Monitoring Anthelmintics Resistance and Assessing Effectiveness of Herbal Anthelmintics against Small Ruminants’ Nematodes Infection in Pakistan
Monitoring Anthelmintics Resistance and Assessing Effectiveness of Herbal Anthelmintics against Small Ruminants’ Nematodes Infection in Pakistan
Abdul Razzaq1*, Muhammad Islam2, Zahra Islam3, Zahida Fatima1, Munib Hussain4 and Farmanullah5,6
1Animal Sciences Division, Pakistan Agricultural Research Council, Islamabad, Pakistan; 2International Center for Agriculture Research in the Dry Areas (ICARDA), NARC, Islamabad, Pakistan; 3Higher Education Commission, Islamabad, Pakistan; 4Animal Health Program, National Agricultural Research Centre, PARC, Islamabad, Pakistan; 5Faculty of Veterinary and Animal Sciences, Lasbela University of Agriculture, Water and Marine Sciences, Uthal, Balochistan, Pakistan; 6Key Laboratory of Agricultural Animal Genetics, Breeding and Reproduction, Education Ministry of China, College of Animal Sciences and Technology, Huazhong Agricultural University, Wuhan 430070, Peoples Republic of China.
Abstract | The gastrointestinal nematodes are common pathogens in grazing sheep/goats throughout the world which impairs productivity and leads to high economic losses. In most part of the world, drug resistance against anthelmintic is now very common. In this context, various alternative control programs including herbal use against these worms is also an important option. To explore the problem, three available synthetic anthelmintic (Oxfendazole alone, oxfendazole-Levamisole combination and Ivermectin) were administered to natural major nematodes (Haemonchus, Trichuris, Strongyloides and Trichostrongylus) infected sheep and goats. Overall results revealed susceptibility of these anthelmintics (97-99% Confidence Interval of Faecal Egg Count Reduction-FECR) against four nematodes and no evidence of resistance recorded. However, three herbal anthelmintics (Atreefal Deedan, Deedani and Kirmar) available in Pakistan were tested against nematodes as an option of alternate remedy. Among these Atreefal Deedan showed highest (90-96%) FECR, followed by Deedani (80-83%) and Kirmar (32-60%). It is concluded that, on small scale assessment no drug resistance observed against few worms in sheep and goats in Pakistan. A broader study is recommended for assessment of drug resistance and also evaluation of available or practiced anthelmintic.
Received | December 05, 2017; Accepted | July 31, 2019; Published | October 26, 2019
*Correspondence | Abdul Razzaq, Animal Sciences Division, Pakistan Agricultural Research Council, Islamabad, Pakistan; Email: abdulrazzaqazrc@gmail.com
Citation | Razzaq, A., M. Islam, Z. Islam, Z. Fatima, M. Hussain and Farmanullah. 2019. Monitoring anthelmintics resistance and assessing effectiveness of herbal anthelmintics against small ruminants’ nematodes infection in Pakistan. Pakistan Journal of Agricultural Research, 32(4): 662-669.
DOI | http://dx.doi.org/10.17582/journal.pjar/2019/32.4.662.669
Keywords | Nematodes, Anthelmintic resistance, Herbal anthelmintic, Sheep, Goats
Introduction
The livestock sector occupies a unique position in the National Agenda of the economic development of Pakistan. This sector plays a vital role in revenue generation of the country and is presumed to play an important role in poverty alleviation to uplift the socioeconomic condition of Pakistan’s rural masses. The animal production operations provide 35% income directly or indirectly to nearly 8 million rural families in Pakistan (Anonymous, 2015-16). In country prospective, livestock contributes approximately 58.6 percent to the agriculture value added and 11.6 percent to the overall GDP during 2015-16. Gross value addition of livestock at constant cost factor of 2005-06 has increased from Rs. 1247 billion (2014-15) to Rs. 1292 billion (2015-16), showing an increase of 3.63 percent over the same period last year (2015). There are about 29.8 and 70.3million heads of sheep and goats, respectively in Pakistan (Anonymous, 2015-16).
