Higher Altitude and Lower Temperature Regulate the Body Mass and Energy Metabolism in Male Eothenomys miletus
Higher Altitude and Lower Temperature Regulate the Body Mass and Energy Metabolism in Male Eothenomys miletus
Yue Ren1, Peng-Fei Liu2, Wan-Long Zhu1,*, Hao Zhang1 and Jin-Hong Cai1
1Key Laboratory of Ecological Adaptive Evolution and Conservation on Animals-Plants in Southwest Mountain Ecosystem of Yunnan Province Higher Institutes College, School of Life Science of Yunnan Normal University, Kunming 650500, China
2College of Life Science and Technology, Longdong University, Qingyang 745000, China
Yue Ren and Peng-Fei Liu have contributed equally to this work.
ABSTRACT
The present study was aimed at examining the roles of altitude and temperature on body mass regulation in Eothenomys miletus from different areas of Hengduan mountain region in different seasons. Body mass, resting metabolic rate (RMR), nonshivering thermogenesis (NST), food intake, serum leptin levels and hypothalamic neuropeptide Y (NPY), agouti aelated peptide (AgRP), pro-opiomelanocortin (POMC), cocaine and amphetamine regulated transcript (CART) expressions were measured. The results showed that body mass and serum leptin levels were lower significantly in winter than that of in summer in five areas. But thermogenic characteristics and food intake were higher significantly in winter than that of in summer in five areas. NPY and AgRP expressions showed significant differences between two seasons, which were higher in winter and lower in summer, but POMC and CART expressions showed no significant differences between winter and summer from all areas. In summer, body mass and serum leptin levels had showed no significant differences among five regions, food intake, RMR, NST and NPY expressions were higher in higher altitude (Xianggelila, XGLL and Deqin, DQ) than that of lower altitude (Ailaoshan, ALS, Jianchuan, JC and Lijiang, LJ). In winter, body mass and serum leptin levels were lower, and food intake, RMR, NST, NPY and AgRP expressions were higher in XGLL and DQ. All of the results suggested that E. miletus can successfully overcome the physiological challenges of an cold temperature in winter by increasing thermogenic capacity, food intake and decreasing body mass and serum leptin levels. Higher altitude can reduce body mass, and increase thermogenic properties and NPY expressions. Differences changes of physiological regulation from five areas were observed in E. miletus, indicating that lower temperature and higher altitude may play an regulation on body mass and energy metabolism in E. miletus.
Article Information
Received 24 August 2017
Revised 26 May 2018
Accepted 27 January 2019
Available online 10 October 2019
Authors’ Contribution
WLZ conceived the study and participated in design, coordination and drafted the manuscript. YR and PFL carried out the studies of body mass, food intake and hormonal, markers. HZ and JHC carried out the studies of metabolic rate.
Key words
Eothenomys miletus, Body mass, Serum leptin levels, Hypothalamic neuropeptides, Food intake.
DOI: https://dx.doi.org/10.17582/journal.pjz/2020.52.1.139.146
* Corresponding author: [email protected]
0030-9923/2020/0139-0001 $ 9.00/0
Copyright 2020 Zoological Society of Pakistan
Introduction
The survival of small animals must acquire and assimilate energy, and allocate energy assimilation for use of maintenance, growth and reproduction as needed (Pablo et al., 2011). The geographical distribution, richness, fitness, survival, adaptation, reproduction and diversity of species are closely related to the characteristics of energy metabolism (Speakman, 2008), such as Meriones unguiculatus in desert population had lower resting metabolic rate (RMR) and total evaporative water loss than mesic population (Shi et al., 2015). The regulation of energy homeostasis depends on the balance between food acquisition and consumption (Zhang et al., 2017). This balance is critical for animal activity, thermogenesis, energy metabolism, and gastrointestinal morphology (Zhu et al., 2012). Energy budget of different regulatory mechanisms may lead to different energy models and life history in small mammals, RMR has been shown to be a minimum metabolic rate for each tissue internal organs to maintain normal metabolism, so RMR has become an important physiological parameters affecting animals’ growth and energy consumption for reproduction (Kristan and Hammond, 2006). The previously study showed some small mammals enhanced RMR and nonshivering thermogenesis (NST) to cope with winter or cold conditions (Zhang and Wang, 2006). During environmental changes, animals changed phenotypic characteristics of morphology, behavior and physiology, including reproduction, thermogenesis, energy intake, and body mass (Bush et al., 2008). Energy intake is affected by many factors, such as environmental temperature, photoperiod, habitat, individual size, classification status and food habits, etc. (Lovegrove, 2005). Seasonal changes of body mass, energy intake and thermogenic capacity in many small mammals are reported (Steinlechner et al., 1983; Mercer, 1998), such as Phodopus sungorus (Klingenspor et al., 2000), Ochotona curzoniae (Wang et al., 1999) and Microtus oeconomus (Wang et al., 1999).
