pyrethroids, axon of a neuron get excited and insects become inactive. They also affect sodium channel (Beeman, 1982) due to which normal neuronal signaling is interrupted (Mujeeb and Shakoori, 2012). They have great knockdown, antifeedant, repellent and residual effect (Hirano, 1989). They are photostable and easily degradable, even at the low dose rate (Barlow et al., 1971; Hadaway, 1972). Numerous insect pests are controlled more efficiently by the use of synthetic pyrethroids than carbamate and organophosphate (Srivastava, 1996). Pyrethroids are rapidly metabolized in mammalian bodies, and thus their toxicity is very restricted (Soderlund et al., 2002). Pyrethroids are used against household, agricultural, stored grain and animal insect pests (Hutson et al., 1981).
Plant materials are used against stored product pests from the ancient time (Aslam et al., 2002). Plant extract and oils are being used in different parts of the world (Burroughs et al., 1988; Koul et al., 2008; Ali et al., 2012). Plant based natural pesticides are used as an alternative insect control measure to protect atmosphere from hazardous residual insecticides (Khan et al., 2013). Many compounds are present in these plant extracts that have effect on insect growth, development and behavior, acting as attractants, antifeedants, repellents, toxins, fumigants and insect growth inhibitors (Singh and Jain, 1987; Champagne et al., 1992; Carlini and Grossi-de-Sa, 2002; Cox, 2004; Kubo, 2006). So the plant extracts have broad spectrum properties and safer to use for sustainable pest management with pesticide free environment (Kiradoo and Srivastava, 2010). Several plants have been proved effective to control stored grain insects (Ratnasekera and Rajapakse, 2009).
Keeping this in mind the present study was designed to explore the efficiency of plant extracts and pyrethroids for the evaluation of repellent and growth inhibitory effect against pulse beetles. So that in future, plant extracts can be used in IPM of stored grain pests.
Materials and Methods
Rearing of homogenous insect culture
Heterogeneous samples of adults of C. chinensis were collected from infested pulses stored in grain market and farmer storages of three District Nankana Sahib, Faisalabad and Multan. The insect culture was reared in plastic jars (1.5 kg capacity) using lentils as food medium. The jars were covered with muslin cloth, tightened with rubber bands to avoid the escape of insects and were placed in cooled incubator at optimum growth conditions 30+ 2°C and 65+5 % R.H. After 3 days, adults were sieved out from the lentils, the grains having egg laying were again put into the jars and were placed again incubator for one month to get homogenous culture of C. chinensis. One week old beetles of C. chinensis from this culture were used for all bioassay studies.
Plant materials and their extracts
Fresh leaves of dhrek, Melia azadirach, seeds of hermal, Pegnum harmala, stem of khar booti, Salsola barysoma, leaves of neem, Azadirachta indica and rhizome of ginger, Zingiber officinale were collected from different locations from district Faisalabad and Nankanasahib.
After drying in shade the plant parts were ground using an electrical grinder into fine powder. The powders were used to make extracts in acetone as solvent. In 250 ml flask, 50 g of plant powder was soaked in 150 ml of acetone. The flasks were placed on rotary shaker for 48 h at 220 rpm. The prepared extract was filtered and used to make different concentrations. After that, the mixture was filtered and filtrates were placed in rotary evaporator to evaporate the acetone from filtrate. The final extract was considered as stock solution to prepare further dilutions for bioassay studies. Different concentrations viz., 5, 10, 15 and 20% were prepared from the stock solution. Like different concentrations of pyrethroids 0.01, 0.02 and 0.03% were also prepared in acetone.
Repellent effect of plant extracts and synthetic pyrethroids
The repellence activity was determined using area preference method (Rehman and Khan, 2014). After cutting the filter papers into two equal halves one half of each paper was treated with each concentration of plant extracts and synthetic pyrethroids. After evaporation of excess solvent two halves were joined together and placed in petri dishes. 50 adults of C. chinensis were released in the center of treated filter papers. Repellency data was recorded after 24, 48 and 72 h.
Percent repellency was calculated following the formula used by Rehman and Khan, 2014.