Sheep and goats are excellent source for meat production in Pakistan, producing 37,000 tons of meat per annum and provide good source of animal protein. There are certain constraints of physiological and infectious origin (Raziq et al., 2010) which limited the growth of this industry in terms of poor production performance and reproductive efficacy; thus affecting the GDP value along with socio-economic status of livestock farmers. Poor animal health and diseases are usually associated to the cause of low farm productivity and indirectly to low profit margin. The other factors which effect their productivity is persistent drought and shortage of range vegetation due to overgrazing/utilization of trees/shrubs as fuel wood.
Among the infectious organisms of parasitic origin, gastrointestinal nematodes are the main cause for production losses in terms of lower milk and meat production in affected animals (Nasreen et al., 2008; Souza et al., 2012; Tasawar et al., 2012; Zeryehun, 2012). The most common feature of gastrointestinal nematodiasis (GIN) is to increase loss of endogenous proteins that leads to retarded growth (Jackson et al., 2009); whereas, heavy worm loads have also been reported for high mortality (Qamar, 2009). Apart from production losses, the other associated losses related to GIN - is financial losses on the purchase of anti-parasitic drugs, veterinary services and other control measures (Thomas et al., 2011).
In last three decades the control of nematodes are relying on chemical treatments and many compounds were very effective but the development of anthelmintic resistance against nematodes in various countries had - been reported (Thomas et al., 2011; Arunkumar, 2012) which is threatening the end of anthelmintic era. To counteract this adverse situation, many studies have been conducted in different parts of the world which showed that there are many medicinal plants that have the potential to be used as anthelmintic (Jabbar et al., 2006). However, majority of the evidences reported in ethno-veterinary sources are based on old people observations, instead of proper experimentation. There are many plants which have been scientifically validated for their anthelmintic properties - based on their traditional uses (Iqbal et al., 2004; Iqbal et al., 2005; Githiori et al., 2006; Iqbal et al., 2006). Ihsanullah (2005) documented that 21 different kind of indigenous plants are being used in ethno-veterinary medicine system of Balochistan for the treatment of different ailments including infections caused by endo-parasites in livestock since last many decades.
Keeping in view the above mentioned scenario, present study was accomplished to investigate the resistance against synthetic anthelmintics and herbal control option of nematodes infections in small ruminants in different ecologies of Pakistan.
Materials and Methods
Selection of site, animals and farmers
The study area was situated in two ecologies of Pakistan i.e. Ziarat valley (30° 22’ 47 N and 67° 43’ 38 E) in Balochistan and second site was Dhulli (32°51’31.02” N 72°11’28.22E) in district Talagang, Punjab Province. At each location four farmers of small ruminant based on range grazing practices were selected during January 2015. Each farmer has mix sheep and goats with a flock size of 60-80 heads. As these animals were expected by having higher nematodes infectivity and according to our knowledge, they were not given any anthelmintics since last 3-4 months.
Experimental design and parasitological techniques
All the female animals (ewes/does) as per higher number of availability between 2-4 year divided in four groups and ear tagged for proper record keeping. The ewes and does at Ziarat were of Harnai and Khurasani breed respectively while at Dhulli, they were White-Shinwar (Afghani cross) and Beetal, respectively. The faecal samples were collected directly from the rectum of these selected animals and examined during January to August 2015 for nematodes infection which was determined through observation of parasitic eggs in faecal sample analysis followed by Faecal Egg count or parasitic larvae through faecal culture (Thienpont et al., 1979; Soulsby, 1982; Urquhart et al., 1996). A total 80 animals (40 ewes and 40 does) were further selected during August 2015 from these flocks at each site based on peak major four nematodes infection i.e., Haemonchus, Trichuris, Strongyloides and Trichostrongylus with infectivity level higher than 2000 FEC. The laboratory results indicated abundance of these four major nematodes equally found in animals at both sites. Other than these many internal parasites were also observed but neglected due to low infection rate. These infected animals were divided in to four equal groups i.e., A, B, C and D. The animals in first three groups were treated with three synthetic anthelmintics comprised of Oxfendazole, Oxfendazole-Levamisole and Ivermectin respectively while last group kept as control of infected animals for comparison (Table 1). These anthelmintics were selected to assess the resistance as most of the farmers being administered to their flocks twice a year since last one or two decades.
