Plant Water Stress Affects the Feeding Performance of American Bollworm, Helicoverpa armigera (Lepidoptera: Noctuidae) on Cotton Plants
Plant Water Stress Affects the Feeding Performance of American Bollworm, Helicoverpa armigera (Lepidoptera: Noctuidae) on Cotton Plants
Muhammad Irfan Ullah1*, Muhammad Arshad1, Muhammad Imran Khan1, Muhammad Afzal1, Azhar Abbas Khan2, Syed Muhammad Ali Zahid1, Muhammad Saqib1, Asad Abdullah1, Saba Kousar1 and Maryam Riaz1
1Department of Entomology, University of Sargodha, 40100, Sargodha, Pakistan; 2College of Agriculture, Bahauddin Zakariya University Bahadur Sub-Campus, Layyah, Pakistan.
Abstract | American bollworm, Helicoverpa armigera (Hub.) (Lepidoptera: Noctuidae) is a major insect pest in cotton crop and is being the most noxious one in cotton growing areas of the world. Knowledge about how water stress modifies the ability of plants to resist against insect feeding is an interesting and important component in an integrated pest management program. In the present study, water stress was applied to field grown cotton with two Bacillus thuringiensis transgenes (CIM-602 and CIM-599) and one non-transgenic genotype (CIM-554) for the performance of H. armigera feeding The difference in leaf injury, relative consumption and growth rate of H. armigera was detected among experimental factors and their interactions. The leaf injury caused by H. armigera was higher (38.5 cm2 and 30.5cm2) on non-transgenic genotype at high and low moisture levels of plants respectively. Similarly, the relative growth and consumption rate of H. armigera was higher on non-transgenic genotype compared to transgenic. Overall the performance of H. armigera feeding was greater on high-watered leaves compared to water stress leaves. The results showed that the performance of H. armigera was superior on non-transgenic compared to transgenic genotypes at high-watered plants.
Received | April 28, 2019; Accepted | May 30, 2019; Published | October 27, 2019
*Correspondence | Muhammad Irfan Ullah, Department of Entomology, University of Sargodha, 40100, Sargodha, Pakistan; Email: [email protected]
Citation | Ullah, M.I., M. Arshad, M.I. Khan, M. Afzal, A.A. Khan, S.M.A. Zahid, M. Saqib, A. Abdullah, S. Kousar and M. Riaz. 2019. Plant water stress affects the feeding performance of american bollworm, helicoverpa armigera (Lepidoptera: Noctuidae) on cotton plants. Pakistan Journal of Agricultural Research, 32(4): 629-635.
DOI | http://dx.doi.org/10.17582/journal.pjar/2019/32.4.629.635
Keywords | Cotton genotypes, Helicoverpa armigera, Water stress, Feeding preference
Introduction
Cotton (Gossypium hirsutum L.) is one of the most valuable crops; contributing an important role in the economy of Pakistan. In cotton production, Pakistan is ranked at 4th position while, 3rd as an exporter of raw cotton, worldwide. During 2015, the total production of cotton was 12.01 million bales in our country (Pakistan Economic Survey, 2015-16). There are many constraints in a lower yield of cotton and among insect pests, fruit-eating Lepidopterous are the major pests worldwide, with the noctuid American bollworm, Helicoverpa armigera (Hub.) being the most noxious one (Nibouche et al., 2007). The early instar larvae of this pest feed on leaves, squares, and flowers of cotton, whereas later instars damage the green cotton bolls (Noor-ul-Ane et al., 2015).
Farmers mostly rely on synthetic insecticides to control the bollworms in the cotton crop. In an attempt to avoid the problems caused by insecticides application in agriculture, some alternative pest control methods have been studied in integrated pest management (IPM) program. Host plant resistance is an important element in modern agriculture (Stout, 2007). The cultivation of resistant plant varieties has ecological benefits in term of reducing the number of insecticide applications and for survival of natural enemies in the field (Boica-Junior et al., 2015). Transgenic cotton cultivars are very important element in IPM (Fitt et al., 2000) having benefits of effective management of targeted pests, cost-effectiveness, higher yield and better biological management (Edge et al., 2001). The quantity and quality of food matters in the performance of all organisms. Environmental variation is a likely factor that may cause droughts at higher intensity (Feng et al., 2013; Spinoni et al., 2014). The period of drought may alter the physiological, morphological, and plant’s biochemical characteristics, which alternatively may affect the response of herbivores to host availability (Chaves et al., 2003).
