Impact of Different Integrated Pest Management Modules on Pest Infestation, Pesticide Residue and Yield in Mango Fruits
Impact of Different Integrated Pest Management Modules on Pest Infestation, Pesticide Residue and Yield in Mango Fruits
Muhammad Asif Farooq1*, Muhammad Jalal Arif1, Muhammad Dildar Gogi1, Bilal Atta2 and Ahmad Nawaz1
1Department of Entomology, Faculty of Agriculture, University of Agriculture, Faisalabad, Punjab, Pakistan; 2Rice Research Institute, Kala Shah Kaku, Punjab, Pakistan.
Abstract | Integrated pest management (IPM) relies on a merger of rational practices and always considered as an effective and environment-friendly approach to managing pests. The present study was conducted at different locations in the Multan District, Punjab Province, Pakistan for two consecutive years 2016-17 to devised four IPM modules (PRMM-1, PRMM-2, PRMM-3, and PRMM-4) for mango to evaluate their impact on insect pests, pesticide residues and yield in mangoes. The modules were based on a combination of different IPM tactics to suppress the pest population with no or minimal use of insecticides. Each module was applied in an area of 0.405 ha of a mango orchard. Fruit samples collected from all modules were subjected to analytical analysis using QuECHERS for sample preparation followed by quantification with GC-ECD. Recoveries of the samples analyzed were ranged from 78-98%. Results revealed that 75.00% samples of pesticide residues mitigation in PRMM-4 followed by PRMM-3 (66.66%), PRMM-2 (33.33%) and PRMM-1 (25.00%). Similarly, the highest number of samples (41.66%) from PRMM-4 exceeded maximum residual limit (MRL) values of the Codex Alimentarius Commission while the lowest (16.66%) were observed in PRMM-1. In addition, all the modules showed a significant difference in pest population reduction of Bactrocera spp., Drosicha mangiferae and Idioscopus clypealis. Although, pesticide contamination was higher in PRMM-1, however PRMM-2 was found best module when compared in terms of pest population reduction (90.79%), average yield and cost-benefit ratio (1:63.28). Conclusively, the pesticide residues can be minimized by applying different control measures with proper integration.
Received | September 08, 2019; Accepted | December 26, 2019; Published | December 29, 2019
*Correspondence | Muhammad Asif Farooq, Department of Entomology, Faculty of Agriculture, University of Agriculture, Faisalabad, Punjab, Pakistan; Email: asiff06@hotmail.com
Citation | Farooq, M.A., Arif, M.J., Gogi, M.D., Atta, B., Nawaz, A. 2019. Impact of different integrated pest management modules on pest infestation, pesticide residue and yield in mango fruits. Journal of Innovative Sciences, 5(2): 72-82.
DOI | http://dx.doi.org/10.17582/journal.jis/2019/5.2.72.82
Keywords | Mango, Integrated pest management, Module comparison, Pest population, Pesticide residues
1. Introduction
A number of insect pests, fruit flies (Bactrocera spp.), mealy bug (Drosicha mangiferae) and hopper (Idioscopus clypealis) cause both qualitative losses in mango (Peña et al., 2002; Nault et al., 2003). Pesticides are used to reduce yield losses and considered as an economical, labor-saving and efficient contrivance of pest management (Damalas and Eleftherohorinos, 2011). Chlorpyrifos, lambda-cyhalothrin, profenophos, deltamethrin, bifenthrin (Gulzar et al., 2015), neonicotinoids and carbamates are recommended for the management of pests causing damage in mango orchards (Aslam et al., 2004). These pesticides or their residues in mango are potential candidates for health hazards in the indigenous population of the country (Farooq et al., 2019). Residues of cypermethrin, cyfluthrin, methyl parathion, dieldrin, monocrotophos, and methamidophos have been detected in different varieties of mango (Shah et al., 2007; Khan et al., 2009). Pesticide residue monitoring and risk evaluations are options and imperious steps to muddle through the scenario and mitigate the health risks associated with pesticide use (Handa et al., 1999; Anwar et al., 2011). Different techniques have been used recently for this work (Vidal et al., 2002; Tao et al., 2009). These techniques performed and excellent job in the detection and separation of pesticide residues from fruits and other matrices (Amvrazi and Tsiropoulos, 2009). Similarly, extraction solvents used for these studies also important because these chemicals provide high polarity and high recovery of pesticide residues (Knežević and Serdar, 2009; Wang et al., 2012).