Table 1: Experimental groups of ewes/does at two sites treated with synthetic anthelmintics.
| Groups | Ziarat site | Dhulli site | Treatment | Dose per kg body wt. | ||
| Ewes | Does | Ewes | Does | |||
| A | 10 | 10 | 10 | 10 | Oxfendazole | 5 mg |
| B | 10 | 10 | 10 | 10 |
Oxfendazole-Levamisole |
7.5 mg |
| C | 10 | 10 | 10 | 10 | Ivermectin | 200ug |
| D | 10 | 10 | 10 | 10 | Control | - |
Similarly, 48 animals (24 ewes and 24 does) were also selected separately from each site based on same major nematodes infection i.e., Haemonchus, Trichuris, Strongyloides and Trichostrongylus with infectivity level higher than 2000 FEC. These infected animals were divided in to four equal groups i.e., A, B, C and D. The animals in first three groups were treated with three herbal anthelmintics comprised of Atreefal Deedan, Deedani and Kirmar respectively while last group kept as infected control for comparison (Table 2).
Faecal samples from all these experimental animals were collected post treatment on day 3rd, 5th, 7th, 10th and 14th for counting the FEC as described by Urquhart et al. (1996). The efficacies of different anthelmintics used in present study were calculated by using the formula as described by Ali (2001).
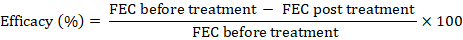
Statistical analysis
The Faecal Egg Reduction percentage was calculated by the formula R%= 100 (1-Xt/Xc) where Xt was treated group and Xc was control group (Coles et al., 1992, 2006). A reduction in eggs per gram of faeces less than 95% with confidence interval between 90% to 95% were taken as an indication (Coles et al., 1992) for the existence of anthelmintic resistant regarding nematodes in treated animals. Calculations were made according to (Coles et al., 1992) using a spreadsheet that was created by Angus Cameron, Aus. Vet. Animal Health Services for the University of Sydney. Their calculations are based on those of the Reso FECR4 test analysis program (Version 2.0).
Results and Discussion
Comparative efficacy and resistance of synthetic anthelmintics against sheep/goats nematodes
All the treated groups of animals showed higher (97-99%) Confidential Interval (CI) in FEC reduction on post-treatment day 14 days (Table 3 and Figure 1). These results revealed there was no resistance of three synthetic anthelmintics against four nematodes of sheep and goats at two target sites. At Ziarat site, the animals in Oxfendazole-levamisol treated group B and Ivermectin group C showed higher CI (99%)
Table 2: Experimental groups of ewes/does at two sites treated with synthetic anthelmintics.
| Groups | Ziarat site | Dhulli site | Treatment | Composition | Dose rate | ||
| Sheep | Goat | Sheep | Goat | ||||
| A | 6 | 6 | 6 | 6 | Atreefal Deedan |
Emblica officianalis, Terminalia bellerica, Terminalia chebula, Embelia robusta, Ipomoea turpethum, Saussurea lappa, Mallotus philippinensis, Lupinus albus, Artemisia absinthium, Darmina turki, Cascuta reflexa, Black salt, Brassica cernua, Citrullus colocynthis, Cyprus scariosus, Zingiber officinale, liquid glucose and sugar |
2 gms/kg body weight once |
| B | 6 | 6 | 6 | 6 | Deedani | Mallolus philppinensis, Embelia ribes, Piper longum | 5 gms/head/day for 3 days |
| C | 6 | 6 | 6 | 6 | Kirmar | Mallolus philppinensis | 3.5 gms/head/day for two days |
| D | 6 | 6 | 6 | 6 | Control | - | - |
Table 3: Mean FECR% and CI in sheep and goats treated with synthetic anthelmintics against nematodes infection at two sites.
| Site | Groups | Post treatment Days | ||||||
|
1st |
3rd |
5th |
7th |
10th |
14th |
CI | ||
| Ziarat | A | 3000±19 | 975±17 (59%) |
540±15 (77%) |
510±12 (80%) |
250±71 (92%) |
185±61 (94%) |
98a |
| B | 3595±15 | 870±12 (73%) |
690±11 (80%) |
540±13 (85%) |
275±11 (92%) |
100±68 (97.5%) |
99a | |
| C | 3155±10 | 1260±82 (59%) |
1000±15 (68%) |
930±62 (70%) |
300±68 (91%) |
255±86 (92%) |
99a | |
| D | 3450±24 | 3535±23 (-5%) |
3545±27 (-3%) |
3705±31 (-6%) |
3650±29 (-6%) |
3710±30 (-8%) |
- | |
| Dhulli | A | 2865±28 | 730±13 (60%) |
580±11 (71%) |
540±13 (74%) |
340±12 (86%) |
250±99 (92.5%) |
97a |
| B | 2955±15 | 1205±10 (59%) |
960±16 (76%) |
884±81 (73%) |
270±68 (92%) |
88±48 (98%) |
99a | |
| C | 3105±97 | 1155±79 (62%) |
915±15 (70%) |
890±77 (71%) |
267±72 (92%) |
216±81 (93%) |
98a | |
| D | 3100±24 | 3285±27 (-7%) |
3340±28 (-10%) |
3405±34 (-7%) |
3420±33 (-8%) |
3510±32 (-13%) |
- | |
CI: Confidence Interval; a susceptible or effective.
