Image Analysis Estimate of Leaf Area Damage Caused by Citrus Leafminer, Phyllocnistis citrella (Lepidoptera: Gracillariidae) Larvae on Different Citrus Cultivars
Image Analysis Estimate of Leaf Area Damage Caused by Citrus Leafminer, Phyllocnistis citrella (Lepidoptera: Gracillariidae) Larvae on Different Citrus Cultivars
Muhammad Arshad1, Muhammad Irfan Ullah1*, Muhammad Afzal1, Mian Anjum Murtaza2, Ejaz Ahraf3, Zahoor Hussain4, Syed Muhammad Ali Zahid1 and Maryam Riaz1
1Department of Entomology, University of Sargodha, 40100, Sargodha, Pakistan; 2Institute of Food Science and Nutrition, University of Sargodha, 40100 Sargodha, Pakistan; 3Department of Agricultural Extension, University of Sargodha, 40100 Sargodha, Pakistan; 4Department of Horticulture, University of Sargodha, 40100, Sargodha, Pakistan.
Abstract | Citrus leafminer (CLM), Phyllocnistis citrella Stainton (Lepidoptera: Gracillariidae) is among the serious insect pests attacking citrus plants in Pakistan. The study was conducted to quantify the leaf area damage of eight citrus cultivars caused by mining activity of CLM during summer 2016. The total leaf area and mine area per leaf were calculated by the image analysis method using Sigma Scan Pro 5.0 software. The results of the present study showed that CLM generated larger mines; 1.64 cm2 on Grapefruit, 1.44 cm2 on Kinnow and 1.40 cm2 on Succari compared to other five cultivars. However, the percent leaf damage due to the feeding of CLM larvae was observed higher 44.2% on Fairchild, 36.5% on seedless Kinnow, 36.3% on Feutrell’s early and 35.8% on Kinnow that showed great susceptibility to CLM. Smallest mines generated by CLM larvae were found on China Lemon and Succari showing the lowest damage percentage compared to other cultivars. Conclusively, the C. tangerines and C. mandarins were the most preferred cultivars by CLM larvae for feeding on the leaf surface. The image analysis method was accurate, relatively simple, easy and inexpensive to calculate the leaf area damage caused by CLM larvae.
Received | April 29, 2019; Accepted | July 21, 2019; Published | September 04, 2019
*Correspondence | Muhammad Irfan Ullah, Department of Entomology, University of Sargodha, Sargodha, Pakistan; Email: muhammad.irfanullah@uos.edu.pk
Citation | Arshad, M., M.I. Ullah, M. Afzal, M.A. Murtaza, E. Ahraf, Z. Hussain, S.M.A. Zahid, M. Riaz. 2019. Image analysis estimate of leaf area damage caused by citrus leafminer, Phyllocnistis citrella (Lepidoptera: Gracillariidae) larvae on different citrus cultivars. Sarhad Journal of Agriculture, 35(3): 948-954.
DOI | http://dx.doi.org/10.17582/journal.sja/2019/35.3.948.954
Keywords | Citrus cultivars, Image analysis, Leaf area, Percent damage, Phyllocnistis citrella, Sigma Scan
Introduction
The citrus leafminer, (CLM) Phyllocnistis citrella, Stainton (Lepidoptera: Gracillariidae) is intrinsic to Southeast Asia and worldwide it has become a major insect pest in most citrus-growing areas (Grafton-Cardwell et al., 2009). Female adult oviposits mostly on young flushes of almost all citrus cultivars as well as other Rutaceae and some ornamental plants (Arshad et al., 2018). CLM larvae start feeding on the epidermis layer of young emerging leaves, by generating serpentine mines (Belasque et al., 2005). The damaged leaves due to CLM feeding become curled and twisted and in case of heavy infestations, stunted growth of plants occurs and ultimately yield reduced (Pena et al., 2000). The CLM larvae mark wounds on leaves which provide a pathway for the bacterium Xanthomonas citri subsp. citri, to enter in leaves and cause citrus canker disease (Christiano et al., 2007; Hall et al., 2010).
