Integrated Management of Root Knot Nematode Meloidogyne graminicola Golden and Birchfield Parasitizing on Wheat
Integrated Management of Root Knot Nematode Meloidogyne graminicola Golden and Birchfield Parasitizing on Wheat
Sumaira Akram1, Sajid Aleem Khan1*, Nazir Javed1 and Saeed Ahmad2
1Department of Plant Pathology, University of Agriculture, Faisalabad, Pakistan
2Institute of Horticultural Sciences, University of Agriculture, Faisalabad, Pakistan
ABSTRACT
M. graminicola Golden and Birchfield (MG) is an important pest of cereal crops. In Pakistan the yield of wheat is extremely low as compared to many other developing countries. Among several factors, responsible for low crop yield, MG is an emerging threat. In vitro hatching percentage of MG was evaluated that was varied significantly at different concentration of chemicals, bio control agents and plant extracts. Among chemicals, cartap was most effective with lowest egg hatching and among biocontrol agents Paecilomyces lilacinus (Thom) Samson (PL) produced highest egg hatching while, Trichoderma harzianum Rifai (TH) caused lowest egg hatching. In plant extracts the highest egg hatching was recorded in clove at S concentration and lowest by neem extracts at S/10 concentration. Cartap and rugby produced 100 % juvenile mortality at S concentration. After 48 h the treatment TH + PL caused highest juvenile mortality of MG and among plant extracts tobacco extract caused the highest juvenile mortality after 48 h. The protective and curative effect of chemicals, biocontrol agents and plant extracts on the development of MG was recorded on the basis of number of eggs on the most susceptible wheat variety cv. ‘Aus -7-58-0850’. In protective effect the highest number of eggs were recorded in rugby treatment. Chemicals were found to be more effective than plant extracts and biocontrol agents. Among biocontrol agents TH produced lowest number of eggs. In curative effect of plant extracts the highest number of eggs were observed in plants treated with in aloe vera extract. Protective and curative treatment with plant extracts, biocontrol agents and chemicals suppressed the disease and enhanced plant height and root weight. All treatments evaluated in this study exhibited suppression of MG. Therefore, these treatments can be used in combination against MG infestation on wheat.
Article Information
Received 11 April 2019
Revised 22 July 2019
Accepted 25 September 2019
Available online 26 March 2020
Authors’ Contribution
SA conducted the study. SAK supervised the research. NJ helped in lay out of experiments. SA helped in statistical analysis.
Key words
Root knot nematode, Trichoderma harzianum, Plant extracts, Egg hatching
DOI: https://dx.doi.org/10.17582/journal.pjz/20190411080438
* Corresponding author: [email protected]
0030-9923/2020/0004-1299 $ 9.00/0
Copyright 2020 Zoological Society of Pakistan
INTRODUCTION
Wheat yield is drastically affected due to the attack of bacteria, virus, fungi and nematodes. Root-knot nematode (Meloidogyne spp.) is the most important emerging plant pathogen prevalent in most countries of the world. In Pakistan, five species of RKN viz, M. hapla, M. arenaria, M. javanica, M. incognita and M. graminicola Golden and Birchfield (MG) are common (Shahina et al., 2009). Plant parasitic nematode MG reduced the yield of several crops approximately 10 to 20 % throughout the world (Bridge et al., 2005).
Root knot nematode is among one of the most threatening pathogens (Al-Hazmi, 1982; Sikora, 1993; Husman, 1996). MG affects the root system of plants and reduce the uptake of water and nutrients (Singh, 1975). Several biotic and abiotic factors affect the growth and reproduction of RKN in the soil. Nematodes can multiply easily on host plants, but innate plant resistance does not allow the development of nematodes and reduces the intensity of infection (Netscher and Luc, 1974). Ear cockle disease of wheat caused by Anguina tritici and root knot nematode are the most important diseases of wheat. Nematodes mostly parasitize on roots and their symptom does not appear on above ground parts of plant. Nematode diseases are mostly neglected because they do not develop any above ground symptoms and there is lack of credible reports in literature (Dahal et al., 1992). MG extensively affects the cereal crops, viz, rice and wheat under diverse climatic conditions and soil types. M. chitwoodi, M. artiellia, M. microtyla, M. ottersoni and M. naasi attack on cereal crops in cool environment (Sikora, 1993) while, M. graminis, M. graminicola, M. spartinae and M. kikuyensis are main species that survive at high temperature (Taylor and Sasser, 1978). MG causes economic loss on lowland and upland wheat and rice crops (Bridge et al., 1990). Many other root knot nematodes such as M. artellia and M. nasii also attack on wheat crop (Davis and Venettee, 2004). Wheat crop is highly infected by MG in Nepal, India and Pakistan (Pokharel et al., 2004; Gaur and Sharma, 1999; Somorro and Hauge, 1992; Padgham et al., 2004). High population of MG increases the severity of MG in wheat and rice cropping systems (Padgham et al., 2004; Gaur and Sharma, 1999).
