Physicochemical Characteristics and Yield of Sugar Beet (Beta vulgaris L.) Cv. “California-Kws” Influenced with Irrigation Intervals
Physicochemical Characteristics and Yield of Sugar Beet (Beta vulgaris L.) Cv. “California-Kws” Influenced with Irrigation Intervals
Imran Khan1*, Muhammad Iqbal1 and Malik Muhammad Hashim2
1Department of Horticulture, Faculty of Agriculture, Gomal University, Dera Ismail Khan, Khyber Pakhtunkhwa, Pakistan; 2Department of Food Science and Technology, Faculty of Agriculture, Gomal University, Dera Ismail Khan, Khyber Pakhtunkhwa, Pakistan.
Abstract | Management practices are very important for increasing yield of sugar beet crop, amongst them irrigation intervals play an important role. The farmers do not have sufficient knowledge about the suitable irrigation time (interval). The aim of study was to explore the best irrigation time for sugar beet crop. Hence, this study examines the physiological response of sugar beet cv. California-kws with different irrigation intervals at the research farm, Faculty of Agriculture, Gomal university Dera Ismail Khan Pakistan during 2013-14 and 2014-15. The study was conducted using Randomized Complete Block Design (RCBD) having six treatments i.e. (I1= 05 days’, I2= 10 days’, I3= 15 days’, I4= 20 days’, I5= 25 days’, and I6= 30 days’’ interval) and three replications. Data were recorded on total chlorophyll content, photosynthesis, plant height (cm), number of leaves plant -1, leaf length (cm), leaf area (cm2), leaf weight plant-1(g), root weight plant-1 (g), root/top ratio, root length (cm), root diameter (cm), sucrose%, tss %, purity %, leaf yield (t ha-1), root yield (t ha-1), sugar yield (t ha-1) and harvesting index. The results indicated significant variation among the treatments for all parameters during both years of study. Among the treatments I3 (15 days’’ interval) improved almost all the studied characters. However, increasing interval improves the sucrose% and TSS% during both years. Hence I3 (15 days’’ interval) is the best for obtaining highest beet yield and quality under Dera Ismail Khan Conditions.
Received | Febraury 27, 2017; Accepted | November 22, 2018; Published | January 18, 2019
*Correspondence | Imran Khan, Department of Horticulture, Faculty of Agriculture, Gomal University, Dera Ismail Khan, Khyber Pakhtunkhwa, Pakistan; Email: imrankhan1441@gmail.com
Citation | Khan, I., M. Iqbal and M.M. Hashim, 2018. Physicochemical characteristics and yield of sugar beet (Beta vulgaris l.) cv. “California-Kws” influenced with irrigation intervals. Sarhad Journal of Agriculture, 35(1): 57-69.
DOI | http://dx.doi.org/10.17582/journal.sja/2019/35.1.57.69
Keywords | Sugar beet, Irrigation intervals, Chlorophyll, Photosynthesis, Yield
Introduction
Sugar beet (Beta vulgaris L.), is one of the most important crops within beet species (Gill and Vear, 1980). It is a biennial, dicotyledonous, herbaceous crop (Smith, 1987), having 18 chromosomes (Elliot and Weston, 1993) and harvested for its sugar during first growing season (Beta vulgaris L.). Sugar beet is successfully grown in more than forty countries (Whitney and Duffus, 1986). It is a temperate crop but also cultivated under arid and semi-arid climatic conditions. It is grown as a winter crop in countries like Pakistan. (Niazi et al., 1997 and 1998) found that wild beet species could be grown successfully in Dera Ismail Khan. Sugar beet is commercially grown in Pakistan in the provinces of Khyber Pakhtunkhwa (K.P.K) and Punjab, whereas it is grown as a vegetable on marginal scale in Sindh and Baluchistan (Ahmad et al., 2012). During 2010-2011, Sugar beet was grown on an area of 0.8 thousand hectares with production of 20.9 thousand tones per annum and average yield of 25.4 tones ha-1 in Pakistan, while in Khyber Pakhtunkhwa (K.P.K), it was grown on 0.4 thousand hectares giving an annual production of 0.4 thousand tones and average yield of 1.0 tones ha-1 (Anonymous, 2011). Khyber Pakhtunkhwa enjoys a unique position throughout Pakistan where sugar beet can be successfully cultivated.
Water shortage significantly reduced sugar beet leaf, root and sugar yield under semi-arid conditions (Kiziloglu et al., 2006) and suitable irrigation program can maximize sugar beet yield. Over or under irrigation may affect crop yields (Reddy et al., 2007). Irrigation regimes had significant effect on sugar beet yield and quality (Mahmoodi et al., 2008). Full irrigation expressed yield (root and sugar) of sugar beet (Yonts, 2011). Pakistan is though an extensively irrigated country, but in some of its dry regions water is insufficient even to provide initial crop requirements. On the other side where water is available throughout the year, farmers do not irrigate properly due to lack of knowledge about the quantitity and timing of irrigation. Farmers irrigate according to the conventional method which prevents efficacy of water use (Kiymaz and Ertek, 2015). Proper irrigation scheduling is essential for water use efficiency during growing season (Passioura, 2007). Due to lack of knowledge of production technology the average yield obtained in Pakistan is very low. Similarly, among the crop production tools, irrigation is one of the main factors (Bakhsh et al., 1999). Crops water requirement from sowing to harvest depend upon plant species and crop growth stage (Raza et al., 2012). Sugar beet has the specialty of producing high yield acre-1 like sugar cane with high recovery (20-25%) in a short period (5-6 months) (Iqbal and Saleem, 2015). In Pakistan first sugar beet factory was established in Charsadda (KPK) in 1963 and four sugar beet factories were operating in KPK that time but now-a-days’, only Premier (Charsadda) and Al-Moiz (Dera Ismail Khan) are functional (Iqbal and Saleem, 2015). Due to continuous decline of available water the sugar cane cultivation in some areas has become a difficult task. To fulfil the demand of sugar, sugar beet is a suitable solution because it can produce two times higher sugar yield per hectare in a short period (5-6 months) with less water demand as compared to sugarcane (Ahmad and Rasool, 2011). Dera Ismail Khan has the potential for sugar beet cultivation, with a sugar beet factory (Almoiz) available in the area, but so for no research work reported in the area compared irrigation intervals effect on sugar beet, therefore, the present study was designed to provide an overview of agronomic aspects of sugar beet to ensure continue sugar production and to suggest a suitable irrigation program to the farmers in the area.