The measurement of leaf area is a very important tool in damage evaluation caused by insect pests and diseases, estimation of micronutrient deficiency in plants, environmental and water stress, the requirement of fertilizer and for effective management techniques (Marcon et al., 2011). Previously, researchers have developed different procedures including mechanical planimeter (Donovan et al., 1958), length × width measurement (Donald, 1963) that work best with the given crop to measure leaf damage caused by various herbivorous insects. Further, some electrical instruments were also used previously for the measurement of undamaged leaf area, length, and width of the leaf (Wolf et al., 1972). Similarly, the leaf area index values were recorded by the direct measurement method like destructive sampling (Potithep et al., 2013). In most of the studies, leaf area consumed by herbivores were measured visually, treated leaves comparison with control and hand tracing of damaged leaves (Stotz et al., 2000; Wheeler and Isman, 2001). Prior studies have been conducted to assess the leaf damage caused by CLM larvae, mostly by visually estimating the percentage of damaged leaf area (Knapp et al., 1995). The accuracy of this visual estimation of leaf damage needs to be determined for quantify the actual damaged area caused by CLM larva because it feeds on the epidermis layer of the leaves and doesn’t make the holes on the leaf surface as other chewing insect pests. These older techniques for measuring leaf area have been replaced with digital image processing methods in a combination of camera and digital scanner (Easlon and Bloom, 2014). In the last decade, due to computing technology advancement, image processing has been developed into a more active area of research. There are comparatively few industrial uses of image processing and widely within the agricultural sector. The plant-insect interaction (Hammond et al., 2000) and many biological studies (Wheeler and Isman, 2001; Sehsah and Hassan, 2015) required the measurement of leaf area and defoliation. These software’s are suitable for a few leaf samples and for small plants (Pandey and Singh, 2011). However, Sigma Scan Pro 5.0 is the most common software to measure the leaf area using a threshold-based pixel count and is suitable for measuring any type and size of the leaves (Atala et al., 2011). The purpose of this study was to estimate the leaf area damage caused by CLM larvae in eight citrus cultivars through image analysis method using Sigma Scan software.
Materials and Methods
Citrus cultivars
The experiment was conducted in citrus nursery plantations at College of Agriculture, University of Sargodha. The following eight citrus cultivars; Citrus mandarins (Kinnow, Seedless Kinnow, Feutrell’s early), Citrus sinensis (Succari, Salustiana), Citrus tangerines (Fairchild), Citrus limon (China lemon) and Citrus paradisi Macfad (Grapefruit) were selected to determine the mine length and percent leaf damage caused by CLM larvae. Ten one-year-old plants were selected from each cultivar and three leaves with newly hatched CLM larvae were selected from each plant and tagged for capturing the image. Any other larva if observed on the leaf surface was removed carefully using a camel hair brush. The experiment was conducted under completely randomized design (CRD) and one plant was considered as 1 replication for a total of 10 plants/cultivar.
Image analysis
An image of each selected leaf was captured by the digital camera (DSC-WX60, 16.2 MP HD, CHINA) at three days interval for one month. For image capturing, the selected leaves were carefully placed on a white background. To avoid light reflection, the white cotton cloth was used as a background placed on the cardboard. The distance between camera lens and object (leaves) were kept constant using camera stand. The distance was neither too close nor too far; it was adjusted in such a way that photographs cover only background and leaf. Images were captured, arranged in numbers and stored in computer hard drive for further analysis. Total leaf area and mine area per leaf were calculated using Sigma Scan Pro 5.0 (Point Richmond, CA, USA) software. When the image was opened in software, trace mode was selected to get the desired portion of leaves and adjust the threshold to get a red leaf image. Hue ranges were set from 47 to 107 and saturation from 0 to 100 as suggested by Richardson et al. (2001) to identify the green pixels (leaves). The measurement set in menu tab was selected to calculate the area of the desired portion of a leaf on a different worksheet. The layout of the whole image analysis process is given in Figure 1. The leaf area and mine area measurement were obtained in pixels. So, a reference object was used to calculate the area in cm2 instead of the pixel at a constant distance. To convert pixel values into cm2, a coin was used as a reference object. A reference object is an object with a known area.