Table 4: Efficacy of herbal anthelmintics (Mean FECR% and CI) against nematodes infection in sheep and goats at two sites.
| Site | Groups | Post treatment Days | ||||||
|
1st |
3rd |
5th |
7th |
10th |
14th |
CI | ||
| Ziarat | A | 1710 ± 53 | 487 ± 91 (72%) | 350 ± 85 (80%) |
148 ± 63 (91%) | 103 ± 58(94%) | 70 ± 46 (96%) | 99a |
| B | 3025±17 | 1445±14 (50%) |
1130±14 (59%) |
1000±12 (64%) |
470±13 (82%) |
440±11 (83%) |
96a | |
| C | 1717 ± 55 | 1543 ± 85(10%) | 1537 ± 81(10%) | 1353 ± 90(21%) | 1140 ± 11(34%) | 691 ± 12 (60%) | 90b | |
| D | 2700±28 | 2840±33 (-1%) |
2690±35 (-4%) |
2820±38 (-4%) |
2510±41 (-7%) |
2535±36(-10%) | - | |
| Dhulli | A | 2655±13 | 1455±16 (41%) |
1050±16 (57%) |
970±10 (61%) |
290±76 (86%) |
175±75 (90%) |
97a |
| B | 2775±21 | 1455±12 (42%) |
1140±13 (53%) |
1010±12 (58%) |
440±13 (81%) |
440±12 (80%) |
96a | |
| C | 1660 ± 71 | 1373 ± 85(17%) | 1247 ± 81(25%) | 1113 ± 10 (33%) | 1020 ± 87(39%) | 1137 ± 11(32%) | 83b | |
| D | 2750±21 | 2936±23 (-1%) |
2989±24(-1.4%) | 3021±22 (2%) |
3173±23 (-11%) |
3323±23 (-5%) |
- | |
CI: Confidence Interval, a susceptible or effective, b resistance or low effective.
than Oxfendazole treated group A (98%). However, the animals in group B present higher Faecal Egg Reduction (97%) followed by A (94%) and C (92%). Similarly, in Dhulli site, animals in Oxfendazole-levamisol treated group B showed higher CI (99%) followed by Ivermectin treated group C (98%) than Oxfendazole treated group A (97%). However, the animals in group B present higher Faecal Egg Reduction (98%) followed by C (934%) and A (92%).
Comparative efficacy of herbal anthelmintics against sheep/goats nematodes
The animals in groups A and B treated with Atreefal Deedan and Deedani showed higher Confidential Interval (96-99%) in FECR while animals in group C treated with Kirmar showed lower (83-90%) reduction after post-treatment of 14 days (Table 4 and Figure 2). The result revealed that, the former two herbal anthelmintics are effective against the sheep/goats nematode infection. However, on the basis of Faecal Egg Reduction percentage only Atreefal Deedan performed above 90% at each target site.
Table 5: Comparative economic values of anthelmintics.
| S. No. | Anthelmintics | Price (Rs.) | Cost/animal (Rs.) |
| 1 | Oxfendazole 1000 ml | 650 | 5.85 |
| 2 | Oxfendazole-Levamisole 1000 ml | 1400 | 28 |
| 3 | Ivermectin 50 ml | 900 | 17 |
| 4 | Ateefal Deedan 100 g | 100 | 2 |
| 5 | Deedani 15 g | 57 | 57 |
| 6 | Kirmar 7 g | 40 | 40 |
Comparative economic values of anthelmintics
The Table 5 presents an economic comparison of the five anthelmintics used in present study. The present study results showed that, all three synthetic and two herbal anthelmintics like Atreefal Deedan and Deedani are effective against four nematodes infection in small ruminants. Among the synthetic anthelmintics “Oxfendazole” is cheaper. However, as the Atreefal Deedan markedly effective and economical among all the anthelmintics. At this movement it is early to recommend this remedy for the control of nematodes in small ruminants in Pakistan. Because there are many other nematodes and even cestodes/ trematodes infecting as mix multiple infection at a same time. In the light of these results, further research is proposed on wider scale to address the entire parasitic range for assessing the efficacy of these available herbal products.