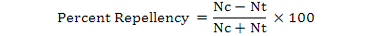
Where, Nc is the No. of insects in control half; and Nt is the No. of insects in treated half.
Progeny inhibition studies
Twenty five pairs of insects were released in the jars containing 50 g of treated grains of each concentration. Acetone was used as a control treatment. After 7 days the released insects were removed from the jars and data regarding population inhibition was recorded after 30 and 60 days (Rehman and Khan, 2014). Percent inhibition rate was calculated using following formula:
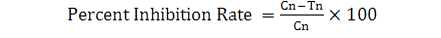
Where, Cn is the No. of progeny in control jars; and Tn is the No. of progeny in treated jars.
Data analysis
Data were subjected to Analysis of Variance for mean repellence and progeny inhibition caused by both plant extracts and pyrethroids will be computed using Statistica-6 following Completely Randomized Design. Means of significant treatments were compared using Tukey’s HSD test at 0.05 probability level, to check the significant difference among treatments.
Results
Repellent activity of plant extracts and pyrethroids
In the case of C. chinensis Faisalabad strain (hereafter used FSD), highest repellency 93% was recorded with the extract of A. indica followed by M. azadirach (90%). The repellent effect of P. hermala and Z. officinale were at par, they caused 82% repellency of the test insect. S. baryosma was least effective of all plant extracts causing 81% repellency (Table I).
In case of C. chinensis Multan strain (MLN), highest repellency 90% was recorded with A. indica followed by M. azadirach (89%) and Z. officinale (86%). P. hermala and S. baryosma caused 76% and 77% repellency respectively. A. indica was most active while P. hermala was least effective of all plant.
The results regarding repellent effect of plant extracts against C. chinensis Nankana Sahib (NNS)strain shows that the highest repellency 94% was recorded with A. indica followed by M. azadirach (92%) and Z. officinale (90%). The highest repellency by P. hermala and S. baryosma were 80% and 78%, respectively.
All plant extract treatments showed highly significant results. The repellent effect of pyrethroids was time and dose dependent. The repellency increase with the increase in concentration and exposure time. Overall results shows that the highest repellency was recorded in NNS strain followed by FSD and MLN strain.
Table II describes the repellent effect of pyrethroids which shows that in case of C. chinensis FSD strain, highest repellency 92% was recorded with deltamethrin which was followed by cypermethrin (90%) and bifenthrin (89%). In the case of C. chinensis MLN strain, highest repellency 90% was recorded with deltamethrin which was followed by cypermethrin (89%) and bifenthrin (82%). In the case of C. chinensis NNS strain, highest repellency (97%) was recorded with deltamethrin which was followed by cypermethrin (89%) and bifenthrin (86%).
All treatments of insecticides showed highly significant results. The repellent effect of pyrethroids was time and dose dependent. The repellency increase with the increase in concentration and exposure time. Out of all pyrethroids, highest repellency was recorded by deltamethrin in case of all strains. Deltamethrin results were most effective in NNS strain followed by FSD and MLN strains. By cypermethrin highest repellency was recorded in FSD while results were approximately same for both NNS and MLN strain. However, with bifethrin repellency was high in FSD strain which was followed by NNS and MLN strains.
Post treatment progeny inhibition of C. chinensis caused by plant extracts
Table III shows that in the case of C. chinensis FSD strain, highest inhibition (79.99%) was recorded with A. indica at 20% concentration after 60 days which was followed by M. azadirach (77.41%), S. baryosma (67.42%), P. hermala (62.90%) and Z. officinale (62.90%) at same concentration and time interval.After 30 days, highest inhibition (59.90%) was recorded with A. indica at 20% concentration which was followed by S. baryosma (58.38%), M. azadirach (57.36%), P. hermala (55.33%) and Z. officinale (50.76%) at same concentration and time interval. Of all the plant extracts used, A. indica was the most active plant with inhibition range 35.54-79.99% and Z. officinale was least effective with 27.41- 62.90% inhibition range.