Materials and Methods
The study was carried out during 2013-14 and 2014-15 at research farm, Faculty of Agriculture, Gomal University Dera Ismail Khan. Before soil preparation, samples were collected at five different points randomly between 0-30 cm depth and were thoroughly mixed to make a composite soil sample for physicochemical analysis as shown in Table 1. Meteorological data i.e. mean temperature; total rainfall and relative humidity of the two growing season of 2013-14 and 2014-15 of the site is shown in Figure 1. Before sowing.
the experimental field was well prepared through two ploughing, leveling, ridging and then divided into the experimental units. Cultural practices were performed throughout growing season. Sugar beet seeds were manually sown on 15th of October and harvesting was done on the 1st week of May during both growing season. The experimental plots were arranged in RCB (Randomized Complete Block) Design having six treatments i.e. (I1= 05 days’, I2= 10 days’, I3= 15 days’, I4=20days’, I5=25 days’, and I6=30 days’ interval) and three replications, comprising of 18 plots. Each plot was separated as 2.0 m to avoid water movement among the treatments. The plant-plant distance was kept as 30 cm. Experimental plots were consisted of five rows each 45 cm apart and 4 m long. The plots were lightly irrigated after sowing (seeding) followed by irrigation as per experimental designed. Flood irrigation method was used to irrigate the plots without submerging the tops of ridges. The field received Nitrogen, phosphorus and potassium @ 120:100:75 kg ha-1, respectively (Ahmed et al., 2010). 2/3 of N and all P and K were applied before ridge formation and rest of N was applied before earthing up. Thinning was carried out at 4-leaf stage and 6-leaf stage to maintain constant plant population.
Total Chlorophyll
Total chlorophyll in the leaves were measured by SPAD-502 Minolta Co. Ltd. Osaka, Japan. To measure leaf chlorophyll content, five plants were taken from the randomly selected plants of each plot.
The reading was made on five fully expanded leaves per plant considering their average as the leaf chlorophyll content of each replication.
Table 1: Soil Physicochemical properties.
| Soil analysis | 2013-14 | 2014-15 |
| EC (ds/m) | 4.07 | 4.06 |
| pH | 7.6 | 7.7 |
| Texture | Clay loam | Clay loam |
| Saturation% | 56 | 55 |
| Organic matter (%) | 0.62 | 0.63 |
| N (%) | 0.04 |
0.06 |
| P ppm | 8 |
8.02 |
| K ppm | 250 |
256 |
Photosynthesis (µmolm-2s-1)
Leaf photosynthesis rate was assessed by photosynthesis meter (CI-340 handheld Photosynthesis System). Leaf photosynthesis rate was measured using photosynthesis meter (CI-340 handheld Photosynthesis System). The measurements were taken by choosing, at random, the five plants from each replication. The reading on Pn (Net photosynthetic rate) and PAR (Photosynthetically Active Radients) were made simultaneously, on five fully expanded leaves per plant considering their average as photosynthesis rate of each replication.
Plant height (cm)
Five plants were randomly selected from each replication and height was measured with measuring tape in cm and mean was computed.
Number of leaves plant-1
Number of leaves plant-1 were counted from randomly selected five plants from each replication and mean was computed.
Leaf length (cm)
Leaf length (leaf base to leaf apex) of randomly selected five plants from each replication was measured with measuring tape in cm and the mean were calculated.
Leaf area plant-1 (cm2)
It was determined as stated by Ahmad et al (2010).
A= 159.52 - 21.95L + 21.33W + 1.59LW + 0.22LL - 1.41WW
Where;
A is leaf area; L is leaf length and W is leaf width.
Leaf weight plant-1
At maturity five plants were chosen at random from each treatment to determine the leaf weight in g by a digital scale (0.01-g precision) and mean was calculated.
Root weight plant-1
At maturity five plants were chosen at random from each treatment to determine the root weight in g by a digital scale (0.01-g precision) and mean was calculated.
Root/top ratio
Five plants were chosen at random from each plot and root: top ratio was calculated by dividing root weight by leaf weight.
Root length (cm)
Five roots were selected from each replication after harvest to record root length (cm) with the help of measuring tape and mean was calculated.
Root diameter (cm)
Five roots were selected from each replication after harvest to record circumference (cm) with the help of measuring tape and then root diameter was calculated using following equation (Ahmad et al. 2010).
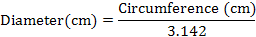
Total soluble solids%
For TSS % reading, five beets were washed and crushed to get juice. Then one drop of juice was placed on a hand refractometer to record TSS (%) reading as reported by Kamal et al. (2003).
Sucrose percentage (%)
To measure the relative sucrose percentage, a subsample of five beets was washed, sliced, and stirred for three minutes after mixing with the distilled water and then was filtered and then subjected to a saccharimeter for recoding sugar percentage as reported by Kamal et al. (2003).
Apparent purity % was determined as a ratio between sucrose % and TSS %.
Root Yield (t ha-1)
At harvest, plants that produced from each plot were collected and cleaned. Roots and tops were separated and weighted in kilograms, then were converted to estimate root yield ton ha-1 as below;
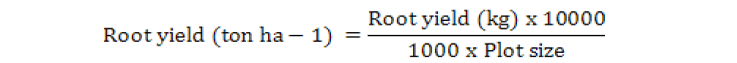
Leaf yield (t ha-1)
At harvest, plants that produced from each plot were collected and cleaned. Leaves were separated and weighted in kilograms, then were converted to estimate leaf yield ton ha-1.
Sugar yield (t ha-1) was calculated by using equation,
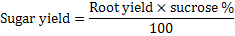
Harvesting index was computed by following formula.
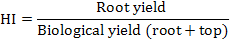
Data analysis
The treatment means were compared at a significance level of 0.05 using least significant difference (LSD) test (Steel and Torrie, 1997). Pearson correlation coefficient was calculated to assess the relationship among various physicochemical properties of sugar beet.
Results and Discussion
Total Chlorophyll
Irrigation intervals affected (P<0.05) the total chlorophyll content during both experimental years (Table 2). Maximum chlorophyll content of (49.62 and 49.61) was recorded in I3 (15 days’ interval) during 2013-14 and 2014-15 and minimum chlorophyll content (47.72 and 47.72) was recorded in I6 (30 days’ interval) during 2013-14 and 2014-15. The minimum chlorophyll content at maximum interval might be due to the reason that drought stress prevents making it, as it lowers the capacity of light harvesting (Lessani and Mojtahedi, 2002; Cornic and Masacci, 1996). Similar results were reported by Leufen et al. (2013).
Photosynthesis (µmolm-2s-1)
Photosynthesis was also affected (P<0.05) by irrigation intervals during both years (Table 2). Maximum photosynthesis (Pn/PAR 32.60/353.67 and 32.63/353.33) was recorded in I3 (15 days’ interval) during 2013-14 and 2014-15.