The target area farmers were administered three synthetic anthelmintics twice a year since last 5-8 years. The results of present study reveals that these three anthelmintics i.e. Oxfendazole, Oxfendazole-Levamisole and Ivermectin are effective rather resistance against four nematodes of sheep and goats at two ecologies of Pakistan. The reason might be that the area farmers used these anthelmintics in rotation basis. Similar findings regarding these anthelmintics efficacies were also experience in different parts of the world (Han-Bo et al., 1997; Swarnkar et al., 1999; Sheferaw and Asha, 2010). In contrast to these few researchers also experienced resistance with some species of nematodes to these anthelmintics (Bartley et al., 2006; Menkir et al., 2006; Mirhadi et al., 2011). These resistances in particular areas might be due to extensive use of anthelmintics. Therefore, it is recommended that the anthelmintics may be administered on rotational basis.
There are limited references available regarding herbal anthelmintics against parasitic infection in sheep and goats. However herbal products tested under this study composed of different botanical plants were reviewed/surveyed on individual plants basis as anthelmintic properties by different researchers such as Artemisia absinthium by Tariq et al. (2009), Embelia ribes by Asadulla et al. (2011). Cuscuta reflexa by Pavan et al. (2012), Terminalia belerica by Manohar et al. (2012), Operculina turpethum (Ipomoea turpethum) by Nitin et al. (2012), Citrullus colocynthis by Borhade et al. (2013) and Emblica officinalis by Satyajit et al. (2013). The combinations of these plants as anthelmintics were also reviewed by different researchers (Githiori et al., 2004; Hussain, 2008; Hordegen et al., 2006; Reddy et al., 2011; Chalapathi et al., 2011; Malvankar, 2012). Keeping in view the situation, it is recommended that a wider study may be launched for further evaluation of efficacy of herbal products based on different parasites and various ecologies of Pakistan.
Conclusions and Recommendations
In small ruminant farming based on rangelands, parasitic infections are generally most prevalent and important health problems. In most part of the world, resistance of worms in sheep and goats against anthelmintics is now very common. The present study results showed no resistance against three anthelmintics. However, the alarming situation needs to assess the resistance of synthetic anthelmintics on wider scale for the future safety and to receive healthy animal production in Pakistan. To overcome this issue, the herbal remedy is very important choice as evident from the present study that Atreefal Deedan markedly effective and cheapest among all the anthelmintics. At this movement it is early or difficult to recommend this remedyfor the control of nematodes in small ruminants. In the light of these results, it is recommended that, further research needed on wider scale, while considering entire parasitic range for assessing the efficacy of these products. Beside these it is clear that, some actions are urgently needed in these areas by providing proper nutrition according to animal physiological conditions and clean hygienic climate control housing to boost up their immunity against the diseases like internal parasites and also for the achievement of higher productivity.
Acknowledgement
The authors particularly like to mention the financial assistance provided by USAID under Agriculture Innovation Program (AIP) in Pakistan. This particular project implemented by the International Livestock Research Institute (ILRI) and the International Maize and Wheat Improvement Centre (CIMMYT). Authors also acknowledge the services of laboratory facilitation for parasites investigation by Animal Health Institute at National Agriculture Research Centre.
Author’s Contribution
Abdul Razzaq: Developed research plan, implement in the field and lab diagnosis, wrote results and discussion.
Muhammad Islam: Wrote abstract, editing whole manuscript.
Zahra Islam: Wrote introduction and methodology.
Zahid Fatima: Data collection from the field and compilation.
Farmanullah: Statiscal analysics and graphs.
References
Ali, M.M. 2001. A synopsis of epidemiology and basic statistics. 2nd Edi. Iftikhar book Co. Tipu Rd. Opp. R.M.C. Rawalpindi, Pakistan. p. 37.