In the case of C. chinensis MLN strain, highest inhibition (66.67%) was recorded with A. indica at 20% concentration after 60 days which was followed by M. azadirach (61.27%), S. baryosma (61.27%), P. hermala (58.41%) and Z. officinale (55.56%) at same concentration and time interval. After 30 days, highest inhibition (58.53%) was recorded with A. indica at 20% concentration, which was followed by M. azadirach (53.88%), S. baryosma (50.77%), P. hermala (49.74%) and Z. officinale (47.15%) at same concentration and time interval. Of all plant extracts used, A. indica was most active plant with inhibition % range 30.56-66.67% and Z. officinale was least effective with 25.90- 55.56%.
In the case of C. chinensis NNS strain, highest inhibition (80.81%) was recorded with A. indica at 20% concentration after 60 days which was followed by M. azadirach (77.49%), S. baryosma (75.34%), P. hermala (63.84%) and Z. officinale (58.30%) at same concentration and time interval. After 30 days, highest inhibition (70.00%) was recorded with A. indica at 20% concentration which was followed by M. azadirach (66.67%), S. baryosma (58.67%), P. hermala (50.00%) and Z. officinale (47.33%) at same concentration and time interval. Of all plant extracts used, A. indica was most active plant with inhibition % range 41.33-80.81% and Z. officinale was least effective with 24.67- 58.30%.
All plant extract treatments showed highly significant results. The effect of plant extracts was time and dose dependent as inhibition percentage was increasing with the increase in concentration and time interval. After 30 days, minimum values of inhibition percentage were recorded. Highest results were recorded at 20% concentration after 60 days of treatments application in case of all geographical strains of C. chinensis used during the study. Population of NNS strain was highly inhibited by plant extracts followed by FSD and MLN strain.
In the case of FSD strain, progeny was highly inhibited (66.67%) was by deltamethrin which was followed by cypermethrin (57.09%) and bifenthrin (49.99%) at 0.03% concentration after 60 days. In the case of C. chinensis MLN strain, highest inhibition (60.32%) was recorded with deltamethrin which was followed by cypermethrin (55.56%) and bifenthrin (45.39%) at same time period and dose rate. In the case of C. chinensis NNS strain, at 0.03% dose and after 60 days of exposure, highest repellency (70.40%) was recorded with deltamethrin which was followed by cypermethrin (61.43%) and bifenthrin (56.05%).
All plant extract treatments showed highly significant results. The effect of pyrethroids was time and dose dependent as progeny inhibition was increasing with the increase in concentration and time interval. After 30 days, minimum values of inhibition percentage were recorded. Highest results were recorded at 0.03% concentration after 60 days of application of treatments in case of all geographical strains of C. chinensis used during the study. Out of all pyrethroids, highest inhibition was recorded by deltamethrin in case of all strains. All pyrethroids were most effective in case of NNS which were followed by FSD and MLN strains.
Discussion
The present research work was conducted to evaluate the repellent and growth inhibitory efficiency of five plant extracts (Azadirachta indica, Melia azadirach, Pegnum hermala, Salsola baryosma and Zingiber officinale) and three synthetic pyrethroids (bifenthrin, cypermethrin and deltamethrin) on three geographical populations of Callosobruchus chinensis collected during 2013 from Faisalabad, Multan and Nankana districts of Punjab, Pakistan. Three concentrations of each plant extract (5, 10, 15 and 20%) and synthetic pyrethroids (0.01, 0.02 and 0.03%) were used in this study. We observed significant results with each treatment. For both repellent and inhibition effects, A. indica and deltamethrin were most efficient. At highest dose rates, more than 90% repellency was recorded with both A. indica and deltamethrin. Upto 80% progeny inhibition was documented with most active A. indica. While more than 50% population of C. chinensis was inhibited with deltamethrin.
Several plants have been reported to control stored grain insects efficiently (Ratnasekera and Rajapakse, 2009). Plant extracts may work as insect growth inhibitors, repellents, antifeedants, fumigants and entomocides (Kubo, 2006; Koul et al., 2008). Pyrethroids are rapidly metabolized in mammalian bodies, and thus their toxicity is very restricted (Soderlund et al., 2002). They are less toxic to the mammals (Gupta and Kumar, 1991). Pyrethroids have great knockdown, antifeedant, repellent and residual effect (Hirano, 1989).