Table 2: Effect of irrigation intervals on Total Chlorophyll, Photosynthesis (Pn/PAR), Plant height of sugar beet cv. California-kws during 2013-14 and 2014-15.
| Treatments (irrigation intervals) | Total Chlorophyll (SPAD) |
Photosynthesis (µmolm-2s-1) |
Plant height (cm) | |||||
| 2013/14 | 2014/15 |
Pn 2013/14 |
PAR 2013/14 |
Pn 2014/15 |
PAR 2014/15 |
2013/14 | 2014/15 | |
|
I1 |
47.73c | 47.74c | 29.70d | 298.33c | 29.667d | 298.33c | 39.67c | 39.69c |
|
I2 |
48.73b | 48.73b | 31.60b | 334.33b | 31.53b | 334.33b | 40.71b | 40.74b |
|
I3 |
49.62a | 49.61a | 32.60a | 353.67a | 32.63a | 353.33a | 42.53 a | 42.55a |
|
I4 |
49.52a | 49.50a | 32.43a | 353.33a | 32.43a | 352.33a | 42.49a | 42.43a |
|
I5 |
48.72b | 48.72b | 30.73c | 331.67b | 30.63c | 331.33b | 39.74c | 39.78c |
|
I6 |
47.72c | 47.72c | 28.70e | 285.00c | 28.60e | 285.67c | 39.52d | 39.43d |
| LSD | 0.2490 | 0.2515 | 0.4622 | 15.859 | 0.2877 | 15.532 | 0.1504 | 0.2222 |
Means in each column with same letter are not significantly different at P≤0.05; (I1=5 days, I2= 10 days, I3= 15 days, I4=20days, I5=25 days, and I6=30 days interval); Pn: Net photosynthesis, PAR: Photosynthetically active radiants.
Table 3: Effect of irrigation intervals on number of leaves plant-1, leaf length, leaf area plant-1, leaf weight plant-1 of sugar beet cv. California-kws during 2013-14 and 2014-15.
| Treatments (irrigation intervals) |
Number of leaves Plant-1 |
Leaf length (cm) |
Leaf area Plant-1 (cm2) |
Leaf weight plant-1 (g) |
||||
| 2013/14 | 2014/15 | 2013/14 | 2014/15 | 2013/14 | 2014/15 | 2013/14 | 2014/15 | |
|
I1 |
42.67 d | 42.73 d | 36.86 b | 36.69 b | 453.05 c | 445.56 cd | 464.67 d | 465.00 d |
|
I2 |
43.40 c | 43.47 c | 36.95 b | 36.79 b | 473.59 b | 470.92 b | 479.06 b | 482.76 b |
|
I3 |
45.67 a | 45.63 a | 37.96 a | 37.84 a | 502.34 a | 499.30 a | 482.45 a | 483.65 a |
|
I4 |
44.43 b | 44.57 b | 37.89 a | 37.81 a | 497.24 a | 497.08 a | 481.65 ab | 482.54 b |
|
I5 |
43.37 c | 43.33c | 36.92 b | 36.76 b | 451.86 c | 450.70 c | 475.53 c | 475.52 c |
|
I6 |
42.33 d | 42.40d | 36.46 c | 36.51 c | 441.98 c | 442.52 d | 454.66 e | 455.32 e |
| LSD | 0.3494 | 0.3953 | 0.1265 | 0.1656 | 12.910 | 6.7800 | 2.9114 | 0.6591 |
Means in each column with same letter are not significantly different at P≤0.05; (I1=5 days, I2= 10 days, I3= 15 days, I4=20days, I5=25 days, and I6=30 days’ interval).
Minimum photosynthesis (Pn/PAR 28.70/285.00 and 28.60/285.67) was recorded in I6 (30 days’ interval) during 2013-14 and 2014-15. As verified, Photosynthesis is highly sensitive to drought (Bloch et al., 2006). Mainly drought damages and limits photosynthesis of plants (Flexas and medrano, 2002). Other researches reported that due to drought photosynthetic activity decreases which lead to stomatal closure (Shangguan et al., 2000; Zlatev and Yordanov, 2004) which allow plants to limit transpiration and CO2 absorption (Nayyar and Gupta, 2006). Ashraf et al. (2007) reported a decrease in photosynthetic activity of all maize cultivars under drought condition. The present findings agreed with those reported by Leufen et al. (2013) who found that Pn is also affected like chlorophyll under temporary water shortage as stressed plant utilize low light as compared to well-watered plants.
Plant height (cm)
Irrigation intervals affected (P<0.05) the plant height during both experimental years (Table 2). Maximum plant height of (42.53 and 42.55 cm) was observed in I3 (15 days’ interval) during 2013-14 and 2014-15 while minimum plant height of (39.52 and 39.43 cm) was recorded in I6 (30 days’ interval) during 2013-14 and 2014-15. These results might be due to adequate available soil moisture within the root zone, highest chlorophyll content and photosynthetic activity in leaves that increased the plant height. Ucan and Gencoglan (2004) found vegetative growth of sugar beet declined severely as water deficit increased. Similar results have been reported previously (Mirabadi et al., 2013; Baloch et al., 2014).
Number of leaves plant-1
Irrigation interval influenced (P<0.05) the number of leaves plant-1 during both study years (Table 3). Least number of leaves plant-1 (42.33 and 42.40) were recorded in I6 (30 days’ interval) during 2013-14 and 2014-15 whereas maximum number of leaves plant-1 (45.67 and 45.63) were recorded in I3 (15 days’ interval) during 2013-14 and 2014-15. Zao (1990) reported leaf as the main assimilation organ of sugar beet. The reduction in leaf number in response to maximum interval can be attributed to the enhancement of leaf abscission due to hormonal imbalance. Consequently, it increased Abscisic acid (ABA) and decreased Indoleacetic acid (IAA) levels in stressed plants (Nandi et al., 2002, Wu et al., 2005). This supports the results of Abayomi (2002) on Sugar beet (Beta vulgaris L) and Mirabadi et al. (2013) on Cantaloupe (Cucumis melo L.).
Leaf length (cm)
Irrigation intervals affected (P<0.05) the leaf length during both experimental years (Table 3). Maximum leaf length of 37.96 and 37.84 was recorded in I3 (15 days’ interval) during 2013-14 and 2014-15. Minimum leaf length of 36.46 and 36.51 was recorded in I6 (30 days’ interval) during 2013-14 and 2014-15. The shortest leaf length at 30 days’ irrigation interval might be due to blocking up of xylem and phloem vessels thus hindering any translocation through them (Khalil and El-Noemani, 2012). This also confirms the results of Abayomi (2002) on sugar beet and Baloch et al. (2014) on wheat.