In wheat and rice cropping system, approximately 40 % reduction in root and shoot length of wheat and rice plants was recorded in MG infested fields (Pokharel et al., 2004). The high yield loss due to nematodes has prompted the search for most reliable and effective management options. Several management strategies have been used to manage MG infestation on wheat plants. Screening of resistance germplasm have been used to manage MG to find resistant germplasm against this devastating nematode (Padgham, 2003; Pokharel et al., 2004). So, far, application of synthetic nematicides is the most effective method to control plant parasitic nematodes. Nematicides are the most common and rapid mean to control root-knot nematode infection. In developing countries farmer mainly rely on the use of chemicals to manage several plant pathogens. Recently, biological control through several antagonistic microbes has gained special significance. Meloidogyne species have been successfully suppressed by using several biological control agents (Murslain et al., 2014; Muhae-ud-Din et al., 2018; Haque et al., 2018). Trichoderma harzianum Rifai was found to be highly effective against M. incognita (Murslain et al., 2014). Paecilomyces lilacinus (Thom) Samson has also been used to control Meloidogyne species. Antimicrobial botanical extracts provide another environment-friendly, safe and reliable option to suppress plant pathogenic nematodes (Mukhtar et al., 2004). Plants are a natural reservoir of antimicrobial compounds (Ngadze, 2014). The control of Meloidogyne spp. using several plant extracts has been reported previously (Samaleiv et al., 2017; Nandi, 2018). However, the use of more than one treatment in combination provides higher disease control than stand-alone applications of treatments. Kumar et al. (2017) reported that combination of organic amendments, Trichoderma spp. and carbofuran was highly effective to control MG. Therefore, more than one treatment should be included as components of integrated disease management strategy of root-knot nematodes.
The project was undertaken with the objective to evaluate different chemicals, biological control agents and antifungal plant extracts on egg hatching and juvenile mortality of MG and to test the protective and curative effect of these treatments in vivo on wheat plants to assess their effect on MG development and plant growth parameters.
MATERIALS AND METHODS
Collection, identification and multiplication RKN
Infected plants were collected from various locations of Faisalabad, Punjab, Pakistan. Infected samples were collected for identification, purification and multiplication of MG. Root samples were collected carefully from plant rhizosphere along with 1 kg of adhering soil. Samples were placed in plastic bags and moistened to ensure nematode survival. Information on locality, date of collection and host were collected. Root and soil samples were processed for isolation of nematodes using Baermann funnel and Hemming tray method. After 24-48 h the number of nematodes were observed under stereomicroscope. The number of nematodes was counted in a counting dish under stereomicroscope. Identification of 2nd stage juveniles of RKN was done on the basis on morphological characters. For further confirmation, perineal pattern micrographs of females were made (Jepson, 1987). Perineal pattern was observed under compound microscope (Eisnback et al., 1981).
Soil preparation
Soil was prepared for experimental use. Sterilized sandy loam soil (70 % sand, 21 % silt, 6 % clay and 3 % organic matter) was mixed thoroughly, air dried and spread on a wooden bench. After drying big stones and debris of plants were removed. Then soil was covered by using plastic sheet for 1 week. For pot trials (in small pots) sterilized sandy loam soil (70 % sand, 21 % silt, 6 % clay, 3 % organic matter) was sterilized in an oven at 120 ºC for 20 min. Soil was stored for two weeks at 25 ºC before using for further experiment. (Talavera and Mizukubo, 2003).
Mass culturing of MG on wheat
To obtain the inoculums for the experiments, mass culturing of nematode MG was carried out on ten-week-old plants on the roots of susceptible cultivar of wheat cv. ‘Aus -7-58-0850’ and maintained in pots having sterilized soil. Pots were inoculated with 2000 J2/ pot near root zone with the help of pipette in three to four holes of 3 cm depth around each plant. Then these holes were covered with soil to prevent drying. After inoculation, plants were kept under field conditions at open place and irrigated regularly to prevent moisture loss.
Effect of chemicals, biocontrol agents and plant extracts
Egg hatching of MG
Three concentrations of each chemical, bio control agents and plant extracts were prepared. To see the effect of chemicals, plant extracts and biocontrol agents on egg hatching, a little drop of egg suspension containing fifty eggs was poured in a counting dish. Five mL concentrations of each biocontrol agents, plant extracts and chemicals were poured in the Petri dishes with the help of pipette (separate pipette was used for each of chemicals, biocontrol agents and plant extracts and concentrations). Each treatment had ten replications and the experiment was repeated twice. Data on hatching was recorded after six days. The egg hatching was assessed according to Abbott’s formula (Abbott, 1925).
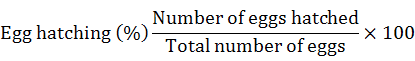
Juvenile mortality of MG
In vitro evaluation of chemicals was carried out at different concentrations for J2 mortality. For the evaluation of mortality 200 J2 were transferred to the Petri plates. Then 1 mL concentration of each chemicals, 1 mL concentration of each bio control agent TH and PL (S, S/5, S/10) and plant extracts at different concentrations were poured in petri plates. Each treatment had ten replications and the experiment was repeated twice. Data on mortality was recorded after 24, 48 and 72 h in a completely randomized design (Hussey and Barker, 1973). Percent Juvenile mortality was recorded according to Abbott’s formula (Abbott, 1925).