IPM has been experimentally proven to be significantly more effective than conventional methods of pest control such as biological, cultural and chemical alone (Pedigo, 1996) and attributes the least risks while generating higher outputs with the least expenses (Clercq et al., 2011). Considerate and applicable use of economic decisions is significant in crop production while dealing with the pest populations to increase the yield, whereas minimizing the management cost in terms of resources and environmental safety (Baker et al., 2002; Tang and Cheke, 2008). IPM is a long term approach which combines highly compatible pest management tactics (Hassan and Bakshi, 2005; Khan et al., 2010) such as cultural (Charles et al., 2000), physical (Atta et al., 2019a), biological (Atta et al., 2019b; Pickett et al., 2010; Rizwan et al., 2019a; 2019b) and also rational chemical control (Pilgrim et al., 2010) to reach an endurable economic levels of pest populations (Tang and Cheke, 2008). It also attributes the least risks while generating higher outputs with the least expenses (Wright et al., 2005; Clercq et al., 2011). The economic decision levels are the major components of any cost-effective IPM program (van Lenteren and Woets, 1988; van Lenteren, 2000). Compatible pest management measures must be integrated to make IPM programs more effective (Grasman et al., 2001). Therefore, the present field study was performed to investigate the pesticide residues in mango fruits and the integration of different techniques to mitigate pesticide residues and their impact on cost-effective mango production.
2. Materials and Methods
2.1 Pesticide residue mitigation modules for mango
Five mango orchards, having commercial mango variety “Chounsa” (heavily infested with D. mangiferae, Bactrocera spp. and I. clypealis) were selected in the Multan district, Pakistan. Four IPM based pesticide residue mitigation modules (PRMM-1, PRMM-2, PRMM-3 and PRMM-4) were designed in comparison to control (Table 1). The IPM module practices were performed on a year-round basis in randomized completely block design (RCBD) with three replications. Conventionally grown mango orchard was also maintained (PRMM-4) with all the regular practices by the farmer while no chemical was applied in control. The pest population was determined from the marked unit area (0.46 m above the ground) on the trunk of the trees for D. mangiferae. The number of larvae in the fruits and the number of adults in the traps were recorded for Bactrocera spp., while the number of nymphs and adults per inflorescence or pest per sweep were observed for I. clypealis. The data was recorded for the year 2016-17. The impact of all modules was observed through pest population reduction at different intervals. Percent population reduction (PPR) over control was calculated using the following formula (Farooq et al., 2019).
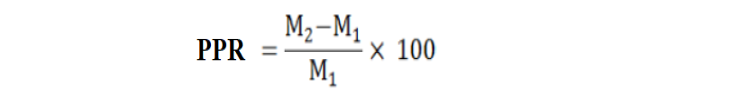
Where;
M1= Average population in treatment; M2 = Average population in control.
2.2 Pesticide residue analysis
A random sampling of mango fruits was performed to obtain 1000g of the sample from each block. Packed and marked fruit samples were transported in ice coolers to the laboratory for analysis (Cook, 2002). Samples were homogenized and only edible parts of the fruits were used for analysis. Samples were stored at -40°C, if the analysis was not performed immediately (Chowdhury et al., 2013).
All solvents and reagents were of HPLC grade such as anhydrous magnesium sulphate (MgSO4), acetonitrile (MeCN), primary secondary amines (PSA) and anhydrous sodium acetate (NaAc) were used for sample preparation. Insecticide reference standards were purchased from SIGMA-ALDRICH®. Insecticides such as lambda-cyhalothrin, cypermethrin, indoxacarb, imidacloprid, pyriproxyfen, acetamiprid, buprofezine, and chlorpyrifos were analyzed (Qin et al., 2015). The purity of all pesticide standards and other chemicals was not less than 98% (Bakırcı et al., 2014).
For extraction and clean up, QUeCHERS (AOAC) method was followed (Zhao et al., 2007). The 100 μl of internal standard was added in accordance with the target pesticides then 6g of MgSO4 and 1.05g NaOac were added in the homogenized sample in 15 ml vial and shaken with hand for at least a minute. The vial with mixture was centrifuged at 5000 rpm for 5 mins, 1.05 ml of supernatant was taken in the vial containing 2 ml of dispersive SPE (primary and secondary amides and MgSO4). The mixture was shaken with the hand and centrifuge for 5 mins at 10×1000 rpm. A supernatant into a lid vial was left for the overnight centrifuge to dry and 100 μl of acetonitrile was added and vortex to re-suspend the supernatant. The sample was placed in a centrifuge for 1 min to separate any possible solids and was transferred into LC vials for analysis (Anastassiades et al., 2003; Martínez-del-Río et al., 2013; Rejczak and Tuzimski, 2015). Extraction and clean-up were performed using the QuECHERS AOAC method and kits were purchased from Agilent Technologies® for mango (5982-5755+5982-5058) where the first number is the part number for Extraction kit while the latter is kit number for clean-up kit.