Anonymous. 2015-16. Pakistan economic survey. Finance division, GoP.
Arunkumar, S. 2012. Immunoprotection in sheep against H. contortus using its thiol-purified excretory/secretory proteins. Vet. Res. Forum. 3 (4): 239–244.
Asadulla, S., Ramandang and Rajasekharan. 2011. Pharmacognosy of Embelia ribes Burm. F. Int. J. Res. Pharm. Chem. 1(4): 1236-1251.
Bartley, D.J., A.A. Donnan, E. Jackson, N. Sargison, G.B.B. Mitchell and F. Jackson. 2006. A small scale survey of ivermectin resistance in sheep nematodes using the faecal egg count reduction test on samples collected from Scottish sheep. Vet. Parasitol. 137: 112-118. https://doi.org/10.1016/j.vetpar.2005.12.014
Chalapathi, V., K. Yamini and V. Gopal. 2011. Anthelminthic activity of Saraswatha Churna-a polyherbal formulation. Int. J. Pharm. Tech. Res. 3(1): 328-329.
Coles, G.C., F. Jackson, W.E. Pomroy, R.K. Prichard, G. Von samson-himmelstjerna, A. Silvestre, M.A. Taylor and J. Vercruysse. 2006. The detection of anthelmintic resistance in nematodes of veterinary importance. Vet. Parasitol. 136: 167-185. https://doi.org/10.1016/j.vetpar.2005.11.019
Coles, G.C., C. Bauer, F.H.M. Borgsteede, S. Geerts, T.R. Klei, M.A. Taylor and P.J. Waller. 1992. World association for the advancement of veterinary Parasitology (W.A.A.V.P.). Methods for the detection of anthelmintic resistance in nematodes of veterinary importance. Vet. Parasitol. 44: 35-44. https://doi.org/10.1016/0304-4017(92)90141-U
Githiori, J.B., S. Anthanasidou and M.S. Thomsborg. 2006. Use of plants in novel approaches for control of gastrointestinal helminthes in livestock with emphasis on small ruminants. Vet. Parasitol. 139(4): 308-20. https://doi.org/10.1016/j.vetpar.2006.04.021
Githiori, J.B., J. Hoglund P.J. Waller and R.L. Baker. 2004. Evaluation of anthelmintic properties of some plants used as livestock dewormers against H. contortus infections in sheep. Para. 129(2): 245-253. https://doi.org/10.1017/S0031182004005566
Han-bo, M., L. Cheng, W.J. Xiang, W.J. Ning, Z.J. Sheng, Y.C. An, B. Han, L. MA, J. Wang, J. Wu, J. Zhou and Y. Yin. 1997. Removal of sheep parasites using ivermactin. Chinese J. Vet. Med. 23: 5-6.
Hordegen, P., J. Cabaret, H. Hertzberg, W. Langhans and V. Maurer. 2006. In vitro screening of six anthelmintic plant products against larval H. contortus with a modified methyl-thiazolyl-tetrazolium reduction assay. J. Ethnopharma. 108(1): 85–89. https://doi.org/10.1016/j.jep.2006.04.013
Ihsanullah, K. 2005. Documentation of ethno-veterinary practices in Baluchistan. M. Sc. Thesis, Dept. Vet. Med. Sindh Agric. Univ. Tandojam, Pak.
Iqbal, Z., M. Lateef, M. Ashraf and A. Jabbar. 2004. Anthelmintic activity of Artemisia brevifolia in sheep. J. Ethanopharam. 93: (2-3): 265-268. https://doi.org/10.1016/j.jep.2004.03.046
Iqbal, Z., M. Lateef, A. Jabbar, M.N. Ghayur and A.H. Gilani. 2006. In vitro and in vivo anthelmintic activity of Nicotinaa tabacum L. leaves against gastrointestinal nematodes of sheep. Phytoth. Res. 20 (1): 46-48. https://doi.org/10.1002/ptr.1800
Iqbal, Z., M. Lateef, A. Jabbar, G. Muhammad and M.N. Khan. 2005. Anthelmintic activity of Calotropis procera (Ait). F. flowers in sheep. J. Ethanopharm. 102: (2) 256-261. https://doi.org/10.1016/j.jep.2005.06.022
Jackson, F., D. Bartley, Y. Bartley and F. Kenyon. 2009. Worm control in sheep in the future. Small Rum. Res., 86(1-3): 40-45. https://doi.org/10.1016/j.smallrumres.2009.09.015
Manohar, V.R., R. Chandrashekar and S.N. Rao. 2012. Phytochemical analysis of Terminalia belerica fruit pulp extracts. World J. Pharm. Pharmaceut. Sci. 1(4): 1376-1383.