Leptin, one of the most important adipose derived hormones (Abelenda et al., 2003), plays a key role in control on food intake and energy expenditure (Friedman and Halaas, 1998). It had been indicated that leptin is a potential signal that mediates the seasonal variations of body mass and energy balance (Brennan and Mantzoros, 2006). The hypothalamic arcuate nucleus (ARC) can regulate food intake under environmental changing (Aguilar et al., 2011). Within the ARC, there are two types of neuropeptides: orexigenic neuropeptides: neuropeptide Y (NPY) and agouti-related protein (AgRP); and anorectic neuropeptides: pro-opiomelanocortin (POMC) and cocaine- and amphetamine-regulated tran-script (CART); the balance between NPY/AgRP and POMC/CART expressions can inhibit food intake and stimulate energy expenditure (Friedman and Halaas, 1998).
Eothenomys miletus is an inherent species in Hengduan mountain region (Zhu et al., 2010; Ren et al., 2019). It has been previously reported that body mass and serum leptin levels were lower in winter compared with that of in summer in E. miletus in Jianchuang area (Zhu et al., 2014, 2017). Altitude varies in the Hengduan mountains region, and its geographical factors restrict water and temperature distribution. Hence, it has two characteristics: geographical diversity and climate diversity (Zhu et al., 2008), all of these may lead to some special physiological and ecological characteristics to E. miletus. The aims of this study were to evaluate the body mass regulation in E. miletus from different areas of Hengduan mountain region in different seasons. We hypothesized that E. miletus would respond to different habitat by changing body mass, serum leptin levels, food intake and adjusting the hypothalamic neuropeptides genes expressions. We predicted that E. miletus may change the physiological responses to regulate body mass, and temperature and altitude may involve in the regulation of body mass in E. miletus under different seasons.
Materials and methods
Samples
E. miletus were obtained from farmland in Ailaoshan (ALS, 24°32´~24°36´N; 101°01´~101°06´E; altitude 2,217m; temperature in summer 27.8oC, temperature in winter 11.5oC), Jianchuan (JC, 26°15´~26°45´N; 99°40´~99°55´E; altitude 2,590m; temperature in summer 23.7oC, temperature in winter 8.4oC), Lijiang (LJ, 26°56´~26°59´N; 100°12´~100°15´E; altitude 2,478m; temperature in summer 21.0oC, temperature in winter 7.9oC), Xianggelila (XXLG, 99°01´~99°03´E; 101°23´~101°25´E; altitude 3,321m; temperature in summer 18.7oC, temperature in winter 2.1oC), Deqin (DQ, 30°33´~30°39´N; 28°13´~28°15´N; altitude 3,459m; temperature in summer 17.2oC, temperature in winter -1.6oC) from Hengduan mountain region, 2016 in summer (June) and winter (December). A total of 96 males included ALS (n=20, summer 10, winter 10), JC (n=23, summer 12, winter 11), LJ (n=20, summer 10, winter 10), XGLL (n=19, summer 10, winter 9) and DQ (n=14, summer 7, winter 7). Adult E. miletus were maintained at a room temperature of 25±1°C, under a photoperiod of 12L:12D (with lights on at 08:00), food (standard mice chow pellets; produced by Kunming Medical University, Kunming) and water were provided ad libitum. All animal procedures were compliance with the Animal Care and Use Committee of School of Life Science, Yunnan Normal University. This study was approved by the Committee (13-0901-011). After the RMR, NST and food intake measurement, subjects were sacrificed by puncture of the posterior vena cava within 4 days after capture for all the two seasons, and blood and tissue samples were taken for measurement of physiological parameters (Wang et al., 2006).