Leaf area (cm2)
Leaf area (cm2) was also affected (P<0.05) by irrigation intervals during both years (Table 3). Maximum leaf area (cm2) (502.34 and 499.30) was recorded in I3 (15 days’ interval) during 2013-14 and 2014-15. Minimum leaf area (cm2) of (441.98 and 442.52) was recorded in I6 (30 days’ interval) during 2013-14 and 2014-15. Ucan and Gencoglan (2004) found LAI values increased with increasing water use. This might be attributed to greater cell elongation and turgidity owing to adequate moisture availability in the soil (Channagoudar and Janawade, 2006). Similarly, a reduction in leaf area due to water stress may represent an increase in xeromorphy (Stocker, 1960). This also supports the results of Abayomi (2002) on Sugar beet (Beta vulgaris L) and Mirabadi et al. (2013) on Cantaloupe (Cucumis melo L.).
Leaf weight plant-1 (g)
Irrigation intervals affected (P<0.05) the leaf weight plant-1 (g) during both experimental years (Table 3). Maximum leaf weight plant-1 (g) of (482.45 and 483.65) was recorded in I3 (15 days’ interval) during 2013-14 and 2014-15. Minimum leaf weight plant-1 (g) of 454.66 and 455.32 was recorded in I6 (30 days’ interval) during 2013-14 and 2014-15. The results might be due to greater leaf size at 15 days’ interval as leaf weight is associated with leaf size. The results are in concord with Ucan and Gencoglan (2004); Hussein et al. (2008) and Farnia and Hashemi (2015). Similar results were also reported in sugar beet (Farnia and Hashemi, 2015), chickpea (Gunes et al., 2006; Rahbarian et al., 2011) and in maize (Ashraf et al., 2007).
Root weight plant-1(g)
Root weight plant-1 (g) was also affected (P<0.05) by irrigation intervals during both years (Table 4). Minimum root weight plant-1 (g) of (1279.2 and 1275.1) was recorded in I6 (30 days’ interval) during 2013-14 and 2014-15. Maximum root weight plant-1 (g) of (1484.6 and 1484.3) was recorded in I3 (15 days’ interval) during 2013-14 and 2014-15. The results might be due to greater root dimensions at 15 days’ interval. The findings are in line with Kassab et al. (2012) who reported that the extension of irrigation days’ reduced the root weight plant-1 in fodder beet. Similarly, Snyman (2004) reported significant decrease in root mass with water stress in Opuntiaficus indica and O. robusta.
Root/top ratio
Irrigation intervals affected (P<0.05) the root/top ratio during both experimental years (Table 4). Maximum root/top ratio of (3.08 and 3.07) was recorded in I3 (15 days’ interval) during 2013-14 and 2014-15. Minimum root/top ratio of (2.81 and 2.80) was recorded in I6 (30 days’ interval) during 2013-14 and 2014-15. Water stress condition have been found to disrupt several physiological processes leading to reduction in growth (Bloch and Hoffmann, 2005), restrict growth and alter the chemical composition of beet under drought. Ucan and Gencoglan (2004) found vegetative growth of sugar beet declined severely as water deficit increased.
Root length (cm)
Root length (cm) was also affected (P<0.05) by irrigation intervals during both years (Table 4). Minimum root length (cm) of (22.26 and 22.33) was recorded in I6 (30 days’ interval) during 2013-14 and 2014-15 whereas maximum root length of (25.30 and 25.34) was recorded in I3 (15 days’ interval) during 2013-14 and 2014-15. The findings show similarity with Kassab et al. (2012) who recorded increase in root length plant-1 (cm) in fodder beet by increasing
Table 4: Effect of irrigation intervals on root weight plant-1, root/top ratio, root length and root diameter of sugar beet cv. California-kws during 2013-14 and 2014-15.
| Treatments (irrigation intervals) |
Root weight plant-1 (g) |
Root/ top ratio | Root length (cm) | Root diameter (cm) | ||||
| 2013/14 | 2014/15 | 2013/14 | 2014/15 | 2013/14 | 2014/15 | 2013/14 | 2014/15 | |
|
I1 |
1364.2 d | 1365.5 e | 2.94 b | 2.94 d | 22.43 c | 22.43 d | 9.99 c | 9.97 c |
|
I2 |
1470.5 b | 1469.7 c | 3.07 a | 3.04 c | 23.41b | 23.44 c | 10.47 b | 10.50 b |
|
I3 |
1484.6 a | 1484.3 a | 3.08 a | 3.07 a | 25.30 a | 25.34 a | 11.55 a | 11.56 a |
|
I4 |
1475.8 b | 1476.9 b | 3.06 a | 3.06 b | 25.25 a | 25.28 a | 11.53 a | 11.54 a |
|
I5 |
1374.1 c | 1373.8 d | 2.89 c | 2.89 e | 23.53 b | 23.61 b | 10.47 b | 10.47 b |
|
I6 |
1279.2 e | 1275.1 f | 2.81 d | 2.80 f | 22.26 c | 22.33 d | 9.53 d | 9.50 d |
| LSD | 7.3212 | 2.7749 | 0.0242 | 4.591e-03 | 0.3220 | 0.1313 | 0.2710 | 0.1461 |
Means in each column with same letter are not significantly different at P≤0.05; (I1=5 days, I2= 10 days, I3= 15 days, I4=20days, I5=25 days, and I6=30 days interval).
Table 5: Effect of irrigation intervals on Sucrose% (Pol%), TSS (Brix%), Purity %, Leaf Yield of sugar beet cv. California-kws during 2013-14 and 2014-15.
| Treatments (irrigation intervals) | Sucrose% (Pol%) | TSS (Brix%) | Purity % |
Leaf yield (t ha-1) |
||||
| 2013/14 | 2014/15 | 2013/14 | 2014/15 | 2013/14 | 2014/15 | 2013/14 | 2014/15 | |
|
I1 |
15.56 d | 15.56 d | 17.85 e | 17.88 e | 87.14 b | 87.01 c | 23.03 d | 23.03 d |
|
I2 |
15.74 c | 15.69 c | 17.92 e | 17.93 d | 87.83 b | 87.49 b | 24.00 b | 23.94 b |
|
I3 |
16.47 b | 16.51 b | 18.36 d | 18.41 c | 89.74 a | 89.70 a | 24.40 a | 24.33 a |
|
I4 |
16.49 b | 16.53 ab | 18.48 c | 18.45 b | 89.23 a | 89.58 a | 24.38 a | 24.31 a |
|
I5 |
16.58 ab | 16.57 ab | 18.58 b | 18.50 a | 89.24 a | 89.55 a | 23.51 c | 23.47 c |
|
I6 |
16.66 a | 16.58 a | 18.69 a | 18.54 a | 89.12 a | 89.46 a | 22.98 d | 22.95 e |
| LSD | 0.1203 | 0.0601 | 0.0741 | 0.0369 | 0.7851 | 0.4476 | 0.1206 | 0.0675 |
Means in each column with same letter are not significantly different at P≤0.05; (I1=5 days, I2= 10 days, I3= 15 days, I4=20days, I5=25 days, and I6=30 days interval).
irrigation amount from 1132 and up to 2106 m3fed-1 per season. Similarly, Snyman (2004) reported significant decrease in root length (cm) with water stress in Opuntiaficus indica and O. robusta.