The foremost emphasis of the study was to elucidate the effect of plant extracts and pyrethroids on repellence activity and progeny inhibition of three different strains of C. chinensis. The major finding of this study was that A. indica plant extract provide more better results and is more effective in managing C. chinensis. Its efficiency was increased with the increase in dose and time interval (Rehman and Khan, 2014). Plants contain some active compounds that are likely to cause insecticidal activities, repellency and progeny inhibition (Jilani and Su, 1983; Schmutterer, 1995). The adverse effects of the A. indicamay be due to its effect on the hormonal system of the insects (Murugan et al., 1999). High repellent effect has been studied by A. indica extract by Pradhan et al. (1963). Upto 30% repellency has been reported by Rehman and Khan (2014). A. indica extract has great effect on the oviposition reduction (Panday et al., 1986). Similarly, reduced progeny emergence of C. chinensis by the use of A. indica leaves extracts has been reported by Rouf et al. (1996), Khalequzzaman and Goni (2009) and Rehman and Khan (2014).
In our research study, the extract of Melia azadirach caused significant repellence as well as growth inhibition of C. chinensis. These results are in concordance with result of Aslam et al. (2002) and Mehdi and Rehman (2008) which reported high progeny inhibition of C. chinensis with the treatment of spices. Saljoqi et al. (2006) reported high toxic and repellent effect of M. azadirach extract on Sitophilus oryzae. Valladares et al. (1999) reported repellent and anti-feedant effect of M. azadirach extract. Khan and Marwat (2004) reported 82.50% repellency of R. dominica with treatment of M. azadirach. Toxic and feeding deterrence activities of M. azadirach has also been reported (Chauhan et al., 1987; Sexena, 1987).
Deltamethrin is one of the pyrethroid which is being used more frequently to control stored product insects (Saleem and Shakoori, 1990; Athanassiou et al., 2004). Resistance in Tribolium castaneum to deltamethrin has been reported by many authors (Champ, 1986; Collins, 1998; Daglish, 1998). Athanassiou et al. (2004) reported that even after 6 months of deltamethrin exposure there was no progeny recorded in T. confusum. Arthur (1996) reported that insecticides will keep on protecting stored product from insect damage. Pyrethroids for their activity are possible to become predominant grain protectant.
In our study P. hermala caused 82% repellency and more than 60% growth inhibition in all strains of C. chinensis. Abbassi et al. (2003) reported that alkaloids present in P. hermala are responsible for their insecticidal activity. Salari et al. (2012) reported low activity of P. hermala after 3 days of exposure against T. castaneum, while Jbilou et al. (2006) reported high insecticidal activity of P. hermala after 32 days of exposure on T. castaneum. High repellent effect has been studied by P. hermala against M. persicae by several authors. Repellent effect of different chemical compounds present in P. hermala on M. persicae has been reported (Gutierrez et al. 1997; Hori, 1998; Bruce et al., 2005).
Our results regarding the toxic effect of S. baryosma are in concordance with the results of Hasan et al. (2005) who reported good toxic effect of S. baryosma and cypermethrin against Trogoderma granarium. 80% repellency has been reported by S. baryosma against Triobolium castaneum (Sagheer et al., 2011) which confirms our results regarding repellent effect of S. baryosma. Z. officinale contain some alkaloids responsible for its inhibition effect (Purseglove, 1972). Carriquiriborde et al. (2009) reported adverse effect of cypermethrin on the growth and survival of Odontesthes bonariensis. Pennetier et al. (2009) proposed that the compounds responsible for repellence and insecticidal effect on combination can have synergistic effect against insect pests.
CONCLUSIONS
Keeping in view the results of the current work, it is concluded that plant extracts and pyrethroids are effective tools for sustainable management of stored product insect pests. But in order to avoid resistance problem these should be used only in recommended doses as well as rotation of insecticides may also be useful. Our aim should not be only to kill insect but our emphasis should be on the suppression of the next progeny of insects. So, further studies should be carried on to prepare botanical insecticide formulations so that they may be properly used by the grain handling agencies.
Statement of conflict of interest
Authors have declared no conflict of interest.