Area of the reference object
The area of a reference object was measured as suggested by Patil and Bodhe (2011).

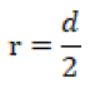
r= radius, d= diameter, the diameter of coin was 2.3cm. So, the radius of the coin was 1.15cm.
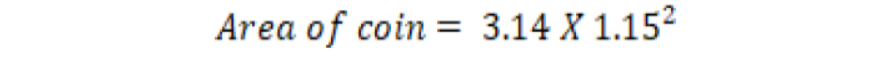
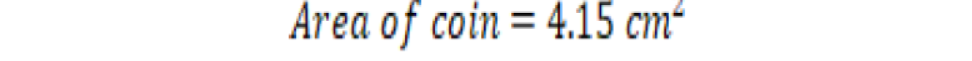
The calculated area of a coin through the above formula was 4.15 in cm2 and 262380 in pixels through Sigma Scan. Hence, 1 cm2 was equal to 63224.1 pixels at a constant distance which we kept between camera lens and object. So, the total leaf area and mine area measured in pixels were converted into cm2 by this method.
Percent leaf damage
The mine area and total leaf area was calculated by image analysis and percent leaf damage was calculated by the formula suggested by Raimondo et al. (2013).
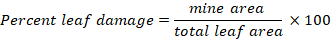
Data analysis
The data for percent leaf damage and mine area per leaf were analyzed by two-factor factorial ANOVA by keeping cultivar and time interval as main factors. Means were separated by Tukey HSD all-pairwise comparison test. All the analyses were performed using SPSS 20.0 software.
Results and Discussion
The mine area generated by CLM larvae was significantly different on citrus cultivars (F = 62.68, P < 0.001) at different time interval (F = 106.6, P < 0.001) at probability level of 5%. Similarly, a significant effect on leaf damage of different citrus cultivars (F = 48.77, P < 0.001) was observed due to CLM feeding at different time intervals (F = 93.36, P < 0.001). The interaction of cultivars and time interval (P < 0.001) was also significant in mine area per leaf and percent leaf damage.
The larger mines generated by CLM larvae were ranged; 1.64 cm2 on Grapefruit, 1.44 cm2 on Kinnow and 1.40 cm2 on Succari leaves. The CLM larvae generated smaller mine of 0.88 cm2 on China lemon and 0.89 cm2 on Salustiana leaves that mean larvae didn’t prefer these cultivars as well (Figure 2). Percent leaf damage of Fairchild due to the feeding of CLM larvae was found higher (44.23%) at 28th day of observation. However, leaves of seedless Kinnow, Feutrell’s early and Kinnow were damaged about 36.5%, 36.3%, and 35.8% respectively at 28th day of sampling. Percent leaf damage of Succari (26.5%) and China Lemon (25.5%) was found lowest compared to other cultivars (Figure 3).
Among all other insect pests in citriculture, the CLM is very important causing severe damage at both nursery and citrus orchards. The present study showed that CLM larvae prefer C. mandarins (Kinnow, Feutrell’s early, seedless Kinnow) and C. tangerines (Fairchild) more compared to other cultivars by generating larger mines. The percent leaf damage was also higher on these cultivars as compared to others. Both the percent leaf damage and mine area were observed minimum in China lemon and Salustiana compared to other cultivars. A possible explanation of variation in damage level may be due to the difference in leaf thickness and certain anatomical modifications (Mathews et al., 2007), various metabolic changes (Smith and Boyko, 2007) or due to different chemical compounds in citrus cultivars that act as repellent or attractant for CLM (Rocchini et al., 2000).
The results of our study showed indirectly the impact of CLM on the productivity of young plants of China Lemon and Salustiana could be lower. In particular, C. mandarins and C. tangerines were susceptible to the infestation, if 30-45% of leaf area loss considered. This percentage may depend on the plant age; the plants were 1-2 years old in our study. In nursery plants, damage caused by CLM delays the normal growth of young plants as well as lessens the canopy development for fruit production. As an alternative, in mature orchards, its medium-term effects on annual leaf balance, flowering pattern, flushing, and yield are not clearly understood. The economic impact on the growth and yield of citrus due to CLM feeding seems to be related on the ability of plants to sprout, the contribution of leaf area to tree growth development annually, flowering as well as fruit-bearing (Stansly et al., 1996).