Root diameter (cm)
Irrigation intervals affected (P<0.05) the root diameter (cm) during both experimental years (Table 4). Maximum root diameter (cm) of (11.55 and 11.56) was recorded in I3 (15 days’ interval) during 2013-14 and 2014-15. Minimum diameter (cm) of (9.53 and 9.50) was recorded in I6 (30 days’ interval) during 2013-14 and 2014-15. Ucan and Gencoglan (2004) found vegetative growth of sugar beet declined severely as water deficit increased. Results are in agreement with M. Al-Barrak (2006) who reported highest value of stem diameter with irrigation every 14 days’ in Canola (Brassica napus L.).
Sucrose %
Sucrose % was also affected (P<0.05) by irrigation intervals during both years (Table 5). Maximum sucrose % of (16.66 and 16.58) was recorded in I6 (30 days’ interval) during 2013-14 and 2014-15. Minimum of (15.56 and 15.56) was recorded in I1 (5 days’ interval) during 2013-14 and 2014-15. The increase in sucrose content in roots by increasing intervals might be due to slower accumulation of water. Similar results were reported by Ucan and Gencoglan (2004), Bloch and Hoffman (2005), Mahmoodi et al. (2008), Topak et al. (2011) and Ghamarnia et al. (2012).
Total Soluble Solids (TSS) %
Irrigation intervals affected (P<0.05) the total soluble solids during both experimental years (Table 5). Maximum TSS % of (18.69 and 18.54) was recorded in I6 (30 days’ interval) during 2013-14 and 2014-15. Minimum of (17.85 and 17.88) was recorded in I1 (5 days’ interval) during 2013-14 and 2014-15. The reduction in TSS due to excess water might be due to the dilution of sugars with excessive moisture contents (Nasir and Mian, 1993). Some researchers reported that total soluble solids values showed high positive correleation with total sucrose content, but it is also accepted as an important quality trait (Gaafer and Rafaie, 2006; Long, 2006; Keshavarzpour and Rashidi, 2011). Mirabadi et al. (2013) reported that water deficit effects the fruit sugar content positively. The results are in alignment with Mahmoodi et al. (2008) who found that sugar beet quality was significantly affected by irrigation regimes.
Purity%
Purity % was also influenced (P<0.05) with irrigation intervals during both years (Table 5). Minimum purity % (87.14 and 87.01) was recorded in I1 (5 days’ interval) during 2013-14 and 2014-15 whereas maximum purity % of (89.74 and 89.70) was recorded in I3 (15 days’ interval) during 2013-14 and 2014-15. The findings agree with Mahmoodi et al. (2008) who found that sugar beet quality was significantly affected by irrigation regimes.
Leaf yield (t ha-1)
Irrigation intervals affected (P<0.05) the leaf yield (t ha-1) during both experimental years (Table 5). Maximum leaf yield (t ha-1) (24.40 and 24.33) was recorded in I3 (15 days’ interval) during 2013-14 and 2014-15. Minimum yield (t ha-1) of (22.98 and 22.95) was recorded in I6 (30 days’ interval) during 2013-14 and 2014-15. The results might be due to adequate available soil moisture within the root zone which led to better uptake of nutrients that increased the various physiological processes and better leaf growth (Gaafer and Refaie, 2006; Rashidi and Seyfi, 2007; Simsek and Comlekcioglu, 2011). The results are in harmony with Kiziloglu et al. (2006) and Mahmoodi et al. (2008) who reported lowest leaf yield under lowest soil water conditions.
Root yield (t ha-1)
Root yield (t ha-1) was also affected (P<0.05) by irrigation intervals during both years (Table 6). Minimum root yield (t ha-1) (60.28 and 60.19) was recorded in I6 (30 days’ interval) during 2013-14 and 2014-15, whereas maximum root yield (t ha-1) of (64.48 and 64.52) was recorded in I3 (15 days’ interval) during 2013-14 and 2014-15. The results might be due to better root growth and size because of the availability of adequate soil moisture at 15 days’ interval. The results are in agreement with Winter (1989), Ucan and Gencoglan (2004), Gaafer and Refaie (2006), Rashidi and Seyfi (2007), Kiziloglu et al. (2008), Mahmoodi et al. (2008), Nourjou (2008), Sayfzadeh and Rashidi (2010), Esmaeili (2011), Simsek and Comlekcioglu (2011), Topak et al. (2011), Yonts (2011), Ghamarnia et al. (2012).
Sugar yield (t ha-1)
Irrigation intervals affected (P<0.05) the sugar yield (t ha-1) during both experimental years (Table 6). Minimum sugar yield (t ha-1) of (9.41 and 9.41) was recorded in I1 (5 days’ interval) during 2013-14 and 2014-15. Maximum sugar yield (t ha-1) of (10.62 and 10.65) was recorded in I3 (15 days’ interval) during 2013-14 and 2014-15. The increase in sugar yield might be due to the increase in root yield at 15 days’ interval. The findings show similarity with Ucan and Gencoglan (2004), Isoda et al. (2007) and Mahmoodi et al. (2008).
Harvesting index
Harvesting index was also influenced (P<0.05) with irrigation intervals during both years (Table 6). Minimum harvesting index of (0.72 and 0.72) was recorded in I6 (30 days’ interval) during 2013-14 and 2014-15, whereas maximum harvesting index of (0.73) was recorded in I3 (15 days’ interval) during 2013-14 and 0.73 was recorded in I3 (15 days’ interval) and I4 (20 days’ interval) during 2014-15. The findings are similar to Baloch et al. (2014) who reported maximum harvesting index in wheat irrigated five times at 15 days’ interval.
Correlation relationship
Pearson correlation coefficient for relationship among various physicochemical properties of sugar beet cv. California-kws for the years 2013-14 and 2014-15, have been shown in Table 7.
Leaf Yield is strongly correlated with Chlorophyll (r=0.971 and 0.970, P≤0.01), Pn (net photosynthetic rate) (r=0.980 and 0.982 P≤0.01, PAR (Photosynthetically active radiant) (r = 0.960 and 0.962 (P≤0.01), plant height (r= 0.937 and 0.946, P≤0.01), leaf area (r= 0.954 and 0.968, P≤0.01), leaf weight (r= 0.922 and 0.928, P≤0.01), root weight (r=0.937 and 0.937, P≤0.01), root length (r=0.947 and 0.946, P≤0.01), root diameter (r=0.946 and 0.954, P≤0.01), Root Yield (r=0.984 and 0.985, P≤0.01). The positive nature of these correlations indicates that any change (decrease or increase) in the growth characters will translate into the yield, which suggests a very high degree of association between growth and yield attributes. Hence the growth characters are considered to be an important determiner of the yield (Msaakpa and Obasi, 2014).