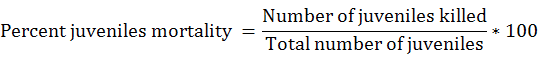
If juveniles did not move while probing with needle they were taken as dead (Abbasi et al., 2008) and were recorded as alive if moved or appeared as winded (El- Rokick and El- Nagdi, 2011).
Pot experiment
Wheat seeds of most susceptible variety cv. ‘Aus -7-58-0850’ were sown in earthen pots (20 cm diam.) in sterilized sandy loam soil (70 % sand, 21 % silt, 6 % clay and 3 % organic matter). Three concentrations S, S/5 and S/10 of each chemical such as rugby and cartap, biocontrol agent’s TH and PL and plant extracts onion (leaves) Allium cepa, neem (leaves) Azadirachta indica, garlic (cloves) Allium sativum, red chilli (fruit containing seeds) Capsicum annuum, clove (seeds), aloe vera (leaves) Aloe vera and tobacco (leaves) Nicotiana tabacum were used. After one month of sowing, selected chemicals, plant extracts and biocontrol agents were amended in soil to check their protective effect against nematode development. 1500 freshly hatched J2 of MG were inoculated in each pot after one week. Inoculation was done by pipette. CRD design was used with ten replications for each treatment. Plants exposed to amended soil for thirty days was uprooted carefully and transplanted into these infested pots. Harvesting was done at maturity of crop and data were recorded on plant and nematode reproductive parameters. For curative effect, Wheat seeds were sown in earthen pots (20 cm diam.) in sterilized sandy loam soil (71 % sand, 20 % silt, 6 % clay and 3 % organic matter). Then pots were inoculated with 1500 freshly hatched juveniles J2 of Meloidogyne graminicola after one month. Three concentrations S, S/5 and S/10 of each chemicals viz, rugby and cartap, biocontrol agents TH and PL and plant extracts onion (leaves), neem (leaves), garlic (cloves), red chilli (fruit containing seeds), clove (seeds), aloe vera (leaves) and tobacco (leaves) were used. One week after inoculation, soil was amended with selected chemicals, plant extracts and bio control agents in completely randomized design. Harvesting was done at maturity of crop. Data were recorded on plant growth and nematode reproductive parameters such as plant height in cm, root weight in g and number of eggs.
Micro plot experiment
Susceptible variety of wheat (Aus -7-58-0850) was grown in micro plots (7 x 3x 5ft) with known infestation level 142 juveniles\100 cm. Three concentrations S, S/5 and S/10 of each chemical such as rugby, cartap, biocontrol agents Trichoderma harzianum (TH), and Paecilomyces lilacinus (PL) and plant extracts onion (leaves), neem (leaves), garlic (cloves), red chilli (fruit containing seeds), clove (seeds), aloe vera (leaves) and tobacco (leaves) were used. Selected chemicals, plant extracts and bio control agents were applied in micro plots after one week of sowing. RCBD design was used with ten replications. Harvesting was done at maturity of crop. Data was recorded on plant growth and nematode reproduction parameters.
Statistical analysis
The data were subjected to analysis of variance (ANOVA) by M-Stat version 1.3. The treatment means were separated using Tukey’s HSD test at p ≤ 0.05 after analysis of variance.
RESULTS
Evaluation of chemicals, biocontrol agents and plant extracts
Effect on egg hatching of MG
The effect of the different concentration of chemicals, biocontrol agents and plant extracts on the egg hatching of root knot nematode MG was tested (Fig. 1). By using chemicals highest egg hatching (14.5) was recorded in cartap at S concentration and lowest egg hatching (10.7) by rugby using S\10 concentration. By using biocontrol agent’s highest egg hatching (5.8) was recorded in PL using S concentration and lowest egg hatching (2.7) by TH using S\10 concentration. By using plant extracts the highest egg hatching (20.8) was recorded in clove at S concentration and lowest egg hatching (9.2) by onion at S\10 concentration. Results were significantly different from each other at p ≤ 0.05.
Effect on juvenile mortality of MG
The effect of different concentration of chemicals, biocontrol agents and plant extracts on juvenile mortality of root knot nematode against MG was assessed. The effect of all concentrations varied significantly on juvenile’s mortality of MG. By using chemicals highest mortality (100.0) was recorded in rugby and cartap using S and S/5 concentration after 12, 24 and 48 h and lowest mortality (72.5) by rugby at S\10 concentration after 12 h (Table I). Among biocontrol agents highest mortality (26.4) was recorded by TH + PL when applied in combination at S concentration after 48 h and lowest mortality (11.8) was exhibited by TH using S\10 concentration after 24 h (Table II). Among plant extracts highest mortality (100.0) was recorded in neem using S concentration after 12, 24 and 48 h and lowest mortality (15.0) by onion using S\10 concentration after 12 h (Table III). Among plant extracts neem was the most effective in causing mortality at S concentration after 48 h and lowest mortality was recorded in bio control agent TH. The results were significantly different from each other at p ≤ 0.05.