Gas Chromatograph equipped with mass spectrometry (MS) was used under specific operational conditions (temperature, flow rate) for optimum behavior and quantitative recoveries (Tao et al., 2009). Residues were identified on the basis of their respective retention times while quantification on the basis of respective peak areas was reported on the basis of sample weight (mg kg-1). All spikes and method blank samples were processed through the analytical method (Zhao et al., 2007). Quantification was based on an external standard calculation using the peak area.
For the determination of recovery, precision and detection limits, pesticide-free samples (blank samples) were used (Lehotay, 2007) conducted by analyzing apple matrices as representative matrices for fruits. Samples were augmented five times at 0.05, 0.1 and 0.50 mg kg-1. The detection limit was derived from the analysis of 10 independent sample blanks fortified at the lowest concentration for acceptable recoveries (between 78% and 98%) and precision (RSD) lower than 20% (Bakırcı and Hışıl, 2011).
2.3 Cost-Benefit Ratio
Cost of all the inputs (fungicides, insecticides, fertilizers, and irrigation), cost of farm mechanization, packaging, transportation, and labor was estimated. The total revenue was calculated from the yield and market price of the product for all PRMMs. All the values were put into the following formula (Lu et al., 1999) to calculate the cost-benefit ratio (CBR).
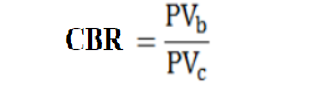
Where;
PVb= All cost received including benefits; PVc= All cost spent.
3. Results and Discussion
All the modules showed a significant difference in the pest population reduction. PRMM-2 showed maximum population reduction for D. mangiferae (91.30%) followed by PRMM-1 (86.27%), PRMM-3 (77.55%) and PRMM-4 (74.46%). Similarly, the maximum population reduction of Bactrocera spp. was observed in PRMM-2 (92.85%) followed by PRMM-1 (81.25%), PRMM-3 (76.47%) and PRMM-4 (73.33%). The analysis revealed a non-significant difference between PRMM-1 and PRMM-2 for population reduction of I. clypealis and caused 83.33% and 88.23% reduction in the population of I. clypealis, respectively. Similarly, PRMM-3 and PRMM-4 showed 73.33% and 68.75% population reduction of I. clypealis, respectively in comparison to control (Table 2).
Overall, maximum pest reduction was observed in PRMM-2 (90.79%) in which different control tactics (cultural, mechanical, and attract and kill methods) including pesticides were integrated. While the minimum pest population reduction was observed in PRMM-4 in which farmer practices (pesticides) were applied to manage the pests. PRMM-2 performed 18.61% better than the chemical method in terms of pest population reduction, while in PRMM-1 (cultural + mechanical + attract and kill methods) pest reduction was 11.43% higher over chemical control method which is in common practice by most of the farmer community in the country (Table 2).
| Module | Insect pest control strategy | |||
| Cultural | Mechanical | Attractants and kill | Chemical | |
| PRMM-1 |
|
Bands of plastic sheets and 4 cm grease applied at the height of 0.46 to 0.62 on the trunk. Bands were applied on the smooth surface of mud and wet FYM (1:1) to collect egg-laying female D. mangiferae. |
|
No application |
| PRMM-2 | -do- | -do- | -do- |
Tracer® (Spinosad) at the rate of 10 ml/acre with 100 liters of water. |
| PRMM-3 | No application | No application | Methyl eugenol + Spinosad (6-8 drops of M.E and 3-4 drops of Spinosad on the pluck of cotton and placed in a trap) 6 traps/ acre, traps refreshed at 12-15 days interval. |
|
| PRMM-4 | No application | No application | No application |
|
| Control | No application | No application | No application | No application |
Table 2: Percent reduction of pest population in pesticide residue mitigation modules in mango orchard.
| Pest |
IPM Module |
||||
| PRMM-1 | PRMM-2 | PRMM-3 | PRMM-4 | Control | |
| Drosicha mangiferae | 86.27 | 91.30 | 77.55 | 74.46 | 0 |
| Idioscopus clypealis | 83.33 | 88.23 | 73.33 | 68.75 | 0 |
|
Bactrocera spp. |
81.25 | 92.85 | 76.47 | 73.33 | 0 |
| Overall Reduction (%) | 83.61 | 90.79 | 75.78 | 72.18 | 0 |
Table 3: Concentration of Pesticide residues quantified in Mango samples collected from different Modules (Maximum-Minimum).