Table 6: Effect of irrigation intervals on root yield (t ha-1), sugar yield (t ha-1) and harvesting index of sugar beet cv. California-kws during 2013-14 and 2014-15.
| Treatments (irrigation intervals) |
Root yield(t ha-1) |
Sugar yield(t ha-1) |
Harvesting Index | |||
| 2013/14 | 2014/15 | 2013/14 | 2014/15 | 2013/14 | 2014/15 | |
|
I1 |
60.46 d | 60.45 d | 9.41 e | 9.41 e | 0.72 b | 0.72 b |
|
I2 |
62.45 b | 62.46 b | 9.83 d | 9.80 d | 0.72 c | 0.72 c |
|
I3 |
64.48 a | 64.52 a | 10.62 a | 10.65 a | 0.73 a | 0.73 a |
|
I4 |
64.40 a | 64.43 a | 10.62 a | 10.65 a | 0.73 a | 0.73 a |
|
I5 |
61.73 c | 61.66 c | 10.24 b | 10.22 b | 0.72 b | 0.72 b |
|
I6 |
60.28 e | 60.19 e | 10.04 c | 9.98 c | 0.72 b | 0.72 b |
| LSD | 0.1663 | 0.1427 | 0.0611 | 0.0546 | 9.220e-04 | 7.388e-04 |
Means in each column with same letter are not significantly different at P≤0.05; (I1=5 days, I2= 10 days, I3= 15 days, I4=20days, I5=25 days, and I6=30 days interval).
Table 7: Pearson correlation coefficients between various physico-chemical properties of sugar beet cv. California-kws (2013-14 and 2014-15).
| 2013-14 | 2014-15 | |||||
| LY | RY | SY | LY | RY | SY | |
| CHL |
0.971** |
0.983** |
0.838* |
0.970** |
0.980** |
0.858* |
| Pn |
0.980** |
0.965** |
0.662 |
0.982** |
0.969** |
0.694 |
| PAR |
0.960** |
0.950** |
0.726 |
0.962** |
0.950** |
0.752 |
| PH |
0.937** |
0.970** |
0.759 |
0.946** |
0.977** |
0.782 |
| NL |
0.900* |
0.940** |
0.775 |
0.916* |
0.955** |
0.809 |
| LL |
0.895* |
0.953** |
0.743 |
0.883* |
0.948** |
0.828* |
| LA |
0.954** |
0.971** |
0.686 |
0.968** |
0.986** |
0.778 |
| LW |
0.922** |
0.888* |
0.575 |
0.928** |
0.878* |
0.563 |
| RW |
0.937** |
0.893* |
0.471 |
0.937** |
0.896* |
0.501 |
| R/T |
0.887* |
0.839* |
0.361 |
0.899* |
0.868* |
0.433 |
| RL |
0.947** |
0.987** |
0.852* |
0.946** |
0.984** |
0.888* |
| RD |
0.946** |
0.981** |
0.772 |
0.954** |
0.983** |
0.802 |
| SUC | 0.178 | 0.266 | 0.785 | 0.231 | 0.323 | 0.803 |
| TSS | 0.015 | 0.103 | 0.669 | 0.136 | 0.230 | 0.741 |
| PUR | 0.458 | 0.538 |
0.922** |
0.339 | 0.428 |
0.865* |
| LY |
0.984** |
0.739 |
0.985** |
0.757 | ||
| RY | 0.806 |
0.823* |
||||
| SY | ||||||
Chl: chlorophyll; Pn: net photosynthetic rate; PAR: Photosynthetically Active Radiants; PH: Plant Height; NL: Number of Leaves; LL: Leaf Length; LA: Leaf Area; LW: Leaf Weight; RW: Root Weight; R/T: Root top Ratio; RL: Root Length; RD: Root Diameter; SUC: sucrose%; TSS: Total Soluble Solids; PUR: Purity; LY: Leaf Yield; RY: Root Yield; SY: Sugar Yield; HI: Harvesting Index; **P <0.01; *P < 0.05.
Similar to Ahmad et al. (2012) who reported that leaf weight has strong association with root yield. Leaf yield also positively correlated with number of leaves (r=0.900 and 0.916, P≤0.05), leaf length (r=0.895 and 0.883, P≤0.05), root/top ratio (r=0.887 and 0.899, P≤0.05) during both years.
Root Yield showed strong correlation with Chlorophyll (r=0.983 and 0.980, P≤0.01), Pn (net photosynthetic rate) (r= 0.965 and 0.969, P≤0.01), PAR (Photosynthetically active radiant) (r=0.950 and 0.950, P≤0.01), plant height (r=0.970 and 0.977, P≤0.01), number of leaves (r=0.940 and 0.955, P≤0.01), leaf length (r=0.953 and o.948, P≤0.01), Leaf area (r=0.971 and 0.986, P≤0.01). Kazakov et al. (1988) reported root yield is correlated with leaf area duration. Root length (r= 0.987 and 0.984, P≤0.01), root diameter (r=0.981 and 0.983, P≤0.01), leaf yield (r=0.984 and 0.985, P≤0.01) and positively correlated with leaf weight (r=0.888 and 0.878, P≤0.05), root weight (r=0.893 and 0.896, P≤0.05) and root/top ratio (r=0.839 and 0.868, P≤0.05) during both years. It is in line with Hozayn et al. (2013) who correlated beet root yield with fresh root weight and root diameter and Ahmad et al. (2012) reported that increase in beet growth characters from a normal size may results in reducing quality of sugar beet.
Sugar Yield is strongly correlated with Purity (r=0.922, P≤0.01) during first year only and is positively correlated with chlorophyll (r=0.838 and 0.858, P≤0.05), leaf length (r=0.743 and 0.828, P≤0.05), root length (r=0.852 and 0.888, P≤0.05) during both year and purity (r=0.865, P≤0.05), Root Yield (r=0.823, P≤0.05) during second year. The results agree with Hozayn et al. (2013) and Ahmad et al. (2012) who reported strong positive correlation of sugar yield with beet root yield. Similarly, Farina and Hashemi (2015) correlated root yield with sugar yield.
Conclusions and Recommendations
The study has investigated the effect of different irrigation intervals on the yield and quality of sugar beet. It is inferred that irrigation at 15 days’ interval was found the most effective irrigation interval with a 7 % and 6 % increase in root and sugar yield t ha1, respectively. However, increasing interval up to 30 days’ improved the sucrose % and T.S.S % but decreased growth and yield.
Acknowledgments
Authors are thankful to Faculty of Agriculture for their technical support both in the field and lab.