Table I. In vitro evaluation of chemicals on juvenile mortality of MG.
|
Treatment |
Concentration |
Time (Hour) |
||
|
12 |
24 |
48 |
||
|
Cartap |
S |
100.0 a |
100.0 a |
100.0 a |
|
S/5 |
100.0 a |
100.0 a |
100.0 a |
|
|
S/10 |
94.7 b |
100.0 a |
100.0 a |
|
|
Rugby |
S |
100.0 a |
100.0 a |
100.0 a |
|
S/5 |
100.0 a |
100.0 a |
100.0 a |
|
|
S/10 |
72.5 c |
82.3 b |
100.0 a |
|
|
Control |
0.0 d |
0.0 c |
0.0 b |
|
Treatment means were separated using Tukey’s HSD test at p ≤ 0.05.
Table II. In vitro effect of biocontrol agents on juvenile mortality of MG.
|
Treatment |
Concentration |
Time (Hour) |
||
|
12 |
24 |
48 |
||
|
TH |
S |
26.2 ab |
25.4 abc |
25.0 abc |
|
S/5 |
15.2 ijk |
15.2 ijk |
19.0 fgh |
|
|
S/10 |
13.0 kl |
11.8 l |
18.0 ghi |
|
|
PL |
S |
24.6 abcd |
24.2 abcd |
23.8 abcde |
|
S/5 |
21.8 def |
17.0 hij |
21.0 cde |
|
|
S/10 |
16.0 hijk |
15.2 ijk |
13.0 kl |
|
|
TH + PL |
S |
23.2 bcde |
23.0 cde |
26.4 a |
|
S/5 |
20.8 efg |
16.0 hijk |
25.0 abc |
|
|
S/10 |
18.4 gh |
14.4jkl |
13.6 kl |
|
|
Control |
0.3 m |
0.4 m |
0.3 m |
|
Treatment means were separated using Tukey’s HSD test at p ≤ 0.05.
TH, Trichoderma harzianum; PL, Paecilomyces lilacinus.
Effect on plant growth and reproductive parameters of MG in pot experiment
Among biocontrol agents, the highest plant height was recorded 43.3 by using TH in protective effect and lowest 33.8 by using PL in curative effect (Fig. 2). The root weight was not significantly different. The highest number of eggs was recorded in PL in curative effect and lowest by TH in curative effect. By using chemicals, the highest plant height was recorded in rugby in protective effect and lowest by using cartap in curative effect (Fig. 3). The root weight was not significantly different. The highest number of eggs was recorded in cartap in curative effect and lowest by cartap in protective effect. By using plant extracts the highest plant height was recorded in red chilli in protective effect and lowest by using clove in curative effect (Fig. 4). The root weight was not significantly different. The highest number of eggs was recorded in aloe vera in curative effect and lowest by onion in protective effect. In curative effect the highest number of eggs was recorded in aloe vera treatment. The results were significantly different from each other.
Plant growth parameters and reproduction of MG in micro plot experiment
The root weight, number of nodes, number of tillers and grain weight/100 seed were not significantly different (Table IV). By using chemicals highest plant height was recorded in cartap and lowest in rugby. The highest number of females was recorded in cartap and lowest in rugby. The number of juveniles was highest in cartap and lowest in rugby. By using bio control agent’s highest plant height was recorded in TH and lowest in PL. The highest number of females was recorded in PL and lowest in TH. The number of juveniles was highest in PL and lowest in TH. By using plant extracts highest plant height was highest in garlic treatment and lowest by in neem treatment.
The highest number of females was recorded in clove and lowest in tobacco. The number of juveniles was highest in garlic and lowest in neem. The results were significantly different from each other at p ≤ 0.05.
Table III. In vitro effect of plant extracts on juvenile mortality of MG.