Measurement of metabolic rates
Metabolic rates were measured by using an AD ML870 open respirometer (AD Instruments, Australia) at 25oC within the TNZ (thermal neutral zone), gas analysis were performed using a ML206 gas analysis instrument, the temperature was controlled by SPX-300 artificial climatic engine (±0.5oC), the metabolic chamber volume was 500ml, flow was 200 ml/min. The voles were stabilized in the metabolic chamber for at least 60 min prior to the RMR measurement, oxygen consumption was recorded for more than 120 min at 1 min intervals. Ten stable consecutive lowest readings were taken to calculate RMR (Zhu et al., 2008). Calculate method of metabolic rate is detailed by Hill (1972) and amelioration:
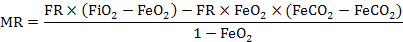
Where, FR is flow rate (ml/min), FiO2 is O2 input fractional concentration (%), FiCO2 is CO2 input fractional concentration (%), FeO2 is O2 excurrent fractional concentration (%) and FeCO2 is CO2 excurrent fractional concentration (%).
The voles stayed in the chamber for another 1 h for NST measurement. Nonshivering thermogenesis (NST) was induced by subcutaneous injection of norepinephrine (NE) (Shanghai Harvest Pharmaceutical Co., Ltd.) and measured at 25oC. Oxygen consumption was recorded at intervals of 10 s. NSTmax was calculated from the stable highest consecutive rate of oxygen consumption over 5 min (Zhu et al., 2010). The doses of NE were approximately 0.8-1.0 mg/kg according to dose-dependent response curves that were carried out before the experiment (Zhu et al., 2010).
Measurement of food intake
Food intake was measured by food equity (Zhao and Cao, 2009). Each animal was put in a metabolic cage (20×15×15cm3) with no nest materials, and fed laboratory mice chow pellets. Animals were fed a fixed quantity at a set time (9.5–10.5 g, 11:00 am), and the next day body mass was assessed, and residual food collected. Residual food was dried in a vacuum dryer until the mass was invariable. Food intake was measured for two days.
Measurement of serum leptin levels
Serum leptin levels were determined by radioimmunoassay (RIA) with the 125I Multi-species Kit (Cat. No. XL-85K, Linco Research Inc.). The lowest level of leptin that can be detected by this assay was 1.0 ng/ml when using a 100 μl sample size. And the inter- and intra-assay variability for leptin RIA were <3.6% and 8.7%, respectively.
Measurements of hypothalamic neuropeptide gene expression
Total RNA was isolated from the hypothalamus by using TRIzol Kit (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. To remove any contaminating DNA, RNA samples were treated with DNase I (Promega, USA) at 37°C for 30 min followed by another cycle of TRIzol extraction to eliminate residual DNase I. An equal amount (3 μg) of total RNA was transcribed into first strand cDNA for each sample using the M-MLV First Strand Kit (Invitrogen) according to the manufacturer’s instructions.
Primers set for β-actin and four hypothalamic genes were used for real-time q-PCR (Zhu et al., 2017). Standard curves were constructed for each gene via serial dilutions of cDNA (1 to 26-fold dilutions). Analysis of standard curves between target genes and β-actin showed that they had similar amplification efficiency, which ensures the validity of the comparative quantity method. Real-time q-PCR was completed using the SYBR Green I qPCR kit (Invitrogen) in the ABI Prism® 7000 Sequence Detection system (Applied Biosystems, Carlsbad CA, USA). Real-time qPCR was carried out in 20 μL reaction agent comprised of 9.5μL RNase-free ddH2O, 9.0 μL Platinum® Quantitative PCR SuperMix-UDG (including Rox), 0.5 μL cDNA templates, 0.5 μL 10 μmoL/L forward primer, and 0.5 μL 10 μmoL/L reserse primer. Each sample was analyzed in triplicate. Thermal cycling conditions were: 50°C for 120 s, 95°C for 120 s, 45 cycles of 95°C for 15 s, and 60°C for 45 s.