| Pesticide | MRLs | PRMM-1 | PRMM-2 | PRMM-3 | PRMM-4 | Control |
Recoveries ± SD |
||
|
Fortification level (mg/kg-1) |
|||||||||
| 0.05 | 0.10 | 0.50 | |||||||
| Lambda cyhalothrin | 0.2 | 0.2037-0.0050 | 0.2425-0.0105 | 0.2675-0.0035 | 0.2679-0.0090 | 0.0023-ND | 91 ± 0.52 | 94 ± 0.36 | 90 ± 0.56 |
| Cypermethrin | 0.7 | 0.0706-0.0069 | 0.7622-0.0095 | 0.8264-0.0143 | 0.7953-0.0154 | 0.0203-0.0145 | 90 ± 0.63 | 95 ± 0.17 | 91 ± 0.54 |
| Indoxacarb | 0.02 | ND-ND | ND-ND | 0.0775-0.0196 | 0.0698-0.0052 | ND-ND | 96 ± 0.12 | 94 ± 0.71 | 85 ± 0.52 |
| Imidacloprid | 0.2 | 0.3434-0.0097 | 0.2325-0.0018 | 0.3376-0.0155 | 0.3630-0.0257 | 0.0109-ND | 89 ± 0.10 | 91 ± 0.33 | 92 ± 0.15 |
| Pyriproxyfen | 0.5 | ND-ND | ND-ND | 0.0406-0.0090 | 0.5864-0.0056 | ND-ND | 90 ± 0.56 | 93 ± 0.31 | 95 ± 0.28 |
| Acetamiprid | 0.01 | 0.1596-0.0063 | 0.0140-0.0052 | 0.0250-0.0068 | 0.0315-0.0047 | 0.0145-ND | 94 ± 0.43 | 97 ± 0.27 | 89 ± 0.26 |
| Buprofezine | 0.9 | 0.9152-0.0064 | 0.9193-0.0368 | 0.9391-0.0491 | 0.9650-0.0016 | ND-ND | 95 ± 0.49 | 93 ± 0.64 | 88 ± 0.51 |
| Chlorpyrifos | 0.05 | 0.0534-0.0031 | 0.0622-0.0054 | 0.0620-0.0066 | 0.0570-0.0035 | 0.0096-0.0075 | 96 ± 0.35 | 94 ± 0.09 | 95 ± 0.26 |
Table 4: Cost-benefit for different pesticide residue mitigation modules in mango fruits.
| Module |
Yield (Kg/Acre) |
Marketable yield (Kg/Acre) |
Gross return (USD) |
Increased yield over control (Kg/acre-1) |
Value of increased yield over control (USD) | Management cost (USD) |
Net profit (USD) |
CBR |
| PRMM-1 | 18341 | 14880 | 6645.29 | 6160 | 2231.88 | 40.94 | 2191.85 | 53.51 |
| PRMM-2 | 19106 | 16287 | 6922.46 | 7567 | 2741.67 | 42.66 | 2699.93 | 63.28 |
| PRMM-3 | 17280 | 13473 | 6260.87 | 4753 | 1722.10 | 55.08 | 1667.03 | 30.27 |
| PRMM-4 | 16723 | 11945 | 6059.06 | 3225 | 1168.48 | 52.18 | 1116.30 | 21.40 |
| Control | 14545 | 8720 | 5269.93 | - | - | - | - | - |
| * 1 USD = 138 PKR (Pakistani Rupees) | ||||||||
The pesticide residue values were compared with MRL values of the Codex Alimentarius Commission. The fruit samples collected from PRMM-4 treated orchard were found highly contaminated (75.00%) with 41.66% samples exceeded MRL, while pesticide contamination in PRMM-1 treated orchard was 25.00% and 8.33% samples were above MRL. Therefore, PRMM-1 samples were the least contaminated with pesticides. PRMM-2 and PRMM-3 showed 33.33% and 66.66% pesticide contamination, respectively. The PRMM-3 was second-most contaminated plot with 33.33% samples exceeding residual limits while PRMM-2 ranked third with 16.66% samples above MRL. The comparison with PRMM-4 (pesticide-treated plot), it was observed that in PRMM-2 was 41.67% less contaminated while PRMM-1 was 50.00% less contaminated with pesticide residues. In addition, the control treatment showed 8.33% contaminated samples with pesticide residues while no sample surpassed the limits. So there was very significant difference in contamination and residual limits in IPM and conventionally grown mango (Table 3; Figure 1). Recoveries for the pesticides analyzed was ranged from 89-96% at 0.05 mg kg-1, 93-97% at 0.10 mg kg-1 and 85-95% at 0.50 mg kg-1 concentration levels with RSD less than 20% (Table 3).