Previously, Huang and Li (1989) reported that the CLM damage above than 20% is necessary for significant losses of citrus yield in China and losses of leaf area below than 20% did not impact significantly on yield. They suggested 0.74 larvae per leaf as the economic threshold level for citrus leafminer. According to Pena et al. (2000), significant yield reduction occurs at 17-23% of leaf area loss in 15year old plants, and 18-85% leaf area loss in 5year old plants due to CLM attack. In Thailand, for 50% infested shoots of Tangerine and Pummelo was the economic threshold, if canker diseases severity was at levels of 31-50% (Morakote and Nanta, 1996). Similarly, Hunsberger et al. (1996) concluded that due to CLM damage 37.7% yield reduction occurs in Thaitti lime in Florida. Keeping in mind the CLM damage level from previous studies, leaf area loss of C. mandarins and C. tangerins were found 30-45% in our study, which means that CLM is playing a major role in citrus yield reduction in Pakistan.
Our findings showed that the percent leaf damage due to CLM feeding was less on C. limon compared to other cultivars which were in accordance with Raimondo et al. (2013). According to their results, the infestation level did not exceed 10% of the total leaf area of damaged plants of C. limon. Our findings also showed that percent leaf damage increased with the passage of time. However, in most of the citrus cultivars, the percent leaf damage was increased up to 22nd days and then remained constant up to the 28th day of observation. It might be possible that most of the larvae were converted into pupae and there was no increase of mine area on the leaf surface. In our study, we kept the number of larvae as one, if the number of larvae or mine increase on the leaf surface the percent leaf damage will increase. Knapp et al. (1995) reported that when more than three larvae present on a single leaf, percent damage reached up to 50%.
We calculated the total leaf area and mine area generated by CLM larvae through image analysis method. The neonate larva of CLM starts feeding on the leaf tissues near the midvein and later make zigzag mines on the leaf surface so, it is difficult to measure the consumed area visually. Previously, Schaffer et al. (1997) have reported that the percent damage of leaf area can be determined visually, but it is more practical of a large number of leaves are being sampled in the field. Furthermore, for visually estimating the damage caused by CLM, the evaluator should be experienced. In contrast, the advantage of leaf damage estimation through image analysis is that it provides a photographic record of the leaf damage. Furthermore, this method has accuracy and high precision whether leaf having maximum width and length, it will not take as much of processing time. At any time, the image can be sorted out to analyze if the images have stored permanently. This method is also viable for area measurement of any type of leaf with the same accuracy (Patil and Bodhi, 2011).
This estimation of leaf area damage using public domain software with digital imaging system was used to train researchers for the accurate measurement of leaf damage. Wheeler and Isman (2001) measured the damaged area caused by Spodoptera litura (F.) (Lepidoptera: Noctuidae) larvae feeding on untreated and treated leaves by plant extracts. Similarly, Sehsah and Hassan (2015) used Image J software version 1.52 to estimate the leaf area losses by different insect herbivores. They also reported that image analysis is an accurate method to calculate the leaf damage caused by CLM. The accuracy of image analyzer methods to estimate leaf area of several crops has been well described in different studies (Strachan et al., 2005; Demarez et al., 2008; Liu and Pattey, 2010). However, it is too important to use an accurate protocol for the validation of basic principles related to leaf area measurement (Wilhelm et al., 2000).
Digital image analysis was proved the easier and less time-consuming method to estimate area and was more useful for narrow leaves (Liu and Pattey, 2010). Practically, it shows performance not only for smaller leaves but also their affordability with nominal training. By using digital image analyzer techniques, it is also possible to get more laborious crop information for limited space and time with an easy acquirement of leaf images (Liu and Pattey, 2010).
Conclusions and Recommendations
The C. mandarins and C. tangerines are more susceptible cultivars for CLM and C. limon (China lemon) is the less preferred cultivar for CLM at nursery level. The imaging process technique is an easy method to measure the mined or damaged area due to the CLM attack. Time-consuming bioenergetics should be study for other herbivorous insects. More detailed studies should be conducted to better evaluate the effective damage due to CLM infestation with and without other parasites (like Xanthomonas axonopodis pv. citri) on the productivity of different citrus cultivars.