Statistical analysis
Data were analyzed using the software package SPSS 15.0. Prior to all statistical analyses, data were examined for assumptions of normality and homogeneity of variance using Kolmogorov-Smirnov and Levene tests, respectively. Body mass, RMR and NST, food intake, serum leptin levels and hypothalamic neuropeptide genes expressions were analyzed by two-way ANOVA (seasons and areas). Results are presented as means±SEM and P < 0.05 was considered to be statistically significant.
Results
Body mass and serum leptin levels
Seasons and areas had a significant effects on body mass in E. miletus among all five regions (seasons: F1,86=21.56, P<0.01; areas: F4,86=9.43, P<0.01; interaction: F4,86=3.89, P<0.05; Fig. 1). Serum leptin levels showed similar trends to body mass (seasons: F1,86=8.63, P<0.01; areas: F4,86=4.25, P<0.01; interaction: F4,86=2.41, P<0.05; Fig. 2). Body mass and serum leptin levels were lower in XXLG and DQ than that of other three regions, and they were lower significantly in winter among all five regions.
Thermogenic characteristics and food intake
Seasons and areas had a significant effects on RMR in E. miletus among all five regions (seasons: F1,86=15.69, P<0.01; areas: F4,86=12.78, P<0.01; interaction: F4,86=5.32, P<0.01; Fig. 3). Similar trends were also found in NST (seasons: F1,86=22.32, P<0.01; areas: F4,86=25.36, P<0.01; interaction: F4,86=4.29, P<0.01; Fig. 4) and food intake (seasons: F1,86=26.69, P<0.01; areas: F4,86=24.32, P<0.01; interaction: F4,86=7.65, P<0.01; Fig. 5). RMR, NST and food intake increased significantly from five areas in winter compared with that of in summer. Whether it’s summer or winter, RMR, NST and food intake were higher in XGLL and DQ than that of ALS, JC and LJ.
Hypothalamic neuropeptide genes expressions
Seasons and areas had significant effects on NPY expressions (seasons: F1,86=4.68, P<0.01; areas: F4,86=3.65, P<0.01; interaction: F4,86=2.12, P<0.05; Fig. 6) and AgRP expressions (seasons: F1,86=3.32, P<0.01; areas: F4,86=2.15, P<0.05; interaction: F4,86=1.02, P>0.05; Fig. 6) in E. miletus among all five regions. But seasons and areas had no significant effects on POMC (seasons: F1,86=1.03, P>0.05; areas: F4,86=0.87, P>0.05; interaction: F4,86=0.32, P>0.05; Fig. 6) and CART expressions (seasons: F1,86=0.83, P>0.05; areas: F4,86=0.74, P>0.05; interaction: F4,86=0.25, P>0.05; Fig. 6).
Discussion
Seasonal variations of body mass were considered to be an adaptive strategy for survival and reproductive success in small mammals (Concannon et al., 2001). Many small mammals reduced body mass to cope with cold-stress or in winter (Merritt et al., 2001). In the present study, body mass was lower significantly among five region in E. miletus in winter, reducing body mass was benefit to reduce the total energy consumption in winter (Bozinovic et al., 2004). Body mass in summer had no significant differences among five areas, which may be related to the higher abundance of food resources and environmental temperature in summer. But in winter, body mass in XGLL and DQ were lower than that of ALS, JC and LJ, which may be related to the altitude difference, altitude in XGLL and DQ were higher than 3000m, but other regions were below 2600m, as the altitude increases, the environmental temperature drops, E. miletus need more energy intake to survival. Body mass declined in XGLL and DQ in E. miletus is considered to be an adaptive mechanism for the reduction of energy requirements when stress occurs (Li and Wang, 2007).