The application cost of all the inputs (insecticides, fungicides, fertilizers, irrigation, farm mechanization, packaging, transportation, and labor) was calculated for all modules. As the modules were compared for the cost of pest management, therefore, only costs of insecticides and IPM (sanitation, sticky band, slippery band, attract and kill tactics such as methyl eugenol combined with Spinosad and GF-120) were used to determine the CBR for all evaluated modules. The results of CBR indicated that PRMM-2 resulted in 1:64.69 followed by that of PRMM-1 (1:54.75), PRMM-3 (1:30.27) and PRMM-4 (1:21.40) (Table 4).
In this experiment, the mango was grown under four different PRMM. In the present study, the integration of soft insecticides in IPM caused 92.66% reduction in the population of Bactrocera spp. This shows that the combinations of multiple control tactics are more reliable for effective pest management in mango. The use of methyl eugenol and protein hydrolysate achieved 83.00% reduction in Bactrocera spp. population (Ndiaye et al., 2008). It has been reported the reduction in infestation in Bactrocera spp. was observed with cultural (90%), MAT (100%), BAT (60%), cover spray of insecticides (50%) and integration of MAT with cultural method (100%) (Patel at el., 2005). Similar results were observed when methyl eugenol and GF-120 were integrated with spinosad in PRMM-2 (90.79%). A combination of MAT, sanitation (as cultural practice) and methyl eugenol was used to suppress the population of Bactrocera spp. in mango (Verghese et al., 2006). The integration of these three control measures resulted in 95.00% reduction in the population of Bactrocera spp. as compared to control (67.00% infestation). These results are slightly different from the results of the present study where a combination of sanitation, mechanical, GF-120, methyl eugenol, and cover spray caused 90.79% reduction in the pest population. Differences in the results are attributed to the fact that these modules were implicated to suppress the population of three major pests of mango in comparison to the other studies where a single pest was target. Maximum control of I. clypealis was obtained with three applications of thiamethoxam, spinosad, and carbaryl at different rates of application but the yield of the individual tree was not more than 125.36 kg per tree (Kumari et al., 2014) while in case of the present study, maximum yield with PRMM-2 (non-chemical methods) was 516 kg per tree. The only difference between the two approaches was the use of chemicals for the suppression of I. clypealis alone (Kumari et al., 2014) while in the present study the objective was to manage all major insect-pests of mango. The minimum rate of return in mango and cashew was 100% using biological and chemical control (George et al., 2013).
The samples from the modules showed a significant difference in the residual concentrations and number of contaminated samples. Chlorpyrifos showed maximum contamination percentage in PRMM-4 with 42.00% samples above MRL, while the maximum percentage sample concentration above residual limits was 17.00% in PRMM-2. Similar results were reported from 40 pesticides including chlorpyrifos (Sumitra et al., 2006). Likewise, vegetable samples from IPM and non-IPM origin exhibited that 20% samples with IPM origin were found contaminated with pesticide residues in comparison of 47% sample contamination with non-IPM origin (Kumari et al., 2012). These results are slightly different from the present study where percentage of contaminated samples was higher but it is in agreement of the statement that agricultural produce with IPM origin is safer in terms of residues. The deviation in the results may be attributed to the fact that results of fruit samples and vegetable samples are being compared.
The comparative results from IPM and non-IPM orchards revealed that only a few samples from non-IPM grown orchard possessed a concentration of cypermethrin (Singh et al., 2009). During the analysis of 150 peach samples for residues, results showed that no sample from IPM orchard exceeded the MRL values while 7% sample conventional orchard were quantified with a concentration above acceptable limits (Tsakiris et al., 2004). These results clearly indicated the pesticide residues mitigation potential of IPM while the difference in percentage of the contaminated samples may be subjected to the target commodity. A comparative analysis of different models (conventional, IPM and organic) described that 82% conventional samples were quantified with residues, 49% samples with IPM origin were contaminated while 23% samples from organic sources were determined with pesticide residues (Baker et al., 2002). A similar trend was observed in the current study that showed pesticide contamination of 75.00% for conventional, 33.33% for IPM and 8.33% for organic samples.
Conclusions
The findings of the present study revealed that mango is contaminated with residues of a variety of pesticides collected from different locations in Multan the District, Pakistan. Mitigation of these pesticide residues is possible by minimizing the use of pesticides in the production of mango using nonchemical methods for pest population suppression. A more extensive study is needed for other fruits and vegetables to assess the scenario of pesticide residues and IPM modules needed to be devised and tested to mitigate residues.