Author’s Contribution
Imran Khan: Designed and performed research, performed statistical analysis and data interpretation and wrote and revised the manuscript.
Muhammad Iqbal: Contributed to research design, participated in experiments and reviewed the manuscript.
Malik Muhammad Hashim: Participated in the research design and revised carefully the manuscript.
References
Abayomi, Y.A., D. Wright. 2002. Sugar beet leaf growth and yield response to soil water deficit. Afr. Crop Sci. J. 10(1): 5l-66. https://doi.org/10.4314/acsj.v10i1.27557
Ahmad, Z., P. Shah, K.M. Kakar, H. El-Sharkawi, P.B.S. Gama, E.A. Khan, T. Honna and S. Yamamoto. 2010. Sugar beet (Beta vulgaris L.) response to different planting methods and row geometries II: Effect on plant growth and quality. J. Food, Agric. Environ. 8(2): 785-791.
Ahmad, S. and A. Rasool. 2011. Evaluation of sugar beet varieties for their adaptability in different soiland environmental conditions of Punjab. Final report (2008-11) Agricultural Linkages Program(ALP), Nat. Agric. Res. Center, Islamabad, Pak.
Ahmad, S., M. Zubair, N. Iqbal, N.M. Cheema and K. Mahmood. 2012. Evaluation of sugar beet hybrid varieties under Thal-kumbi soil series of Pakistan. Int. J. Agric. Biol. 14(4): 605-608.
Ashraf, M., S.H. Nawazish and H. Athar. 2007. Are chlorophyll fluorescence and photosynthetic capacity potential physiological determinants of drought tolerance in maize (Zea mays L.). Pak. J. Bot. 39: 1123–1131.
Bakhsh, I., I.U. Awan and M.S. Baloch. 1999. Effect of various irrigation frequencies on yield and yield components of sunflower. Pak. J. Biol. Sci. 2: 194-195. https://doi.org/10.3923/pjbs.1999.194.195
Bloch, D. and C.M. Hoffman. 2005. seasonal development of genotypic differences in Sugar beet (Beta vulgaris L.) and their interaction with water supply. J. Argon. Crop Sci. 191: 263-272. https://doi.org/10.1111/j.1439-037X.2005.00150.x
Bloch, D., C.M. Hoffmann and B. Marlander. 2006. Impact of water supply on photosynthesis, water use and carbon isotope discrimination of sugar beet genotypes. Eur. J. Agron. 24: 218–225. https://doi.org/10.1016/j.eja.2005.08.004
Cornic, G. and A. Masacci. 1996. Leaf photosynthesis under drought stress. In: Baker NR (ed) Photosynthesis and the environment. Kluwer, Dordrecht. pp. 347–366.
Elliot, M.C and G.D. Weston. 1993. Biology and physiology of the sugar beet plant. In: Cooke DA, Scott Rk, eds. The sugar beet crop: Science into practice. Chapman and Hall Publishing, London. 37-63.
Esmaeili, M.A. 2011. Evaluation of the effects of water stress and different levels of nitrogen on sugar beet (Beta vulgaris). Int. J. Biol. 3: 89-93. https://doi.org/10.5539/ijb.v3n2p89
Fabeiro, C., F. Martın and J.A. de Juan. 2002. Production of muskmelon (Cucumismelo L.) under controlled deficit irrigation in a semi-arid climate. Agric. Water Manage. 54: 93–105. https://doi.org/10.1016/S0378-3774(01)00151-2
Farnia, A. and G. Hashemi. 2015. Correlation between yield and other treats in sugar beet (Beta vulgaris L.) under application of different biofertilizers and irrigation. Int. J. Biosci. 6(3): 146-152. https://doi.org/10.12692/ijb/6.3.146-152
Flexas. J. and H. Medrano. 2002. Drought-inhibition of photosynthesis in C3 plants: stomatal and non-stomatal limitation revisited. Ann. Bot. 89: 183–189. https://doi.org/10.1093/aob/mcf027
Ghamarnia, H., I. Arji, S. Sepehri, S. Norozpour and E. Khodaei. 2012. Evaluation and comparison of drip and conventional irrigation methods on sugar beets in a semiarid region. J. Irri. Drain. Eng. 138: 90-97. https://doi.org/10.1061/(ASCE)IR.1943-4774.0000362
Gunes, A., N. Cicek, A. Inal, M. Alpaslan, F. Eraslan, E. Guneri and T. Guzelordu. 2006. Genotypic response of chickpea (Cicer arietinum L.) cultivars to drought stress implemented at pre and postanthesis stages and its relations with nutrient uptake and efficiency. Plant Soil Environ. 52: 368–376. https://doi.org/10.17221/3454-PSE
Hussein, M.M., O.M. Kassab and A.A. Ellil. 2008. Evaluating water stress influence on growth and photosynthetic pigments of two sugar beet varieties. Res. J. Agric. Biol. Sci. 4(6): 936–941.
Iqbal, M.A. and A.M. Saleem. 2015. Sugar beet potential to beat sugarcane as a sugar crop in Pakistan. Am. Euras. J. Agric. Environ. Sci. 15 (1): 36-44.
Isoda, A., H. Konishi and P. Wang. 2007. Effect of different irrigation methods on yield and water use efficiency of sugar beet (Beta vulgaris) in the arid area of China. Hort. Res. Chiba Univ. (Japan). 61: 7-10.
Kamal, A. J., A.A. Shad, M. Younas, I. Mohammad and D. Khan. 2003. Selection and evaluation of exotic genotypes of sugar beet (Beta vulgaris L.) in Peshawar valley. Asian J. Plant Sci. 2(8): 655-660. https://doi.org/10.3923/ajps.2003.655.660
Kazakov, E.A., S.M. Kazakova and B.I. Gulyaev. 1988. Effect of soil moisture on formation and necrosis of sugar beet leaf apparatus. Fiziologiya i Biockimiya Kul turnykh, Rastenii. 20: 431-438.
Khalil, S.E. and A.A. El-Noemani. 2012. Effect of irrigation intervals and exogenous proline application in improving tolerance of garden cress plant (Lepidium sativum L.) to water stress. J. Appl. Sci. Res. 8(1): 157-167.
Kiymaz, S. and A. Ertek. 2015. Yield and quality of sugar beet (Beta vulgaris L.) at different water and nitrogen levels under the climatic conditions of Kirsehir, Turk. Agric. Water Mange. 158: 156-165. https://doi.org/10.1016/j.agwat.2015.05.004
Kiziloglu, F.M., M. Turan, U. Sahin, Y. Kuslu and A. Dursun. 2008. Effects of untreated and treated wastewater irrigation on some chemical properties of cauliflower (Brassica olerecea L. var. botrytis) and red cabbage (Brassica olerecea L. var. rubra) grown on calcareous soil in Turkey. Agric. Water Manag. 95: 716-724.