It has been reported that the BMR varies with the seasons changing (Rene et al., 2014). The current study showed that E. miletus increased RMR in winter than that of summer, which may be related to lower temperature in winter, higher metabolic rate was required to maintain survival in order to adapt to cold conditions. NST is an effective and economical mode of thermogenesis for small mammals under cold exposure (Zhang and Wang, 2007). Previously studies have shown that NST had seasonal changes (Wang et al., 2006). In the present study, NST of E. miletus showed seasonal variations, which was related to the lower temperature in winter, so E. miletus requires higher thermogenesis to maintain life activities. Whether it’s summer or winter, RMR and NST were higher in
XGLL and DQ than that of ALS, JC and LJ, suggesting that higher altitude environments lead to lower temperature, E. miletus in XGLL and DQ increased thermogenic capacity to maintain the survival and stability of body temperature. Small mammals also showed seasonal variations of energy intake (Heldmaier et al., 1982). In seasonal acclimatized animals, animals increased food intake in winter as compared to that in summer (Zhang and Wang, 2006). In our study, food intake increased significantly from five areas in winter compared with that of in summer, which may be related to an increase of energy consumption in winter. RMR and NST increased significantly in winter, E. miletus need to increase food intake for the energy consumption. Food intake were higher in XGLL and DQ than that of ALS, JC and LJ, which was also related to the higher altitude decreased the environmental temperature, E. miletus need to increase food intake under lower temperature to compensate for the increase of energy consumption.
Leptin plays an important role in the regulation of body mass in small mammals (Abelenda et al., 2003). Leptin can regulate body mass mainly through the regulation of energy intake and energy consumption (Concannon et al., 2001). Current researches showed that serum leptin levels decreased significantly in winter among five regions, suggesting that lower levels of leptin can promote food intake, suggesting that leptin may involve in the regulation of body mass under different seasons. Serum leptin levels had no significant differences in summer among five regions, indicating that food resources were abundant in summer, E. miletus can get enough food, and the body mass increased, so no significant differences were found in serum leptin levels. Hypothalamic neuropeptide genes were essential for the maintenance of body mass and energy metabolism (Tang et al., 2009). Our results showed that NPY and AgRP expressions in winter were higher than that of in summer, POMC and CART expressions had no significant differences among five regions. Increasing NPY and AgRP expressions can increase food intake for E. miletus in winter. POMC and CART expressions did not decrease in winter, because higher POMC and CART expression levels could increase thermogenesis in winter (Zhu et al., 2017).
Conclusion
In conclusion, E. miletus can successfully overcome the physiological challenges of an cold environment in winter by increasing thermogenic capacity, food intake and decreasing body mass and serum leptin levels. Differences changes of physiological regulation from five areas were observed in E. miletus, indicating that cold temperature and higher altitude may play an regulation on body mass and energy metabolism in E. miletus.
Acknowledgments
This research was financially supported by National Science Foundation of China (No. 31560126; 31760118). We wish to thank Pro. Burkart Engesser at Historisches Museum Basel, Switzerland for correcting the English usage in the draft. Thank you for the anonymous reviewers and the editor of the journal for their valuable comments.
Statement of conflict of interest
The authors declare no conflict of interest.
Reference
Abelenda, M., Ledesma, A. and Rial, E., 2003. Leptin administration to cold acclimated rats reduces both food intake and brown adipose tissue thermogenesis. J. Therm. Biol., 28: 525-530. https://doi.org/10.1016/S0306-4565(03)00053-6
Aguilar, A.J., Conde-Sieira, M., López-Patiño, M.A., Míguez, J.M. and Soengas, J.L., 2011. In vitro leptin treatment of rainbow trout hypothalamus and hindbrain affects glucosensing and gene expression of neuropeptides involved in food intake regulation. Peptides, 32: 232-240. https://doi.org/10.1016/j.peptides.2010.11.007
Brennan, A.M. and Mantzoros, C.S., 2006. Drug insight: The role of leptin in human physiology and path physiology-emerging clinical applications. Nat. Clin. Pract. Endocrinol. Metab., 2: 318-327. https://doi.org/10.1038/ncpendmet0196
Bozinovic, F., Bacigalupe, L.D., Vasquez, R.A. and Kenagy, G.J., 2004. Cost of living in free-ranging degus (Octodondegus): Seasonal dynamics of energy expenditure. Comp. Biochem. Physiol., 137: 597-604. https://doi.org/10.1016/j.cbpb.2003.11.014
Bush, N.G., Brown, M. and Downs, C.T., 2008. Seasonal effects on thermoregulatory responses of the Rock Kestrel, Falco rupicolis. J. Therm. Biol., 33: 404-412. https://doi.org/10.1016/j.jtherbio.2008.06.009
Concannon, P., Levac, K., Rawson, R., Tennant, B. and Benadoun, A., 2001. Seasonal changes in serum leptin, food intake, and body weight in photo entrained woodchucks. Am. J. Physiol., 281: 951-959.