List of Abbreviations
IPM: Integrated pest management; QuECHERS: Quick Easy Cheap Effective Rugged Safe; GC-ECD: Gas Chromatography with Electron Capture Detector; PRMM: Pesticide Residue Mitigation Module; MRL: Maximum Residual Limit; PPR: Percent Population Reduction; M1: Average Population in Treatment; M2: Average Population in Control; HPLC: High Performance Liquid Chromatography; MgSO4: Magnesium Sulphate; MeCN: Acetonitrile; PSA: Primary Secondary Amines; NaAc: Sodium Acetate; AOAC: Association of Official Analytical Chemists; NaOac: Sodium Acetate; SPE: Solid Phase Extraction; MS: Mass Spectrometry; RSD: Relative Standard Deviation; CBR: Cost-Benefit Ratio; PVb: All cost received including benefits; PVc: All cost spent; MAT: Male Annihilation Technology; BAT: Male Annihilation Technology; Cont.: Contaminated samples; ND: Not Detected.
Acknowledgment
The authors would like to thank Hi-Tech. Laboratory, University of Agriculture, Faisalabad and Higher Education Commission of Pakistan for their technical and financial support to conduct this study. This study was conducted under the project funded by the Higher Education Commission of Pakistan (Grant # 4673).
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this article.
Author’s Contribution
MAF designed and conducted the experiment, collected and analyzed the data, and wrote the manuscript. MJA helped in apprehending the idea of this research, designing the layout of the experiment and improving the write-up, format, and language of this manuscript. MDG, BA and AN contributed in data sets for analysis, reviewed the final manuscript and made the format of this manuscript according to the format of this journal. This final manuscript was ultimately perused, scrutinized and approved for final submission by all the authors.
References
Amvrazi, E.G. and Tsiropoulos, N.G., 2009. Application of single-drop microextraction coupled with gas chromatography for the determination of multiclass pesticides in vegetables with nitrogen phosphorus and electron capture detection. Journal of Chromatography A. 12: 2789-2797. https://doi.org/10.1016/j.chroma.2008.07.070
Anastassiades, M., Lehotay, S.J., Stajnbaher, D. and Schenck, F.J., 2003. Fast and easy multiresidue method employing acetonitrile extraction/partitioning and dispersive solid-phase extraction for the determination of pesticide residues in produce. Journal of AOAC International. 86: 412-431. https://doi.org/10.1093/jaoac/86.2.412
Aslam, M., Razaq, M., Shahand, S.A. and Ahmed, F., 2004. Comparative efficacy of different insecticides against sucking pests of cotton. Journal of Research (Science). 15: 53-58.
Atta, B., Rizwan, M., Sabir, A.M., Gogi, M.D. and Ali, K., 2019a. Damage potential of Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae) on wheat grains stored in hermetic and non-hermetic storage bags. International Journal of Tropical Insect Science. pp. 1-11. https://doi.org/10.1007/s42690-019-00047-0
Atta, B., Rizwan, M., Sabir, A.M., Gogi, M.D., Farooq, M.A. and Batta, Y.A., 2019b. Efficacy of entomopathogenic fungi against brown planthopper Nilaparvata lugens (Stål) (Homoptera: Delphacidae) under controlled conditions. Gesunde Pflanzen. pp. 1-12. https://doi.org/10.1007/s10343-019-00490-6
Baker, B.P., Benbrook, C.M., Groth, E. and Benbrook, K.L., 2002. Pesticide residues in conventional, integrated pest management (IPM)-grown and organic foods: insights from three US data sets. Food Additives and Contaminants. 19: 427-446. https://doi.org/10.1080/02652030110113799
Bakırcı, G.T. and Hışıl, Y., 2011. Fast and simple extraction of pesticide residues in selected fruits and vegetables using tetrafluoroethane and toluene followed by ultrahigh-performance liquid chromatography/ tandem mass spectrometry. Food Chemistry. 135: 1901-1913. https://doi.org/10.1016/j.foodchem.2012.06.051