|
Treatment |
Concentration |
Time (Hour) |
||
|
12 |
24 |
48 |
||
|
Tobacco |
S |
34.5 defg |
36.5 bcde |
44.0 a |
|
S/5 |
33.6 defgh |
31.0 ghij |
34.5 defg |
|
|
S/10 |
19.5 o |
29.5 hijk |
31.0 ghij |
|
|
Aloe vera |
S |
39.5 b |
37.0 bcd |
34.0 defg |
|
|
S/5 |
34.5 defg |
29.0 ijk |
32.5 efghi |
|
S/10 |
32.5 defg |
27.5 jklm |
32.5 efghi |
|
|
Neem |
S |
100.0 op |
100 lmn |
100 ijkl |
|
S/5 |
85.0 no |
75.6 op |
87.5 hijk |
|
|
S/10 |
70.5 mn |
50.5 hijk |
65.5 b |
|
|
Clove |
S |
39.5 b |
39.0 bc |
39.9 bc |
|
S/5 |
36.4 bcde |
29.0 ijkl |
39.0 bc |
|
|
S/10 |
32.3 bcde |
26.5 klm |
34.5 defg |
|
|
Red chilli |
S |
31.6 ghij |
35.5 bcdef |
36.5 bcde |
|
S/5 |
31.0 ghij |
34.0 defg |
34.5 defg |
|
|
S/10 |
21.0 no |
29.0 ijkl |
31.5 fghij |
|
|
Garlic |
S |
29.0 ijkl |
31.0 ijkl |
35.5 bcdef |
|
S/5 |
28.9 ijklm |
30.2 ijkl |
34.9 cdefg |
|
|
S/10 |
26.5 ijkl |
29.0 ijkl |
29.0 ijkl |
|
|
Onion |
S |
29.0 ijkl |
34.5 defg |
35.9 defg |
|
S/5 |
19.5 o |
30.5 ijkl |
34.5 defg |
|
|
S/10 |
15.0 p |
29.0 ijkl |
29.2 ijkl |
|
|
Control |
- |
0.1 q |
0.3 q |
0.6 q |
Treatment means were separated using Tukey’s HSD test at p ≤ 0.05.
DISCUSSION
Root knot nematode MG is a very important group of plant parasitic nematode occurring in all regions but more present in those areas having humid and hot environment. RKN not only affect the yield but also decrease market value and quality of fruits. In the present investigation synthetic chemicals, biocontrol agents and plant extracts were evaluated for their suppressive effect on MG. The nematicides evaluated in this study exhibited in vitro and in egg hatching and caused juvenile mortality. It has been stated that the most successful control of MG is through application of nematicides and flooding the soil (Le et al., 2009). The suppressive potential of nematicides is attributed to toxicity of active ingredients. The toxicity of nematicides has been previously reported against MG (Khan et al., 2012).
Biocontrol agent’s TH and PL showed egg hatching inhibition and juvenile mortality in vitro. Both biocontrol agents were antagonistic against MG. Soils naturally contain a reservoir of microbes including antagonistic microbes having the ability to reduce the reproduction of Meloidogyne spp. (Sikora, 1992). Suppressive soils reduced the reproduction of MG due to the microbial diversity (Bent et al., 2008). High suppression of nematodes was observed by endophytic fungi and bacteria that infect nematode eggs (Whipps and Davies, 2000). Members of Trichoderma and Purpureocillium genera reduced the population of Meloidogyne spp. (Dababat et al., 2006;
Table IV. Combined efficacy of chemicals, bio control agents and plant extracts against MG in micro plots.
|
Treatment |
Root weight (g) |
Number of nodes |
Number of tillers |
Plant height (cm) |
Grain weight/100 seed |
Number of females |
Number of juveniles |
|
Cartap |
0.83 a |
4 a |
10 a |
87.7 a |
4.13 a |
133.0 a |
1373 a |
|
Rugby |
0.85 a |
4 a |
10 a |
83.8 a |
4.13 a |
127.8 b |
1215 b |
|
Control |
0.73 a |
4 a |
10 a |
86.0 ab |
3.99 a |
0.3 c |
0.60 c |
|
PL |
0.85 a |
4 a |
10 a |
88.4 a |
4.12 a |
135.8 a |
1383 a |
|
TH |
0.85 a |
4 b |
10 a |
93.5 a |
4.19 a |
130.2 b |
1374 a |
|
Control |
0.66 b |
4 b |
10 a |
61.7 b |
4.07a |
0.3 c |
0.4 b |
|
Onion |
0.86 a |
4 a |
10a |
84.6 ab |
4.13 a |
130.2 cd |
1374 a |
|
Tobacco |
0.88 a |
4 a |
10 a |
77.5 b |
4.00 a |
127.8 d |
1215 d |
|
Red chilli |
0.86 a |
4 a |
10 a |
79.6 b |
4.12 a |
135.0 ab |
1315 c |
|
Aloe vera |
0.82 a |
4 a |
10 a |
84.7 ab |
4.09 a |
133.0 abc |
1373 a |
|
Garlic |
0.85 a |
4 a |
10 a |
99.3 a |
4.17 a |
135.8 a |
1383 a |
|
Neem |
0.82 a |
4 a |
10 a |
75.2 b |
4.18 a |
131.0 bcd |
1192 e |
|
Clove |
0.86 a |
4 a |
10 a |
86.0 ab |
3.99 a |
135.6 a |
1345 b |
|
Control |
0.70 b |
4 a |
10 a |
63.7 c |
4.10 a |
0.2 e |
0.5 f |
Treatment means were separated using Tukey’s HSD test at p ≤ 0.05.
For abbreviations, see Table II.