Friedman, J.M. and Halaas, J.L., 1998. Leptin and the regulation of body weight in mammals. Nature, 395: 763-770. https://doi.org/10.1038/27376
Heldmaier, G., Steinlechner, S. and Rafael, J., 1982. Nonshivering thermogenesis and cold resistance during seasonal acclimatization in the Djungarian hamster. J. Comp. Physiol., 149: 1-9.
Hill, R.W., 1972. Determination of oxygen consumption by use of the paramagnetic oxygen analyzer. J. appl. Physiol., 33: 261-263. https://doi.org/10.1152/jappl.1972.33.2.261
Klingenspor, M., Niggemann, H. and Heldmaier, G., 2000. Modulation of leptin sensitivity by short photoperiod acclimation in the Djungarian hamster, Phodopus sungorus. J. comp. Physiol., 170: 37-43. https://doi.org/10.1007/s003600050005
Kristan, D.M. and Hammond, K.A., 2006. Effects of three simultaneous demands on glucose transport, resting metabolism and morphology of laboratory mice. J. comp. Physiol., 176: 139-151. https://doi.org/10.1007/s00360-005-0036-9
Li, X.S. and Wang, D.H., 2007. Photoperiod and temperature can regulate body mass, serum leptin concentration, and uncoupling protein 1 in Brandt’s Voles (Lasiopodomys brandtii) and Mongolian Gerbils (Meriones unguiculatus). Physiol. Biochem. Zool., 80: 326-334. https://doi.org/10.1086/513189
Lovegrove, B.G., 2005. Seasonal thermoregulatory responses in mammals. J. comp. Physiol., 175: 231-247. https://doi.org/10.1007/s00360-005-0477-1
Mercer, J.G., 1998. Regulation of appetite and body weight in seasonal mammals. Comp. Biochem. Physiol., 119: 295-303.
Merritt, D., Ferrarese, L. and Joseph, S.H., 2001. No supermassive black hole in M33? Science, 293: 1116-1118. https://doi.org/10.1126/science.1063896
Pablo, A.C., Marcela, F., Pablo, S. Quijano, S.A. and Nespolo, R.F., 2011. Bioenergetics and intestinal phenotypic flexibility in the microbiotherid marsupial (Dromiciops gliroides) from the temperate forest in South America. Comp. Biochem. Physiolo., 160: 117-124. https://doi.org/10.1016/j.cbpa.2011.05.014
Ren, X.Y., Zhang, D. and Zhu, W.L. 2019. Geometric morphometry of skulls characteristics of nine species of Eothenomys. Pakistan J. Zool., 51: 467-474.
Rene Q., Villavicencio, C.P., Addis, E., and Wingfield, J.C. and Vasquez, R.A., 2014. Seasonal variations of basal cortisol and high stress response to captivity in Octodon degus, a mammalian model species. Gen. comp. Endocrinol., 197: 65-72. https://doi.org/10.1016/j.ygcen.2013.12.007
Shi, Y.L., Chi, Q.S., Liu, W., Fu, H.P. and Wang, D.H., 2015. Environmental metabolomics reveal geographic variation in aerobic metabolism and metabolic substrates in Mongolian gerbils (Meriones unguiculatus). Comp. Biochem. Physiol., 14: 42-52.