Keshavarzpour, F. and M. Rashidi. 2011. Response of crop yield and yield components of cantaloupe to drought stress. W. A. Sci. J. 3: 382- 385.
Leufen, G., G. Noga and M. Hunsche. 2013. Physiological response of sugar beet (Beta vulgaris L.) genotypes to a temporary water deficit, as evaluated with a multiparameter fluorescence sensor. Acta. Physiol. Plant. 35: 1763–1774. https://doi.org/10.1007/s11738-012-1213-6
Long, L.R., B.K. Walsh and J.D. Midmore. 2006. Irrigation scheduling to increase muskmelon fruit biomass and soluble solids concentration. Hort. Sci. 41(2): 369-367.
Al-Barrak, M.K. 2006. Irrigation interval and nitrogen level effects on growth and yield of canola (Brassica napus L.). Sci. J. King Faisal Univ. (Basic Appl. Sci.). 7(1): 87-103.
Mahmoodi, R., H. Maralian and A. Aghabarati. 2008. Effects of limited irrigation on root yield and quality of sugar beet (Beta vulgaris L). Afri. J. Biotech. 7 (24): 4475-4478.
Mirabadi, A.A., M. Lotfi and M.R. Roozban. 2013. Impact of water-deficit stress on growth, yield and sugar content of cantaloupe (Cucumis melo L.). Int. J. Agric. Crop Sci. 5 (22): 2778-2782.
Mohammadian, R., M. Moghaddam, H. Rahimian and S.Y. Sadeghian. 2004. Effect of early season drought stress on growth characteristics of sugar beet genotype. Turk. J. Agric. For. 357-368.
Msaakpa, T.S and M.O. Obasi. 2014. Correlated studies between growth and yield characters of castor bean (Ricinus communis L). Int. J. Sci. Res. Publ. ISSN. 4(7): 2250-3153.
Nandi, R.K., M. Dem., J.K. Malty and G. Sounda. 2002. Response of onion to different levels of irrigation and fertilizer. Crop Res. 23(2): 317-320.
Nasir, M.A and I.U.H, Mian 1993. Mango yield and qualtiy as affected by irrigation intervals. Pak. J. Agric. Res. 14(4): 324-328.
Nayyar, H. and D. Gupta. 2006. Differential sensitivity of C3 and C4 plants to water deficit stress: Association with oxidative stress and antioxidants. Environ. Exp. Bot. 58: 106-113. https://doi.org/10.1016/j.envexpbot.2005.06.021
Niazi, B.H., J. Rozema, M. Salim and M. Yasin. 1998. Field performance of fodder beet and seabeet germination, growth and ion relations under saline soil conditions. Pak. J. Soil Sci. 14: 21–26.
Nourjou, A. 2008. The effects of water deficit on yield and yield components of sugar beet and water productivity. Iran. J. Irrig. Drain. 2: 31-42.
Passioura, J. 2007. The drought environment: physical, biological and agricultural perspectives. J. Exp. Bot. 58: 113-117. https://doi.org/10.1093/jxb/erl212
Rahbarian, R., R. Khavari-Nejad, A. Ganjeali, A. Bagheri and F. Najafi. 2011. Drought stress effects on ohotosynthesis, chlorophyll fluorescence and water relations in tolerant and susceptible chickpea (Cicer arietinum L.) Genotypes. Acta. Bio. Cracov. Ser. Bot. 53 (1): 47-56. https://doi.org/10.2478/v10182-011-0007-2
Raza, M.H., G.U. Sadozai, M.S. Baloch, E.A. Khan, I. Din and K. Wasim. 2012. Effect of irrigation levels on growth and yield of mungbean. Pak. J. Nutr. 11 (10): 974-977. https://doi.org/10.3923/pjn.2012.974.977
Ribas, F., M.J. Cabello, M. Moreno, A. Moreno and L. Lopez-Bellido. 2003. Influencia del riego y de la aplicacio´n de potasioen la produccio´n de melo´n (Cucumis melo L.). II. Calidad. Span. J. Agric. Res. 1 (1): 79–90. https://doi.org/10.5424/sjar/2003011-11
Shangguan, Z.P., M.A. Shao and J. Dyckmans. 2000. Nitrogen nutrition and water stress effects on leaf photosynthetic gas exchange and water use efficiency in winter wheat. Environ. Exp. Bot. 44: 141-149. https://doi.org/10.1016/S0098-8472(00)00064-2
Smith, G.A. 1987. Sugar beet: principles of cultivar development. Fehr WR, ed. MacMillian Publishing Company. 577-625.
Snyman, H.A. 2004. Effects of various water applications strategies on root development of Opuntia ficus-indica and O. robusta. J. PACD. 35-61.
Steel, R.G.D., J.H. Torrie and D. Dickey. 1997. Principles and procedure of statistics. A biometrical approach 3rd Ed. McGraw hill book co. Inc., New York. pp. 352-358.
Stocker, O. 1960. Physiological and morphological changes in plants due to water deficiency. In: Plant water relationships in arid and semiarid conditions. Arid Zone Res. Rev. Res., UNESCO, Paris, Vol. 15, pp. 63-104.
Topak, R., S. Suheri and B. Acar. 2011. Effect of different drip irrigation regimes on sugar beet (Beta vulgaris L.) yield, quality and water use efficiency in Middle Anatolian, Turkey. Irrig. Sci. 29: 79-89. https://doi.org/10.1007/s00271-010-0219-3
Viard, F., J. Bernard and B. Desplanque. 2002. Crop-weed interactions in the Beta vulgaris complex at a local scale: allelic diversity and gene flow within sugar beet fields. Theor. Appl. Gen. 104: 688-697. https://doi.org/10.1007/s001220100737
Winter, S.R. 1989. Sugar beet yield and quality response to irrigation, row width and stand density. J. Sugar beet Res. 26: 26-33. https://doi.org/10.5274/jsbr.26.1.26
Wu, Q., R. Xia and Y. Zou. 2005. Reactive oxygen metabolism in mycorrhizal and nonmycorrhizal citrus (Poncirus trifoliate) seedlings subjected to water stress. J. Plant Physio. 163: 417-425. https://doi.org/10.1016/j.jplph.2005.04.024
Yonts, C.D. 2011. Development of season long deficit irrigation strategies for sugarbeet. Int. Sugar J. 113: 728-731. https://doi.org/10.5274/ASSBT.2011.14
Zao, D.L. 1990. The study on photosynthesis of sugar beet. China Beet. 3:2-5.
Zlatev, Z.S and I.T. Yordanov. 2004. Effects of soil drought on photosynthesis and chlorophyll fluorescence in bean plants. Bulg. J. Plant Physiol. 30: 3-18.