Affokpon et al., 2011; Wilson and Jackson, 2013). In support of our work Murslain et al. (2014) reported the suppression of M. incognita by using TH. Biological control agents such as Fusarium and Trichoderma were effective for management of MG in rice (Le et al., 2008). In lab experiment the use B. megaterium culture delayed egg hatching and reduced J2 population (Padgham et al., 2004). The mixtures of P. fluorescens strains with PF1+TDK1+PY15 applied as bacterial suspensions as a seed treatment reduced the population of MG (Seenivasan et al., 2012).
Plant extracts evaluated in this study caused egg hatching inhibition and juvenile mortality of MG. In support of our findings Haroon et al. (2018) evaluated the effect of four plant extracts Azadirachta indica, Moringa oleifera, Lantana camara, and Glycyrrhiza glabra and reported that all plant extracts decreased egg hatching of Meloidogyne spp. The mortality of root-knot nematodes can be attributed to toxic compounds in botanical extracts that inhibit the development of nematodes (Chopra et al., 1963). Among toxic compounds found in botanical extracts some of them like phenolics (Hasan and Saxena, 1974), fatty acids (Tarjan and Cheo, 1956; Loos, 1958), and alkaloids were inhibitory against nematodes. Therefore, the efficacy of indigenous botanical extracts should be further investigated at field level.
The protective and curative efficacy of botanical extracts, biocontrol agents and plant extracts were also evaluated in microplots to reduce the number of eggs and plant growth promoting effect. The curative inhibitory effect of nematicides phorate, carbofuran, carbosulfan and chlorpyrifos against MG has been on egg mass production and nematode population and increment in plant growth has been reported previously (Khan et al., 2012). The reported significant increment in plant root weight. The protective and curative treatment with TH and PL reduced the number of eggs and caused an increment in plant height and root weight. In agreement with our findings Narasimhamurthy et al. (2017) reported the biocontrol potential and plant growth promoting effect of PL and TH in field conditions against MG. The nematicidal efficacy of plant extracts such as Azadiracta indica, Curcuma longa, Zingiber officinale and Eucalyptus globules and plant growth promoting effect has been reported previously against MG under pot conditions (Shukla and Chand, 2017). The application of P. fluorescens, TH and carbofuran in soil reduced the (40-46 %) gall formation, (45-57 %) egg mass production and (56-64 %) soil population of MG and increased 37-42 % plant growth (Ziaul, 2013). The treatments investigated in present study has exhibited suppression of MG on wheat. Therefore, these treatments should be further investigated at large scale and as components of integrated disease management program of MG.
CONCLUSION
Botanical extracts, biocontrol agents and chemicals used in this study were effective to cause juvenile mortality, egg hatching inhibition, reduction in nematode reproduction parameters and growth promotion of the plants. Antagonistic agents TH and PL, neem and tobacco extracts and cartap with highest egg hatching inhibition and mortality can be used in combinations or as components of integrated disease management program of MG.
Statement of conflict of interest
The authors declare no potential conflicts of interest that could jeopardize this work.
REFERENCES
Abbasi, M.W., Ahmad, N., Zaki, M.J. and Shaukat, S.S., 2008. Effect of Barleria acanthoides vahl: On root knot nematode infection and growth of infected okra and brinjal plants. Pak. J. Bot., 40: 2193-2198.
Abbott, W.S., 1925. A method of computing the effectiveness of an insecticide. J. econ. Ent., 18: 265-267. https://doi.org/10.1093/jee/18.2.265a
Affokpon, A., Coyne, D.L., Htay, C.C., Agbede, R.D., Lawouin, L. and Coosemans, J., 2011. Biocontrol potential of native Trichoderma isolates against root knot nematodes in West African vegetable production systems. Soil Biol. Biochem., 13: 600-608. https://doi.org/10.1016/j.soilbio.2010.11.029
Al-Hazmi, A.S., 1982. Biology of Meloidogyne platani Hirschmann parasitic onsycamor, Platanus occidentalis. J. Nematol., 14: 154-161.
Bridge, J., Plowright, R.A. and Peng, D., 2005. Nematode parasites of rice. In: Plant parasitic nematodes in subtropical and tropical agriculture (eds. M. Luc, R.A. Sikora, and J. Bridge), 2nd Edition. CABI Publishing, Wallingford., UK. 87-130. https://doi.org/10.1079/9780851997278.0087
Bridge, J.L., Michel and Sikora, R.A., 1990. Nematode parasites of rice. In: Plant parasitic nematodes in subtropical and tropical agriculture (eds. M. Luc, R.A. Sikora and J. Bridge), CABI., U.K. 69-108.
Bent, E., Loffredo, A., McKenry, M.V., Becker, J.O. and Borneman, J., 2008. Detection and investigation of soil biological activity against Meloidogyne incognita. J. Nematol., 40: 109-118.
Chopra, C.N., Chopra, I.C. and Verma, B.S., 1963. Glossary of Indian medicinal plants, Publication and Information, Directorate New Delhi.
Dahal, G., Amatya, P. and Manandhar, H.K., 1992. Bibliographic database on plant pathology in Nepal. J. Nematol., 13: 53-64.