Bakırcı, G.T., Acay, D.B.Y., Bakirci, F. and Otles, S., 2014. Pesticide residues in fruits and vegetables from the Aegean region, Turkey. Food Chemistry. 160: 379-392. https://doi.org/10.1016/j.foodchem.2014.02.051
Chowdhury, M.A.Z., Fakhruddin, A.N.M., Islam, M.N., Moniruzzaman, M., Gan, S.H. and Alam, M.K., 2013. Detection of the residues of nineteen pesticides in fresh vegetable samples using gas chromatography-mass spectrometry. Food Control. 34: 457-465. https://doi.org/10.1016/j.foodcont.2013.05.006
Clercq, P., Mason, P.G. and Babendreier, D., 2011. Benefits and risks of exotic biological control agents. BioControl. 56: 681-698. https://doi.org/10.1007/s10526-011-9372-8
Damalas, C.A. and Eleftherohorinos, I.G., 2011. Pesticide exposure, safety issues, and risk assessment indicators. International Journal of Environmental Research and Public Health. 8: 1402-1419. https://doi.org/10.3390/ijerph8051402
Farooq, M.A., Arif, M.J., Gogi, M.D., Nawaz, A. and Atta, B., 2019. Comparative efficacy of different Pesticide Residue Mitigation Modules in Mango. American Journal of Biomedical Science and Research. 4(4): 214-219. https://doi.org/10.34297/AJBSR.2019.04.000801
Grasman, J., van Herwaarden, O.A., Hemerik, L. and van Lenteren, J.C., 2001. A two component model of host–parasitoid interactions: determination of the size of inundative releases of parasitoids in biological pest control. Mathematical Biosciences. 169: 207-216. https://doi.org/10.1016/S0025-5564(00)00051-1
Handa, S.K., Agnihotri, N.P. and Kulshrestha, G., 1999. Pesticide residue, significance, management and analysis. Research Periodicals and Book Publishing House, Texas.
Hassan, R. and Bakshi, K., 2005. Integrated pest management: means of sustainable agricultural development in developing countries. Pakistan Journal of Social Sciences. 3: 603-613.
Khan, B.A., Farid, A., Asi, M.R., Shah, H. and Badshah, A.K., 2009. Determination of residues of trichlorfon and dimethoate on guava using HPLC. Food Chemistry. 114: 286-288. https://doi.org/10.1016/j.foodchem.2008.08.092
Khan, Z.R., Midega, C.A.O., Bruce, T.J.A., Hooper, A.M. and Pickett, J.A., 2010. Exploiting phytochemicals for developing a ‘push–pull’ crop protection strategy for cereal farmers in Africa. Journal of Experimental Botany. 61: 4185-4196. https://doi.org/10.1093/jxb/erq229
Knežević, Z. and Serdar M., 2009. Screening of fresh fruit and vegetable for pesticide residues on croation market. Food Control. 20: 419-422. https://doi.org/10.1016/j.foodcont.2008.07.014
Kumari, B.R., Ranga, R.G.V., Sahrawat, K.L. and Rajasekhar, P., 2012. Evaluation of integrated pest management in reducing insecticide residues in plant, soil and water. Indian Journal of Plant Protection. 40: 268-272.
Lehotay, S.J., 2007. Determination of pesticide residues in foods by acetonitrile extraction and partitioning with magnesium sulfate: Collaborative study. Journal of AOAC International. 90: 485-520. https://doi.org/10.1093/jaoac/90.2.1SUP
Lu, Y.C., Watkins, B. and Teasdale, J., 1999. Economic analysis of sustainable agricultural cropping systems for Mid-Atlantic States. Journal of Sustainable Agriculture. 15: 77-93. https://doi.org/10.1300/J064v15n02_09
Martínez-del-Río, J., Vidal, J.L.M. and Frenich, A.G., 2013. Economic evaluation of pesticide residue analysis of vegetables. Trends in Analytical Chemistry. 44: 2-8. https://doi.org/10.1016/j.trac.2012.11.008
Nault, B.A., Speese, J., Jolly, D. and Groves, R.L., 2003. Seasonal patterns of adult thrips dispersal and implications for management in eastern Virginia tomato fields. Crop Protection. 22: 505-512. https://doi.org/10.1016/S0261-2194(02)00203-X
Patel, R.K., Verghese, A., Patel, V.M., Joshi, B.K., Stonehouse, J.M. and Mumford, J.D., 2005. Bait, lure and cultural IPM of fruit flies in mangoes in Gujarat. Pest Management in Horticultural Ecosystems. 11: 155-158.
Pedigo, L.P., 1996. Entomology and pest management, second ed., Prentice-Hall, Englewood Cliffs, NJ. pp. 679.