References
Abbassi, K., Atay-Kadiri, Z. and Ghaout, S., 2003. Biological effects of alkaloids extracted from three plants of Moroccan arid areas on the desert locust. Physiol. Ent., 28: 232-236. https://doi.org/10.1046/j.1365-3032.2003.00329.x
Ali, A., Ahmad, F., Biondi, A., Wang, Y. and Desneux, N., 2012. Potential for using Datura alba leaf extracts against two major stored grains pests, the rice weevil Sitophillus oryzae and Khapra beetle Trogoderma granarium. J. Pestic. Sci., 85: 359-366. https://doi.org/10.1007/s10340-012-0426-1
Arthur, F.H., 1996. Grain protectants: current status and prospects for the future. J. Stored Prod. Res., 32: 293-302. https://doi.org/10.1016/S0022-474X(96)00033-1
Aslam, M., Khan, K.A. and Bajwa, M.Z.H., 2002. Potency of some spices against Callosobruchus chinensis L. J. biol. Sci., 2: 449-452. https://doi.org/10.3923/jbs.2002.449.452
Athanassiou, C.G., Kavallieratos, N.G., Vayias, B.J., Dimizas, C.B., Papagregoriou, A.S. and Buchelos, C.T., 2004. Residual toxicity of beta cyfluthrin, alpha cypermethrin and deltamethrin against Tribolium confusum Jacquelin du Val (Coleoptera: Tenebrionidae) on stored wheat. Appl. Ent. Zool., 39: 195-202. https://doi.org/10.1303/aez.2004.195
Barlow, F., Elliotte, M., Farnham, A.W., Hadaway, A.B., Janes, N.F., Needham, P.H. and Wickham, J.C., 1971. Insecticide activity of the pyrethrins and related compounds essential features of insecticide activity in chryseinthemates and related cyclopropane esters. Pestic. Sci., 2: 115-118. https://doi.org/10.1002/ps.2780020306
Beeman, R.W., 1982. Recent advances in mode of action of insecticides. Annu. Rev. Ent., 27: 253-281. https://doi.org/10.1146/annurev.en.27.010182.001345
Bruce, T.J.A., Birkett, M.A., Blande, J., Hooper, A.M., Martin, J.L. and Khambay, B., 2005. Response of economically important aphids to components of Hemizygia petiolata essential oil. Pest Manage. Sci., 61: 1115-1121. https://doi.org/10.1002/ps.1102
Carlini, C.R. and Grossi-de-Sa, M.F., 2002. Plant toxic proteins with insecticidal properties– A review on the potentialities as bio-insecticide. Toxicology, 40: 1515-1539.
Carriquiriborde, P., Diaz, J., Lopez, G.C., Ronco, A.E. and Somoza, G.M., 2009. Effects of cypermethrin chronic exposure and water temperature on survival, growth, sex differentiation, and gonadal developmental stages of Odontesthes bonariensis (Teleostei). Chemosphere, 76: 374-380. https://doi.org/10.1016/j.chemosphere.2009.03.039
Champagne, D.E., Koul, O., Isman, M.B., Scudder, G.G.E. and Towers, G.H.N., 1992. Biological activity of limonoids from the Rutales, Phytochemistry, 31: 377-394. https://doi.org/10.1016/0031-9422(92)90003-9
Collins, P.J., 1998. Inheritance of resistance to pyrethroid insecticides in Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae). J. Stored Prod. Res., 34: 395-401. https://doi.org/10.1016/S0022-474X(98)00020-4
Cox, P.D., 2004. Potential for using semiochemicals to protect stored products from insect infestation. J. Stored Prod. Res., 40: 1-25. https://doi.org/10.1016/S0022-474X(02)00078-4
Daglish, G.J., 1998. Efficacy of six grain protectants applied alone or in combination against three species of Coleoptera. J. Stored Prod. Res., 34: 263-268. https://doi.org/10.1016/S0022-474X(98)00007-1
Gupta, P.K. and Kumar, S., 1991.Cumulative toxicity of deltamethrin in mice. J. environ. Biol., 12: 45-50.
Gutierrez, C., Fereres, A., Reina, M., Cabrera, R. and Gonzalez, C.A., 1997. Behavioral and sublethal effects of structurally related lower terpenes on Myzus persicae. J. chem. Ecol., 23: 1641-1650. https://doi.org/10.1023/B:JOEC.0000006428.00568.c5
Hadaway, A.B., 1972. Toxicity of insecticide to tsetse flies. Bull. World Hlth. Org., 46: 353-362.