Acknowledgments
We are thankful to the Department of Horticulture, University of Sargodha for providing a necessary arrangement for the study.
Author’s Contribution
MA and MIU conceived the idea and planned the experiment. MA, MAM, and EA performed statistical analyses. ZH and SMAZ technically revised the manuscript. MR provided technical assistance and proofread the manuscript.
Novelty Statement
We quantified the actual damaged area through image analysis method because it feeds on the epidermis layer of the leaves and, thus the visual damage estimation is not accurate in case of CLM.
References
Arshad, M., M.I. Ullah and M. Afzal. 2018. Citrus leafminer Phyllocnistis citrella and its parasitoids in Sargodha region of Pakistan. EPPO Bull. 48: 309-313. https://doi.org/10.1111/epp.12479
Atala, C., C. Cordero and E. Gianoli. 2011. Drought and leaf damage limit the search for support in the climbing plant Ipomoea purpurea (L.) Roth (Convolvulaceae). Gayana Bot. 68: 207-212. https://doi.org/10.4067/S0717-66432011000200011
Belasque, J., A. Parra-Pedrazzoli, J. Rodrigues, P. Yammamoto, M. Chagas, J. Parra and J. Hartung. 2005. Adult citrus leafminer (Phyllocnistis citrella) are not vectors for citrus canker in experimental microcosms. Plant Dis. 89: 590-594. https://doi.org/10.1094/PD-89-0590
Christiano, R., M. Dalla Pria, W.J. Junior, J. Parra, L. Amorim and A. Bergamin Filho. 2007. Effect of citrus leafminer damage, mechanical damage and inoculum concentration on severity of symptoms of Asiatic citrus canker in Tahiti lime. Crop Prot. 26: 59-65. https://doi.org/10.1016/j.cropro.2006.03.016
Demarez, V., S. Duthoit, F. Baret, M. Weiss and G. Dedieu. 2008. Estimation of leaf area and clumping indexes of crops with hemispherical photographs. Agric. For. Meteorol. 148: 644-655. https://doi.org/10.1016/j.agrformet.2007.11.015
Donald, C.M. 1963. Competition among crop and pasture plants. Adv. Agron. 15: 1-118. https://doi.org/10.1016/S0065-2113(08)60397-1
Donovan, L., A. Magee and W. Kalbfleisch. 1958. A photoelectric device for measurement of leaf areas. Can. J. Plant Sci. 38: 490-494. https://doi.org/10.4141/cjps58-076
Easlon, H.M. and A.J. Bloom. 2014. Easy Leaf Area: Automated digital image analysis for rapid and accurate measurement of leaf area. Appl. Plant Sci. 2: 1400033. https://doi.org/10.3732/apps.1400033
Hall, D.G., T.R. Gottwald and C.H. Bock. 2010. Exacerbation of citrus canker by citrus leafminer Phyllocnistis citrella in Florida. Fla. Entomol. 93: 558-566. https://doi.org/10.1653/024.093.0413
Hammond, R.B., L.G. Higley, L.P. Pedigo, L. Bledsoe, S.M. Spomer and T.A. DeGooyer. 2000. Simulated insect defoliation on soybean: Influence of row width. J. Econ. Entomol. 93: 1429-1436. https://doi.org/10.1603/0022-0493-93.5.1429
Hunsberger, A.G.B., J.E. Pena and B. Schaffer. 1996. Relationships of citrus leafminer density to citrus damage and yield. Paper presented at the managing the citrus leafminer, Proc. Int. Conf. Univ. Florida, Gainesville; pp. 86.
Knapp, J.L., L.G. Albrigo, H.W. Browning, R.C. Bullock, J.B. Heppner, D.G. Hall, M.A. Hoy, R. Nguyen, J.E. Pena and P.A. Stansly. 1995. Citrus leafminer, Phyllocnistis citrella Stainton: Current status in Florida, Ins. Food Agric. Sc. Univ. Florida, Gainesville, FL.