Peña, J.E., Sharp, J.L. and Wysoki, M., 2002. Tropical fruit pests and pollinators: Biology, economic importance, natural enemies and control. CAB Publishing. New York, NY. https://doi.org/10.1079/9780851994345.0000
Pickett, J.A., Hamilton, M.L., Hooper, A.M., Khan, Z.R. and Midega, C.A.O., 2010. Companion cropping to manage parasitic plants. Annual Review of Phytopathology. 48: 161-177. https://doi.org/10.1146/annurev-phyto-073009-114433
Pilgrim, E.S., Macleod, C.J.A., Blackwell, M.S.A., Bol, R., Hogan, D.V., Chadwick, D.R., Cardenas, L., Misselbrook, T.H., Haygarth, P.M., Brazier, R.E., Hobbs, P., Hodgson, C., Jarvis, S., Dungait, J., Murray, P.J. and Firbank, L.G., 2010. Interactions amongst agricultural production and other ecosystem services delivered from European temperate grassland systems. Advances in Agronomy. 109: 117-154. https://doi.org/10.1016/B978-0-12-385040-9.00004-9
Qin, G., Li, Y., Chen, Y., Sun, Q., Zuo, B., He, F., Shen, N., Jia, G. and Ding, G., 2015. Pesticide residues determination in China vegetables in 2010-2013 applying gas chromatography with mass spectrometry. Food Research International. 72: 161-167. https://doi.org/10.1016/j.foodres.2015.03.036
Rejczak, T. and Tuzimski, T., 2015. A review of recent developments and trends in the QuEChERS sample preparation approach. Open Chemistry. 13: 980-1010. https://doi.org/10.1515/chem-2015-0109
Rizwan, M., Atta, B., Rizwan, M., Sabir, A.M., Shah, Z.U. and Hussain, M., 2019a. Effect of the entomopathogenic fungus, Beauveria bassiana, combined with diatomaceous earth on the red flour beetle, Tribolium castaneum (Herbst) (Tenebrionidae: Coleoptera). Egyptian Journal of Biological Pest Control. 29: 27-26. https://doi.org/10.1186/s41938-019-0131-y
Rizwan, M., Atta, B., Sabir, A.M., Yaqub, M. and Qadir, A., 2019b. Evaluation of the entomopathogenic fungi as a non-traditional control of the rice leaf roller, Cnaphalocrocis medinalis (Guenee) (Lepidoptera: Pyralidae) under controlled conditions. Egyptian Journal of Biological Pest Control. 29: 10-14. https://doi.org/10.1186/s41938-019-0111-2
Singh, S.B., Mukherjee, I., Maisnam, J., Kumar, P., Gopal, M. and Kulshrestha, G., 2009. Determination of pesticide residues in integrated pest management and nonintegrated pest management samples of Apple (Malus pumila Mill.). Journal of Agricultural and Food Chemistry. 57: 11277-11283. https://doi.org/10.1021/jf903624v
Tang, S. and Cheke, R.A., 2008. Models for integrated pest control and their biological implications. Mathematical Biosciences. 215: 115-125. https://doi.org/10.1016/j.mbs.2008.06.008
Tsakiris, I.N., Danis, T.G., Stratis, I.A., Nikitovic, D., Dialyna, I.A., Alegakis, A.K. and Tsatsakis, A.M., 2004. Monitoring of pesticide residues in fresh peaches produced under conventional and integrated crop management cultivation. Food Additives and Contaminants. 21: 670-677. https://doi.org/10.1080/02652030410001698715
van Lenteren, J.C., 2000. Measures of success in biological control of arthropods by augmentation of natural enemies. In: Wratten, S. and Gurr, G. (Eds.). Measures of Success in Biological Control, Kluwer, Dordrecht. pp. 77-103. https://doi.org/10.1007/978-94-011-4014-0_3
van Lenteren, J.C. and Woets, J., 1988. Biological and integrated control in greenhouses. Annual Review of Entomology. 33: 239-269. https://doi.org/10.1146/annurev.en.33.010188.001323
Vidal, J.L.M., Arrebola, F.J. and Mateu-Sánchez, M., 2002. Application of gas chromatography-tandem mass spectrometry to the analysis of pesticides in fruits and vegetables. Journal of Chromatography A. 959: 203-213. https://doi.org/10.1016/S0021-9673(02)00444-2
Wang, J.L., Xia, Q., Zhang, A.P., Hu, X.Y. and Lin, C.M., 2012. Determination of organophosphorus pesticide residues in vegetables by an enzyme inhibition method using α-naphthyl acetate esterase extracted from wheat flour. Journal of Zhejiang University Science B. 13: 267-273. https://doi.org/10.1631/jzus.B11a0180
Wright, M.G., Hoffmann, M.P., Kuhar, T.P., Gardner, J. and Pitcher, S.A., 2005. Evaluating risks of biological control introductions: A probabilistic risk-assessment approach. Biological Control. 35: 338-347. https://doi.org/10.1016/j.biocontrol.2005.02.002