Dababat, A.A., Sikora, R.A. and Hauschild, R., 2006. Use of Trichoderma harzianum and Trichoderma viridae for biological control of Meloidogyne incognita on tomato. Commun. Agric. appl. biol. Sci., 71: 953-961.
Davis, E.E. and Venettee, R.C., 2004. British root knot nematode: Meloidogyne artiellia Franklin (Nematode: Meloidogynidae). Dept. of Entomology., University of Minnesota. 1-19.
Eisenback, J.D., Hirschmann, H., Sasser, J.N. and Triantaphyllou, A.C., 1981. A guide to the four most species of root knot nematodes Meloidogyne spp. with a pictorial key. North Carolina State Uni. Graphics and USAID., Raleigh. pp. 48.
El- Rokick, K.G. and El- Nagdi, W.M., 2011. Dual effect of leaf extracts of Eucalyptus citriodora on controlling purslane and root knot nematode in sunflower. J. Pl. Prot. Res., 51: 121-129. https://doi.org/10.2478/v10045-011-0021-0
Gaur, H.S. and Sharma, S.N., 1999. Relative efficacy of bioassay and extraction of juveniles from soils for detection and estimation of populations levels of the root knot nematodes. Meloidogyne graminicola and M. tritocoryzae. Annls. Pl. Prot., 7: 75-79.
Haque, Z., Khan, M.R. and Ahamad, F., 2018. Relative antagonistic potential of some rhizosphere biocontrol agents for the management of rice root-knot nematode, Meloidogyne graminicola. Biol. Contr., 126: 109-116. https://doi.org/10.1016/j.biocontrol.2018.07.018
Haroon, S.A., Hassan, B.A.A., Hamad, F.M.I. and Rady, M.F., 2018. The efficiency of some natural alternatives in root-knot nematode control. Adv. Pl. agric. Res. 8: 355-362. https://doi.org/10.15406/apar.2018.08.00337
Hasan, N. and Saxena, S.K., 1974. Effects of different concentrations of commonly occurring phenolic compounds on the larval hatching of Meloidogyne incognita (Kofoid and White) Chitwood. Ist Ann. Meet. Soc. Adv. Bot. Munit., 27-28.
Husman, S., 1996. TeloneII and Temik efficiency on root knot nematodes in cotton. Cotton Report, College of Agriculture., Series. pp. 103.
Hussey, R.S. and Barker, K.R., 1973. A comparison of methods of collecting inocula of Meloidogyne spp including a new technique. Pl. Dis. Rep. 57: 1025–1028.
Jepson, S.B., 1987. Identification of root knot nematodes (Meloidogyne species). CAB Inter, Wallingford., UK,
Khan, M.R., Zaidi, B. and Haque, Z., 2012. Nematicides control rice root-knot, caused by Meloidogyne graminicola. Phytopathol. Mediterr., 51: 298-306.
Kumar, D., Khilari, K., Kumar, N. and Kumar, S., 2017. Integrated disease management of rice root knot nematode (Meloidogyne graminicola) through organic amendments, Trichoderma spp. and carbofuran. J. Pharma. Phytochem., 6: 2509-2515.
Le, H.T., Padgham, J.L. and Sikora, R.A., 2008. Combinations of fungal and bacterial antagonists for biological control of the rice root knot nematode Meloidogyne graminicola. Proceeding of conference held at Hohenheim University, Stuttgart, Germany pp. 31-36.
Le, H.T., Padgham, J.L. and Sikora, R.A., 2009. Biological control of the rice root knot nematode Meloidogyne graminicola on rice using endophytic and rhizosphere fungi. Int. J. Pest. Manage., 55: 31-36. https://doi.org/10.1080/09670870802450235
Loos, C.A., 1958. Certain fatty acids and hexadecyclamine as nematicides. Pl. Dis. Rep., 42: 1179-1186.
Muhae-ud-Din, G., Moosa, A., Ghummen, U.F., Jabran, M., Abbas, A., Naveed, M., Jabbar, A. and Ali, M.A., 2018. Host status of commonly planted ornamentals to Meloidogyne incognita and management through endophytic bacteria. Pakistan J. Zool., 50: 1393-1402. https://doi.org/10.17582/journal.pjz/2018.50.4.1393.1402
Mukhtar, T., Ahmed, R. and Ahmed, M.S., 2004. Some studies on the control of citrus nematode (Tylenchulus semipenetrans) by leaf extracts of three plants and their effects on plant growth variables. Asian J. Pl. Sci., 3: 544-548. https://doi.org/10.3923/ajps.2004.544.548
Murslain, M., Javed, N., Khan, S.A., Khan, H.U., Abbas, H. and Kamran, M., 2014. Combined efficacy of Moringa oleifera leaves and a fungus, Trichoderma harzianum against Meloidogyne javanica on eggplant. Pakistan J. Zool., 46: 827-832.
Nandi, B., 2018. Control of root-knot nematode, Meloidogyne incognita (Kofoid and White) Chitwood on tomato plant with some plant extracts. Indian J. Nematol., 48: 42-52.