Water deficit stress alters the plant metabolism (Beck et al., 2007), and physiological processes being factors affecting the herbivores to host plant preferences, and their growth and development (Showler, 2012). Due to alterations in plants’ traits, insect growth and their preference to suitable host may affect (Huberty and Denno, 2004; Hale et al., 2005; Gutbrodt et al., 2011; Gutbrodt et al., 2012; Oliveira et al., 2014). Previously researchers have reported different aspects about the impact of water-stressed plants on the survival and host selectivity of lepidopterous; showing favorable response (Gutbrodt et al., 2011), no response (Estiarte et al., 1994) and unfavorable response to the insect (Lambert and Heatherly, 1995; Inbar et al., 2001).
Thereby, the changes in the water status of the host plants due to water stress can also affect the performance and ability of H. armigera to feed on cotton plants. In the present study, we sought to investigate the performance of H. armigera on water-stressed plants with different cotton genotypes. Our experimental setup comprised of two water regimes: high or well-watered and low-watered or stressed plants and cotton genotypes: transgenic and non-transgenic. We analyzed the leaf injury and feeding indices parameters of H. armigera on different cotton genotypes with different water status.
Materials and Methods
The bioassay was conducted in the Laboratory of Entomology, College of Agriculture, University of Sargodha, Pakistan.
Cotton plants
Two transgenic cotton genotypes: CIM-602, CIM-599, and one non-transgenic CIM-554 were sown in the research area (32°07’51.3”N 72°41’36.2”E) of University. Hand dibbling method was used for sowing of seeds with 2.5ft row-to-row distance and plant-to-plant distance was kept 6-8 inches with total ridge length of 78ft for each. During planting, commercial fertilizer 2-1-1 (N:P:K) was applied at 5-7g/plant and when the plants were 10-days old, 20ml nitrogen dilution prepared with 20g urea per liter of water was applied at weekly interval. A regular irrigation regime was applied to plants until use in the experiment. Irrigation was controlled for plants for different water status; high-watered and low-watered.
Insect
Third and fourth instars of H. armigera larvae were collected from a cotton field located at Central Cotton Research Institute Multan (30°08’55.8”N 71°26’21.6”E) and reared in the Entomology laboratory at controlled conditions (25±2°C temperature with 80±5% relative humidity). The larval culture was reared in 100-ml plastic transparent pots; kept 1 larva per pot due to cannibalistic behavior of bollworms. The young larvae were fed with cotton leaves and replaced daily. However, at later instars, buds and soft bolls were also provided. The pupae were transferred to 500ml plastic transparent pots. The adults were transferred to glass cages (30cm x 30cm x 30cm) with 10-15 couples per cage and were fed 10% honey/water solution (wt/vol). The white paper was lined in cages for oviposition and the eggs were collected daily.
Water status
Till the flowering stage of crop, the moisture level for all three cotton genotypes was kept constant. Ten days before performing the bioassay in the laboratory, the plants were allotted to the treatments at different level of water. The soil moisture level was determined with tensiometers (Hangzhou Mindfull Technology Co., Ltd, China). For low-watered plants, the soil moisture level was maintained at around 0.4–0.5 PSI. For high-watered plants, water potential was maintained at 0.8–0.10 PSI and there was no evidence of leaf wilting.