Hasan, M., Siddique, M.A., Sagheer, M. and Aleem, M., 2005. Comparative efficacy of ethanol extracts of Amaranthus viridis L. and Salsola baryosma (Schultes) and Cypermethrin against Trogoderma granarium (Everts). Pak. J. agric. Sci., 42: 61-63.
Hirano, M., 1989. Characteristics of pyrethroid for insect pest control in agriculture. Pestic. Sci., 27: 353-360. https://doi.org/10.1002/ps.2780270404
Hori, M., 1998. Repellency of rosemary oil against Myzus persicae in a laboratory and in a screen house. J. chem. Ecol., 24: 1425-1432. https://doi.org/10.1023/A:1020947414051
Hutson, D.H., Gaughan, L.C. and Casida, J.E., 1981.Metabolism of the cis- and trans-isomers of cypermethrin in mice. Pestic. Sci., 12: 385-398. https://doi.org/10.1002/ps.2780120405
Jilani, G. and Helen, C.F., 1983. Laboratory studies on several plant materials as insect residual deterrent for production of cereal grain. J. econ. Ent., 76: 154-157.
Jilani, G. and Su, H.C.F., 1983. Laboratory studies on several plants materials as insect repellent for protection of cereal grains. J. econ. Ent., 76: 154-157.
Khalequzzaman, M. and Goni, S.H.M.O., 2009. Toxic potentials of some plant powders on survival and development of Callosobuchus maculatus (F.) and Callosobuchus chinensis. J. Life Earth Sci. 3: 1-6.
Khan, S.M. and Marwat, A.A., 2004. Effect of bakain (Melia azadarach) and Ak (Calatropis procera) against lesser grain borer Rhyzopertha dominica F. J. Res. Sci., 15: 319-324.
Khan, F.Z.A., Sagheer, M., Mansoor-ul-Hasan, Saeed, S., Ali, K.. Gul, H.T., Bukhari, S.A. and Manzoor, S.A., 2013. Toxicological and repellent potential of some plant extracts against stored product insect pest, Tribolium castaneum (Herbst.) (Coleoptera: Tenebrionidae). Int. J. Biosci., 3: 280-286. https://doi.org/10.12692/ijb/3.9.280-286
Koul, O., Walia, S. and Dhaliwal, G.S., 2008. Essential oils as green pesticides: potential and constraints. Biopestic. Int., 4: 63-84
Kubo, I., 2006. New concept to search for alternate insect control agents from plants. In: Naturally occurring bioactive compounds 3 (eds. M. Rai, and M. Carpinella). Elsevier, Amsterdam, The Netherlands. pp. 61-80. https://doi.org/10.1016/S1572-557X(06)03004-2
Mehdi, S.H.A. and Rahman, M.K., 2008. Insecticidal effect of some spices on Callosobruchus maculatus in black gram seeds. Univ. J. Zool. 27: 47-50.
Mujeeb, K.A. and Shakoori, A.R., 2012. Effect of fury, a synthetic pyrethroid, on esterases of different developmental stages of stored grain pest, red flour beetle, Tribolium castaneum (Herbst.)-spectrophotometric analysis. Pakistan J. Zool., 44: 601-613.
Murugan, K., Kumar, V.S., Jeyabalan, D., Babu, R., Nathan, S.S. and Sivaramakrishnan, S., 1999. Interactive effect of neem products on the control of pulse beetle, Callosobruchus maculatus (F). Neem Newsl., 15: 41-44.
Pandey, N.D., Mathur, K.K., Pandy, S. and Tripathi, R.A., 1986. Effect of some plant extracts against pulse beetle, Callosobruchus chinensis (L). Indian J. Ent., 48: 85-90.
Pennetier, C., Costantini, C., Corbel, V., Licciardi, S., Dabire, R.K., Lapied, B., Chandre, F. and Mougard, J.M., 2009. Synergy between repellents and organophosphates on bed nets: efficacy and behavioural response of natural free-flying. An. Gambiae mosquitoes. PLoS One, 4: 1-8. https://doi.org/10.1371/journal.pone.0007896
Purseglove, J.W., 1972. Tropical crops. Monocotyledons, volume 2, Longman Group Ltd, London, pp. 633.