Liu, J. and E. Pattey. 2010. Retrieval of leaf area index from top-of-canopy digital photography over agricultural crops. Agric. For. Meteorol. 150: 1485-1490. https://doi.org/10.1016/j.agrformet.2010.08.002
Marcon, M., K. Mariano, R.A. Braga, C.M. Paglis, M.S. Scalco and G.W. Horgan. 2011. Estimation of total leaf area in perennial plants using image analysis. Rev. Bras. Eng. Agríc. Ambient. 15: 96-101. https://doi.org/10.1590/S1415-43662011000100014
Mathews, C.R., M.W. Brown and D.G. Bottrell. 2007. Leaf extract oral nectaries enhance biological control of a key economic pest Grapholita molesta (Lepidoptera: Tortricidae) in peach (Rosales: Rosaceae). Environ. Entomol. 36: 383-389. https://doi.org/10.1093/ee/36.2.383
Pandey, S.K. and H. Singh. 2011. A simple, cost-effective method for leaf area estimation. J. Bot. 2011: 1-6. https://doi.org/10.1155/2011/658240
Pena, J.E., A. Hunsberger and B. Schaffer. 2000. Citrus leafminer (Lepidoptera: Gracillariidae) density: effect on yield of ‘Tahiti’lime. J. Econ. Entomol. 93: 374-379. https://doi.org/10.1603/0022-0493-93.2.374
Potithep, S., S. Nagai, K.N. Nasahara, H. Muraoka and R. Suzuki. 2013. Two separate periods of the LAI–VIs relationships using in situ measurements in a deciduous broadleaf forest. Agric. For. Meteorol. 169: 148-155. https://doi.org/10.1016/j.agrformet.2012.09.003
Raimondo, F., P. Trifilò and M.A. Lo Gullo. 2013. Does citrus leafminer impair hydraulics and fitness of citrus host plants? Tree Physiol. 33: 1319-1327. https://doi.org/10.1093/treephys/tpt094
Richardson, M.D., D.E. Karcher and L.C. Purcell. 2001. Quantifying turfgrass cover using digital image analysis. Crop Sci. 41: 1884-1888. https://doi.org/10.2135/cropsci2001.1884
Rocchini, L.A., B.S. Lindgren and R.G. Bennett. 2000. Effects of resin flow and monoterpene composition on susceptibility of lodgepole pine to attack by Douglas-firpitch moth, Synanthedon novaroensis (Lepidoptera:Sesiidae). J. Appl. Entomol. 124: 87-92. https://doi.org/10.1046/j.1439-0418.2000.00449.x
Schaffer, B., J.E. Pena, A.M. Coils and A. Hunsberger. 1997. Citrus leafminer (Lepidoptera: Gracillariidae) in lime: Assessment of leaf damage and effects on photosynthesis. Crop Prot. 16: 337-343. https://doi.org/10.1016/S0261-2194(97)00003-3
Smith, C.M. and E.V. Boyko. 2007. The molecular bases of plant resistance and defense responses to aphid feeding: current status. Entomol. Exp. Appl. 122: 1-16. https://doi.org/10.1111/j.1570-7458.2006.00503.x
Stotz, H.U., B.R. Pittendrigh, J. Kroymann, K. Weniger, J. Fritsche, A. Bauke and T. Mitchell-Olds. 2000. Induced plant defense responses against chewing insects. Ethylene signaling reduces resistance of Arabidopsis against Egyptian cotton worm but not diamondback moth. Plant Physiol. 124: 1007-1018. https://doi.org/10.1104/pp.124.3.1007
Wheeler, D.A. and M.B. Isman. 2001. Antifeedant and toxic activity of Trichilia americana extract against the larvae of Spodoptera litura. Entomol. Exp. Appl. 98: 9-16. https://doi.org/10.1046/j.1570-7458.2001.00751.x
Wilhelm, W., K. Ruwe and M.R. Schlemmer. 2000. Comparison of three leaf area index meters in a corn canopy. Crop Sci. 40: 1179-1183. https://doi.org/10.2135/cropsci2000.4041179x