Bioassay
The performance of H. armigera was assessed by feeding the larvae on leaves collected from cotton plants having different moisture level. On daily basis, the leaves from both water-stressed and high-watered plants from each genotype were collected and kept transferred in ice boxes to Entomology laboratory. Disk-size leaves were cut and placed in Petri plates and 1 larva of third instar with almost same size were released in each plate. Each treatment was replicated thrice and 10 larvae were tested in each replication. Leaf area before and after larval feeding was measured using leaf area meter (LI-COR model LI-3000, Lincoln, NE, USA). The weight of both leaf and larvae was recorded by high precision weight balance. Data were recorded at 24, 48 and 72 hours and the equations of growth indices parameters were derived as suggested by Waldbauer (1968).
Relative Consumption Rate (RCR)
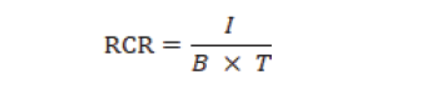
Where;
I is the dry weight of food consumed; T is the duration of the feeding period (days) and B is the insect dry weight gain.
Relative Growth Rate (RGR)
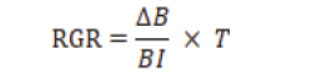
Where,
ΔB = change in body weight of insect (mg); BI = initial larval weight and T = feeding period (days).
Leaf Injury (LI)
Leaf injury was expressed as the total consumption of leaf area and was calculated by the following formula:
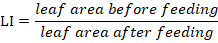
Statistical analysis
Data for feeding indices parameters were tested for normality and were log transformed prior to analysis. However, the untransformed means are given in the figures. Data were analyzed by two-factor factorial ANOVA by keeping cotton genotypes and water status as main factors and means were separated by the least significant difference test at 5% probability level. All the analyses were performed using Minitab 17.0 software.
Results and Discussion
The results showed that the relative growth rate of H. armigera was not significantly (F2, 53 = 0.74, P < 0.001) affected feeding on different cotton genotypes. Similarly, the different water status had no significant (F1, 53 = 0.43, P > 0.05) effect on the growth rate of H. armigera. For relative consumption rate of H. armigera, the cotton genotypes had significant (F2, 53 = 42.23, P < 0.001) effect. Leaf injury caused by H. armigera feeding was significantly (F2, 53 = 92.23, P < 0.001) different on cotton genotypes, and also with different water status of plants (F1, 53 = 124.4, P > 0.001). The relative growth rate of H. armigera was higher (1.65 mg/mg/day) feeding on non-Bt CIM-554 followed by 1.45mg/mg/day on Bt CIM-602 and 1.14 mg/mg/day on Bt CIM-599 when the water status was high. The growth rate of H. armigera was lower feeding on low watered or stressed leaves compared to high watered. The maximum growth rate was 1.39 mg/mg/day on non-Bt CIM-554 and the minimum was 0.97 mg/mg/day on Bt CIM-599 (Figure 1). Similar findings were found in case of relative consumption rate of H. armigera. The highest consumption rate was found on no-Bt CIM-554 (15.4 mg/mg/ day) on high-watered leaves and 14.4 mg/mg/day on low-watered leaves. However, the lowest consumption rate was found on Bt CIM-599; 9.88mg/mg/day on high-watered leaves and 8.2mg/mg/day on low-watered leaves (Figure 2). The leaf injury caused by H. armigera was found high (38.5 cm2) on non-Bt CIM-554 at high-watered leaves and 30.5cm2 at low-watered leaves. The leaf injury was low on transgenic genotypes compared to non-transgenic. However, the lowest injury of 14.5cm2 was found on Bt CIM-599 at high-watered leaves and 10.4cm2 at low-water leaves (Figure 3).
The results showed that H. armigera preferred non-Bt CIM-554 more than transgenic genotypes. The leaf injury and growth parameters of H.armigera were found higher feeding on non-trangenic genotype. Our findings are in accordance with Chitkowski et al. (2003) and Prasad et al. (2009) who stated the highest damage level on non-transgenic cultivars due to the feeding of H. armigera. The growth parameters of H. armigera were high feeding on non-transgenic genotype; indicated the more preference compared to transgenic cultivars.