Qayyum, H.A. and Zafar, M.A., 1978. Research on stored grain pests in Pakistan. Final Rep. PL480 Project, Department of Entomology, University Agriculture, Faisalabad, pp. 67-112.
Ratnasekera, D. and Rajapakse, R., 2012. The potential use of indigenous plant materials against Callosobruchuschinensis L. and Callosobruchus maculatus L. (Coleoptera, Bruchidae) in stored legumes in Sri Lanka. J. Biopestic., 5: 88-94.
Rehman, H.U. and Khan, S.M., 2014. Growth inhibition and repellent effect of neem seed powder on pulse beetle, Callosobruchus chinensis L. (Coleoptera: Bruchidae). Pak. J. Sci., 66: 301-305.
Righi-Assia, A., Khelil, M., Medjdoub-Bensaad, F. and Righi, K., 2010. Efficacy of oils and powders of some medicinal plants in biological control of the pea weevil (Callosobruchus chinensis L.). Afr. J. agric. Res., 5: 1474-1481.
Rouf, F.M., Sardar, M.A. and Ahmed, K.S., 1996. Individual and combined effect of some plant materials for protection of lentil seeds against pulse beetle, Callosobruchus Chinensis L. Bangladesh J. Ent., 6: 13-21.
Sagheer, M., Mansoor-ul-Hasan, Latif, M.A. and Iqbal, J., 2011. Evaluation of some indigenous medicinal plants as a source of toxicant, repellent and growth inhibitors against Tribolium castaneum (Coleoptera: Tenebrionidae). Pak. Entomol., 33: 87-91.
Salari, E., Ahmadi, K., Dehyaghobi, R.Z., Purhematy, A. and Takalloozadeh, H. M., 2012. Toxic and repellent effect of harmal (Peganum harmala L.) acetonic extract on several aphids and Tribolium castaneum (Herbst). Chil. J. agric. Res., 72: 147-151. https://doi.org/10.4067/S0718-58392012000100023
Saljoqi, A.U.R., Afridi, M.K., Khan, S.H. and Rehman, S., 2006. Effects of six plant extracts on rice weevil Sitophilus oryzae L. in the stored wheat grain. J. Agric. biol. Sci., 1: 1-5.
Schmutterer, H., 1995. The neem tree, source of unique natural products for integrated pest management, medicine, industry and other purposes, VCH Weinheim, New York, Basel, Cambridge, Tokyo, 696.
Sexena, R.C., 1987. Antifeedants in tropical pest management. Insect Sci. Applic., 8: 731-736. https://doi.org/10.1017/s1742758400022840
Soderlund, D.M., Clark, J.M., Sheets, L.P., Mullin, L.S., Piccirillo, V.J., Sargent, D., Stevens, J.T. and Weiner, M.L., 2002. Mechanisms of pyrethroid neurotoxicity: implications for cumulative risk assessment. Toxicology, 171: 3-59. https://doi.org/10.1016/S0300-483X(01)00569-8
Srivastava, K.P., 1996. A text book of applied entomology, Kalyani Publisher, India, New Delhi.
Tapondjou, L.A., Adler, C., Bouda, H. and Fontem, D.A., 2002. Efficacy of powder and essential oil from Chenopodium ambrosioides leaves as post-harvest grain protectants against six stored product beetles. J. stored Prod. Res., 38: 395-402. https://doi.org/10.1016/S0022-474X(01)00044-3
Valladares, G., Salvo, A. and Videla, M., 1999. Moscas minadoras en cultivos de Argentina. Horticul. Argent., 18: 56-61.
Wekesa, I., Onek, L.A., Deng, A.L., Hasanali, A. and Othira, J.O., 2011. Toxicity and repellant potency of Hyptis spicigera extracts on Sitophilus zeamais Motschulsky (Coleoptera: Curculionidae). J. stored Prod. Postharv. Res., 2: 113-119.