Narasimhamurthy, H.B., Ravindra, H. and Mukesh Sehgal, S.D.E., 2017. Bio-management of rice root-knot nematode (Meloidogyne graminicola). J. Entomol. Zool. Stud., 5: 1433-1439.
Netscher, C. and Luc, M., 1974. Nematodes associated with crops vegetables in Mauritania. Agron. Trop., 29: 697-701.
Ngadze, E., 2014. In vitro and greenhouse evaluation of botanical extracts for antifungal activity against Phytophthora infestans. J. Biopestic., 7:199-204.
Padgham, J., Mazid, L., Duxbury, J.M., Abawi, G.S. and Hossain, M., 2004. Yield loss caused by Meloidogyne graminicola on lowland rain fed rice in Bangladesh. J. Nematol., 36: 42-48.
Padgham, J.L., 2003. Impact of the rice root knot nematode (Meloidogyne graminicola) on lowland rainfed rice in Northern western Bangladesh. Ph.D. dissertation, Cornell University, Ithaca., NY. pp: 148.
Pokharel, R.R., Abawi, G.S., Duxbury, J.M. and Smart, C., 2004. Characterization of root knot nematodes recovered from rice wheat fields in Nepal. J. Nematol., 36: 341-342.
Samaliev, H., Markova, D., Nikolova, M. and Baicheva, O., 2017. Management of root-knot nematode Meloidogyne hapla on strawberry plant with some plant extracts. Russian parasitol. J., 4: 42.
Shahina, F., Zarina, B., Firoza, K., Soomro, M.H. and Javed, N., 2009. Research on root knot nematode, Meloidogyne by Pakistani scientists: A review with description of species prevalent in Pakistan. Pak. J. Nematol., 27: 1-115.
Shukla, A. and Chand, R., 2017. Studies on the bio-efficacy of botanicals against rice root-knot nematode, Meloidogyne graminicola development. Annls Pl. Biol. Crop Res., 1: 35-40.
Sikora, R.A. 1992. Management of the antagonistic potential in agricultural ecosystems for the biological control of plant parasitic nematodes. Annu. Rev. Phytopathol., 30: 245-270. https://doi.org/10.1146/annurev.py.30.090192.001333
Sikora, R.A., 1993. Nematode parasites of food legumes. In: Plant parasitic nematodes in subtropical and tropical agriculture (eds. M. Luc, R.A. Sikora, J. Bridge), Institute of Parasitology, CAB International, Wallingford, UK, pp. 629.
Singh, N.D., 1975. Effect of inoculums levels and plant age on pathogenecity of Meloidogyne incognita and Rotylenchulus reniformis to tomato and lettuce. Pl. Dis. Rep. 9: 905-908.
Soomro, M.H. and Hauge, N.G.M., 1992. Relationship between inoculums density of Meloidogyne graminicola, growth of rice seedling and development of the nematode. Pak. J. Nematol., 11:103-114.
Seenivasan, N., David, P.M., Vivekanandan, M. and Samiyappan, R., 2012. Biological control of rice root knot nematode Meloidogyne graminicola through mixture of Pseudomonas fluorescens strains. Biocont. Sci. Technol., 6: 611-632. https://doi.org/10.1080/09583157.2012.675052
Talavera, M. and Mizukubo, T., 2003. Infulence of soil conditions, spore densities and nematode age on Pasturia penetrans attachment to Meloidogyne incognita. Spanish J. agric. Res. 1: 57-63. https://doi.org/10.5424/sjar/2003014-54
Tarjan, A.C. and Cheo, A.C., 1956. Nematicidal value of some fatty acid. Bull Rohde Island Agric. Exp. Stat., pp. 41.
Taylor, A.L and Sasser, J.N., 1978. Biology identification and control of root knot nematodes., North Carolina State University Graphics, Raleigh, NC, USA. pp. 111.
Whitehead, A.G. and Hemming, A.K., 1965. Comparison of quantitative method of extracting small vermiform nematodes from soil. Annls. appl. Biol., 55: 25-38. https://doi.org/10.1111/j.1744-7348.1965.tb07864.x
Whipps, J.M. and Davies, K.G., 2000. Success in biological control of plant pathogens and nematodes by microorganisms. In: Measures of success in biological control (eds. G. Gurr and S.D. Wratten). Kluwer Academic Publishers Dordrecht. The Netherlands. pp. 231-269. https://doi.org/10.1007/978-94-011-4014-0_8
Wilson, M.J., and Jackson, T.A., 2013. Progress in the commercialization of bio nematicides. Bio Control. 58: 715–722.
Ziaul, 2013. Development of integrated nematode management module for rice root knot disease caused by Meloidogyne graminicola: A success story for endemic area. Conference Proceeding National Symposium on Nematode. A Friend and Foe to Agri-horticultural Crops, at solan, (H.P.), India.
To share on other social networks, click on any share button. What are these?