Environmental conditions may alter the plant development, affecting plant resistance to insect pests (Smith, 2005). Several environmental changes, especially through flood or drought, can significantly alter the temperature, soil conditions, or even both. These climatic changes disturb the growth and metabolic process of plants, which ultimately affect the resistance level in plants toward biotic and abiotic stress (Smith, 2005). Further, the physio-chemical changes in water-stressed plants may directly inhibit the growth and development insects (Mattson and Haack, 1991). The present study showed maximum leaf injury on low-watered or stressed leaves compared to high-watered. The level of consumption in bollworm’s larvae is important, therefore the leaf injury plays a major role in the performance and development of larvae. The water-stressed leaves of different cotton genotypes affected the consumption rate of H. armigera larvae. The growth of H. armigera was low on low-watered leaves including the consumption rate and relative growth rate. Our findings are also supported by Inbar et al. (2001) who stated reduced weight gain by H. zea larvae when they fed upon water-stressed leaves of tomato. However, species with different level of host specialization can show a variable response to consumption of water stress plants. According to Gutbrodt et al. (2011), water-stressed leaves of Alliaria petiolata (Bieb.) reduced the consumption rate of Pieris brassicae (L.) larvae while the polyphagous Spodoptera littoralis showed increased consumption on water-stressed leaves. Furthermore, both species consumed a large number of water-stressed plants of Brassica oleracea L. (Gutbrodt et al., 2012). Regarding preference of lepidopteran pests on water-stressed plants, Flint et al. (1994) stated that Pectinophora gossypiella (Saund.) damaged 32% more bolls on plants irrigated every two weeks compared to weekly irrigated plants.
The H. armigera larvae showed poor performance on water-stressed leaves might be due to lower availability of nitrogen availability or due to elevated allelochemicals (McMillin and Wagner, 1995; Inbar et al., 2001). Similarly, S. exigua (Hübner) larvae showed reduced growth when they reared on water-stressed Solanum lycopersicum L. plants (English-Loeb et al., 1997). The findings are more interesting and important subject to water-stressed plants shows variable responses to lepidopteran species. Therefore, the suitability and quality of host plants for insects may vary according to different rainfall levels and it will be difficult to determine the pest status, especially in cotton crop, which is a host of many lepidopterous species.
A significant interaction was found between cotton genotypes and water status for H. armigera in our study. The non-transgenic genotype was preferred more by H. armigera compared to transgenic cotton on high-watered plants. These findings are in similar to Mao et al. (2004), Grinnan et al. (2013) and Noor-ul-Ane et al. (2015). There is a dire need to identify the resistant/tolerant cotton genotypes against abiotic stress (water stress) and biotic stress (herbivores) (Sinclair, 2011). Identification of resistant genotypes against both abiotic and biotic stress could be helpful for breeders to develop some new cultivars that could perform well in the future climate (Long and Ort, 2010).
Conclusions and Recommendatons
Our results showed that transgenic genotypes are resistant to H. armigera compared to non-transgenic and this pest caused more damage to high-watered plants. Further investigations should be conducted to better understand the insect-plant interactions under increased drought frequency in the field which might influence the pest status. Additionally, the drought conditions affect the pest status or not in the presence or absence of potential competitors.
Acknowledgment
We thank higher authorities of Central Cotton Research Institute, Multan for the technical assistance in insect collection.
Author’s Contribution
MIU conceived the idea, MIK, SMAZ, MS conducted the experiment, MA, AAK, AA analyzed the data, SK, MR prepared the initial draft, M Afzal reviewed the manuscript.