Menkir, M.S., A. Uggla and P.J. Waller. 2006. Anthelmintic resistance of nematode parasites of small ruminants in eastern Ethiopia: Exploitation of refugia to restore anthelmintic efficacy. Vet. Parasitol. 135: 337-346. https://doi.org/10.1016/j.vetpar.2005.09.005
Mirhadi, K., G. Yagoob and S. Saeid. 2011. The effect of Ivermectin pour-on administration against natural N. spathiger infections and prevalent rate of that in cattle. Afr. J. Micro. Res. 5(23): 3858-3861. https://doi.org/10.5897/AJMR11.446
Nasreen, S., M.R. Khan, S. Peerzada and S.A. Andrabi. 2008. Efficacy of different Anthelmintic formulations against helminth infection in sheep. Online J. Vetscan, 3(2): 29. Available from www.vetscan.co
Pavan BU, Suggala VS, Chandrashekhar DU. In vitro anthelmintic activity of stems of Cuscuta re flexa. Int J Bioassays. 2012; 01(08): 18–19.
Qamar, M.F. 2009. Epidemiology, sero-diagnosis, economic losses and control of haemonchosis in sheep and goats. Ph. D. Thesis, Dept. Parasitol. Univ. Vet. Anim. Sci. Lahore, Pak. pp. 63-113.
Raziq, A., M. Younas and Z. Rehman. 2010. Prospects of livestock production in Baluchistan. Pak. Vet. J. 30(3): 181-186.
Reddy, N.L.N., K. Yamini and V. Gopal. 2011. Anthelmintic activity of aqueous and ethanolic extract of Trikatu churna. J. App. Pharmaceut. Sci. 01(03): 140-142.
Satyajit, G., Patil, A.A. Deshmukh, R.P. Amol and A.A. Jagadale. 2013. Phytochemical characterization and estimation of percent extractability of Emblica officinalis fruit extract. Int. J. Nat. Prod. Res. 2(1): 20-24.
Sheferaw, D. and A. Asha. 2010. Efficacy of selected anthelmintics against gastrointestinal nematodes of sheep owned by smallholder farmers in Wolaita, Southern Ethiopia. Ethiop. Vet. J. 14(2): 31-38. https://doi.org/10.4314/evj.v14i2.63882
Souza, M.F., M. Pimentel-Neto, R.M. Silva, A.C.B. Farias and M.P. Guimaraes. 2012. Gastrointestinal parasites of sheep, municipality of Lajes, Rio Grande do Norte, Brazil. Rev. Bras. Parasitol. Vet. 21(1): 71-73. https://doi.org/10.1590/S1984-29612012000100015
Swarnkar, C.P., F.A. Khan, D. Singh and P.S.K. Bhagwan. 1999. Further studies on anthelmintic resistance in sheep at an organized farm in arid region of Rajasthan. Vet. Parasitol. 82: 81-84. https://doi.org/10.1016/S0304-4017(98)00265-9
Tariq, K.A., M.Z. Chishti, F. Ahmad and A.S. Shawl. 2009. Anthelmintic activity of extracts of Artemisia absinthium against ovine nematodes. Vet. Para. 160(1-2): 83–88. https://doi.org/10.1016/j.vetpar.2008.10.084
Thienpont, D., F. Rochette and O.F.J. Vanparijs. 1979. Diagnosing helminthiasis through coprological examination. Janssen Res. Found., Beerse Belgium. p. 36.
Thomas, P., G. Barbara, P.H. James, M. Grace and W. Theo-de. 2011. Gastrointestinal nematode control practices on lowland sheep farms in Ireland with reference to selection for anthelmintic resistance. Ir. Vet. J. 64(1): 4. https://doi.org/10.1186/2046-0481-64-4
Zeryehun, T. 2012. Helminthosis of sheep and goats in and around Haramaya, Southeastern Ethiopia. J. Vet. Med. Anim. Health. 4(3): 48-55.