Speakman, J.R., 2008. The physiological cost of reproduction in small mammals. Philos. Trans. R. Soc. Lond. B: Biol. Sci., 363: 375-398. https://doi.org/10.1098/rstb.2007.2145
Steinlechner S., Heldmaier, G. and Becker, H., 1983. The seasonal cycle of body weight in the Djungarian hamster: Photoperiod control and the influence of starvation and melatonin. Oecologia, 60: 401-405. https://doi.org/10.1007/BF00376859
Tang, G.B., Cui, J.G. and Wang, D.H., 2009. Role of hypoleptinemia during cold adaptation in Brandt’s voles (Lasiopodomys brandtii). Am. J. Physiol., 297: 1293-1301.
Wang, D.H., Sun, R.Y., Wang, Z.W. and Liu, J.K., 1999. Effects of temperature and photoperiod on thermogenesis in plateau pikas (Ochotona curzoniae) and root voles (Microtus oeconomus). J. comp. Physiol., 169: 77-83. https://doi.org/10.1007/s003600050196
Wang, J.M., Zhang, Y.M. and Wang, D.H., 2006. Seasonal regulations of energetic, serum concentrations of leptin, and uncoupling protein 1 content of brown adipose tissue in root voles (Microtus oeconomus) form the Qinghai-tibtan plateau. J. comp. Physiol., 176: 663-671. https://doi.org/10.1007/s00360-006-0089-4
Zhang, X.Y. and Wang, D.H., 2006. Energy metabolic, thermogenesis and body mass regulation in Brandt’s voles (Lasiopodomys brandtii) during cold acclimation and rewarming. Horm. Behav., 50: 61-69. https://doi.org/10.1016/j.yhbeh.2006.01.005
Zhang, Z.Q. and Wang, D.H., 2007. Seasonal changes in thermogenesis and body mass in wild Mongolian gerbils (Meriones unguiculatus). Comp. Biochem. Physiol., 148: 346-353. https://doi.org/10.1016/j.cbpa.2007.05.012
Zhang, L., Yang, F., Wang, Z.K. and Zhu, W.L., 2017. Role of thermal physiology and bioenergetics on adaptation in tree shrew (Tupaia belangeri): The experiment test. Scient. Rep., 7: 41352. https://doi.org/10.1038/srep41352
Zhao, Z.J. and Cao, J., 2009. Effect of fur removal on the thermal conductance and energy budget in lactating Swiss mice. J. exp. Biol., 212: 2541-2549. https://doi.org/10.1242/jeb.029603
Zhu, W.L., Jia, T., Lian, X., Wang, Z.K., 2008. Evaporative water loss and energy metabolic in two small mammals, voles (Eothenomys miletus) and mice (Apodemus chevrieri) in Hengduan mountains region. J. Therm. Biol., 33: 324-331. https://doi.org/10.1016/j.jtherbio.2008.04.002
Zhu, W.L., Cai, J.H., Lian, X. and Wang, Z.K., 2010. Adaptive character of metabolism in Eothenomys miletus in Hengduan mountains region during cold acclimation. J. Therm. Biol., 35: 417-421. https://doi.org/10.1016/j.jtherbio.2010.09.002
Zhu, W.L., Zhang, H. and Wang, Z.K., 2012. Seasonal changes in body mass and thermogenesis in tree shrews (Tupaia belangeri) the roles of photoperiod and cold. J. Therm. Biol., 37: 479-484. https://doi.org/10.1016/j.jtherbio.2012.04.007
Zhu, W.L., Zhang, H., Zhang, L., Yu, T.T. and Wang, Z.K., 2014. Thermogenic properties of Yunnan red-backed voles (Eothenomys miletus) from the Hengduan mountain region. Anim. Biol., 64: 59-73. https://doi.org/10.1163/15707563-00002430
Zhu, W.L., Zhang, D., Hou, D.M. and Yang, G., 2017. Roles of hypothalamic neuropeptide gene expression in body mass regulation in Eothenomys miletus (Mammalia: Rodentia: Cricetidae). Eur. Zool. J., 84: 322-333. https://doi.org/10.1080/24750263.2017.1334840
To share on other social networks, click on any share button. What are these?