References
Beck, E.H., S. Fettig, C. Knake, K. Hartig and T. Bhattarai. 2007. Specific and unspecific responses of plants to cold and drought stress. J. Biosci. 32: 501-510. https://doi.org/10.1007/s12038-007-0049-5
Boica-Junior, AL., E.N. Costa, B.H.S. De-Souza, A.G. Da-Silva and A.F. Chiorato. 2015. Infestation of Caliothrips phaseoli (Thysanoptera: Thripidae) on bean cultivars grown in the winter, rainy and dry seasons in Brazil. Environ. Entomol. 44(4): 1108–1115. https://doi.org/10.1093/ee/nvv100
Chaves, M.M., J.P. Maroco and J.S. Pereira. 2003. Understanding plant responses to drought: from genes to the whole plant. Funct. Plant Biol. 30: 239–264. https://doi.org/10.1071/FP02076
Chitkowski, R.L., S.G. Turnipseed, M.J. Sullivan and W.G. Bridges. 2003. Field and laboratory evaluations of transgenic cottons expressing one or two Bacillus thuringiensis var. kurstaki Berliner proteins for management of Noctuid (Lepdoptera) pests. J. Econ. Ento. 96: 755-762. https://doi.org/10.1093/jee/96.3.755
Edge, J.M., J.H. Benedict, J.P. Carroll and H.K. Reding. 2001. Bollgard cotton: an assessment of global economic environmental and social benefits. J. Cotton Sci. 5: 121–136.
English-Loeb, G., M.J. Stout and S.S. Duffey. 1997. Drought stress in tomatoes: Changes in plant chemistry and potential nonlinear consequences for insect herbivores. Oikos. 79: 456–468. https://doi.org/10.2307/3546888
Estiarte, M., I. Filella, J. Serra and J. Pen˜uelas. 1994. Effects of nutrient and water stress on leaf phenolic content of peppers and susceptibility to generalist herbivore Helicoverpa amigera (Hubner). Oecologia. 99: 387–391. https://doi.org/10.1007/BF00627753
Feng, X., A. Porporato and I. Rodriguez-Iturbe. 2013. Changes in rainfall seasonality in the tropics. Nat. Clim. Chang. 3: 811–815. https://doi.org/10.1038/nclimate1907
Fitt, G.P., L.J. Wilson and G.G. Kennedy. 2000. Genetic engineering in IPM: Bt cotton in Emerging technologies for integrated management concepts, research and implementation. pp. 108-125. Proc. Conf. Raleigh, North Carolina, USA, March 8-10: 1999.
Flint, H.M., F.D. Wilson, D. Hendrix, J. Leggett, S.E. Naranjo, T.J. Henneberry and J.W. Radin. 1994. The effect of plant water stress on beneficial and pest insects including the pink bollworm and the sweet potato whitefly in two short-season cultivars of cotton. Southwest. Entomol. 19: 11–22.
Grinnan, R., T.E. Jr. Carter and M.T.J. Johnson. 2013. The effects of drought and herbivory on plant–herbivore interactions across 16 soybean genotypes in a field experiment. Ecol. Entomol. 38: 290–302. https://doi.org/10.1111/een.12017
Gutbrodt, B., K. Mody and S. Dorn. 2011. Drought changes plant chemistry and causes contrasting responses in lepidopteran herbivores. Oikos. 120: 1732–1740. https://doi.org/10.1111/j.1600-0706.2011.19558.x
Gutbrodt, B., S. Dorn and K. Mody. 2012. Drought stress affects constitutive but not induced herbivore resistance in apple plants. Arthropod Plant Interact. 6: 171–179. https://doi.org/10.1007/s11829-011-9173-0
Hale, B.K., D.A. Herms, R.C. Hansen, T.P. Clausen and D. Arnold. 2005. Effects of drought stress and nutrient availability on dry matter allocation, phenolic glycosides, and rapid induced resistance of poplar to two lymantriid defoliators. J. Chem. Ecol. 31: 2601–2620. https://doi.org/10.1007/s10886-005-7616-8
Huberty, A.F. and R.F. Denno. 2004. Plant water stress and its consequences for herbivorous insects: A new synthesis. Ecol. 85: 1383–1398. https://doi.org/10.1890/03-0352
Inbar, M., H. Doostdar and R.T. Mayer. 2001. Suitability of stressed and vigorous plants to various insect herbivores. Oikos. 94: 228–235. https://doi.org/10.1034/j.1600-0706.2001.940203.x
Lambert, L. and L.G. Heatherly. 1995. Influence of irrigation on susceptibility of selected soybean genotypes to soybean looper. Crop Sci. 35: 1657–1660. https://doi.org/10.2135/cropsci1995.0011183X003500060024x
Long, S.P. and D.R. Ort. 2010. More than taking the heat: crops and global change. Curr. Opin. Plant Biol. 13: 241–248. https://doi.org/10.1016/j.pbi.2010.04.008
Mao, L., L.E. Jett, R.N. Story, A.M. Hammond, J.K. Peterson and D.R. Labonte. 2004. Influence of drought stress on sweet potato resistance to sweet potato weevil, Cylas formicarius (Coleoptera: Apoinidae), and storage root chemistry. Fla. Entomol. 87: 261–267. https://doi.org/10.1653/0015-4040(2004)087[0261:IODSOS]2.0.CO;2
Mattson, W.J. and R.A. Haack. 1991. The role of drought in outbreaks of plant-eating insects. Bio. Sci. 37: 110–118. https://doi.org/10.2307/1310365
McMillin, J.D. and M.R. Wagner. 1995. Season and intensity of water stress: host-plant effects on larval survival and fecundity of Neodiprion gillettei (Hymenoptera: Diprionidae). Environ. Entomol. 24: 1251–1257. https://doi.org/10.1093/ee/24.5.1251
Nibouche, S., E. Goze, R. Babin, J. Beyo and T. Brevault. 2007. Modeling Helicoverpa armigera (Hu¨bner) (Lepidoptera: Noctuidae) damages on cotton. Environ. Entomol. 36(1): 151-156. https://doi.org/10.1603/0046-225X(2007)36[151:MHAHLN]2.0.CO;2
Noor-ul-Ane M., M.J. Arif, M.D. Gogi and M.A. Khan. 2015. Evaluation of different integrated pest management modules to control Helicoverpa for adaptation to climate change. Int. J. Agric. Biol. 17(3): 483-490. https://doi.org/10.17957/IJAB/17.3.14.236
Oliveira, M.D., C.S.A. Silva-Torres, J.B. Torres and J.E.M. Oliveira. 2014. Population growth and within-plant distribution of the striped mealybug Ferrisia virgata (Cockerell) (Hemiptera, Pseudococcidae) on cotton. Rev. Bras. Entomol. 58: 71–76. https://doi.org/10.1590/S0085-56262014000100012
Pakistan Economic Survey. 2015-16. Ministry of Finance, Government of Pakistan, Islamabad, http://www.finance.gov.pk/survey/chapters_16/02_Agriculture.pdf
Prasad, N.V.V.S.D., M. Rao and N.H. Rao. 2009. Performance of Bt cotton and non Bt cotton hybrids against pest complex under unprotected conditions. J. Biopest. 2(1): 107-110.
Showler, A.T. 2012. Drought and arthropod pests of crops. p. 131-154. In D.F. Neves and J.D. Sanz (eds.) Drought: New research, Nova Sci., 978-1-62100-769-2 Hauppauge, New York, USA.
Sinclair, T.R. 2011. Challenges in breeding for yield increase for drought. Trends Plant. Sci. 16: 289–293. https://doi.org/10.1016/j.tplants.2011.02.008
Smith, C.M. 2005. Plant resistance to arthropods: molecular and conventional approaches. Springer, Dordrecht, NLD. https://doi.org/10.1007/1-4020-3702-3
Spinoni, J., G. Naumann, H. Carrao, P. Barbosa and J. Vogt. 2014. World drought frequency, duration and severity for 1951–2010. Int. J. Climatol. 34: 2792–2804. https://doi.org/10.1002/joc.3875
Stout, M.J. 2007. Types and mechanisms of rapidly induced plant resistance to herbivorous arthropods. p. 89–107. In D. Walters, A. Newton and G. Lyon (eds.), Induced resistance for plant defense. Blackwell, Oxford, U. K. https://doi.org/10.1002/9780470995983.ch5
Waldbauer, G.P. 1968. The consumption and utilization of food by insects. Adv. Insect Physiol. 5: 229–287. https://doi.org/10.1016/S0065-2806(08)60230-1
To share on other social networks, click on any share button. What are these?







