Growth and Pigmentation of Goldfish (Carassius auratus L, 1758) Fed on a Diet Supplemented with Purslane (Portulaca sp.) Extract
Growth and Pigmentation of Goldfish (Carassius auratus L, 1758) Fed on a Diet Supplemented with Purslane (Portulaca sp.) Extract
Dilek Şahin1*, Meryem ÖZ2, Orhan Aral2, Mehmet Bahtiyar2 and Selda Taşçi2
1Sinop University Vocational School, 57000 Sinop, Turkey.
2Sinop University Fisheries Faculty, 57000 Sinop, Turkey.
ABSTRACT
This study aimed to investigate the effects of the purslane extract on growth and pigmentation of goldfish (Carassius auratus L, 1758). The study was carried out with 4 experimental groups of which composed of control (0%, T0) and different amount of purslane plant extracts (3%, T3; 6%, T6; 9%, T9) and each group had 3 replicates. The fish with an average live weight of 2.55±0.08 g were selected randomly from a fish stock of 250 fish and were 13 fish were placed in each aquarium (15 L). The growth, survival and coloring parameters were determined at the end of 60th day of the study. The best growth parameters were recorded in the T9 group. The best weight gain, specific growth rate and feed conversation ratio values occurred at 0.815 g, 0.462 % and 0.86, respectively were observed in the group fed with 9% of purslane extract. The survival rate parameters indicated no death in any experimental group of goldfish fry. The purslane extract had a positive effect on coloring, and the highest pigmentation for goldfish was obtained in T6 and T9 (Hue (Habº) angle values (86.73±0.32 and 77.64±0.47, respectively). The results revealed that purslane extract had a positive effect on the growth and coloring parameters of goldfish. The results recorded in this study can be used database in aquaculture for future studies carried out in aquaculture.
Article Information
Received 18 March 2021
Revised 18 May 2021
Accepted 10 June 2021
Available online 29 July 2021
(early access)
Published 20 November 2021
Authors’ Contribution
DŞ, MÖ and OA designed the study. DŞ, MÖ, MB and ST performed the experiment and data collection. DŞ and MÖ wrote the article and performed the data analysis.
Key words
Carassius auratus, Development, Pigmentation, Portulaca sp., Aquaculture
DOI: https://dx.doi.org/10.17582/journal.pjz/20210318090307
* Corresponding author: dsahin@sinop.edu.tr
0030-9923/2022/0001-0175 $ 9.00/0
Copyright 2022 Zoological Society of Pakistan
INTRODUCTION
The feed additives and raw materials have significant effects on growth, reproduction and coloring of fish in ornamental fish farming. Therefore, aquaculture in all over the world significantly relies on fish meal and fish oil production. The aquaculture in Turkey is highly dependent of imported feed. Approximately, 80% of raw material (fish meal, soymeal, cornmeal etc.) and additives (vitamins, minerals, carotenoids, hormones etc.) used in the fish feed are imported from other countries (Yeşilayer et al., 2013). Therefore, studies recently focused on reproduction, coloring parameters, survival rates and disease resistance of fish fed with high nutritive value feeds (Yeşilayer et al., 2011a, 2020; Karsli et al., 2016, 2018).
Purslane, which is rich in antioxidants, vitamins, minerals, omega-3 fatty acids, carbohydrates, protein and fiber, is used for health and medical purposes for thousands of years (Uddin et al., 2014; Kartikasari et al., 2017). The species has a cosmopolitan distribution, but it is more present in the Mediterranean area, mainly in the arid and semi-arid lands of northern Africa and southern Europe. P. oleracea is largely cultivated. Purslane is also used for cooking or used as a pickle (Uddin et al., 2014). The purslane naturally is found in Turkey; thus, is considered an available economic feed additive and raw material in aquaculture. Purslane can be used in fish feed both by drying and extracting.
The extract of purslane contains 4.9% protein, 3.8% ash, 0.82% fiber and 40.4 mg 100g-1 total carotenoid, while the dried powder purslane contains 18.58% protein, 16.5% ash, %17.9% fiber, 110.97 mg 100g-1 total carotenoid on dry weight basis (Hannan et al., 2014). In ornamental fish diet, a proper balanced formulation not only improves digestibility and growth performance, but also it lowers the water pollution caused due to uneaten feed (Devi et al., 2019). When these references are evaluated, purslane can be used as a feed for both aquarium fish and edible fish farming.
Growth performance and reproduction are the most significant parameters to determine the sustainability of the population in aquarium fish farming. Breeders in commercial fish farms mostly focus on the maximum growth performance, gonad maturation, grown to market size in a short time and coloration for a culture of aquarium fish (Sales and Janssens, 2003). Goldfish is a modified variety of Carassius auratus and is the most preferred species due to easy breeding, long life, and variety of colors. Goldfish are omnivorous in feeding habits. This study was conducted to investigate the effects of purslane extracts on the growth and coloration of goldfish (Carassius auratus Linnaeus, 1758) which is very popular in the aquarium fish markets.
MATERIALS AND METHODS
Experimental setup and feeding management
The study was carried out with 4 experimental groups of which composed of control (0%, T0) and different amount of purslane plant extracts (3%, T3; 6%, T6; 9%, T9) and each group had 3 replicates. The fish with an average live weight of 2.55±0.08 g were selected randomly from a fish stock of 250 fish and were 13 fish were placed in each aquarium (15 L). The fish were fed twice a day (09:00, 15:00) for 2 months with four diets till satiation. Waste materials were syphoned out every day and reduced water was added.
In each experimental treatment group, fish were acclimatized to aquarium and feeding conditions for a week. Temperature of the aquarium unit was set to 24±1ºC throughout the experiments using an air conditioner. Oxygen was set to over 5 mg L-1, pH between pH 6.0 and 8.5 and ammonia level below 1 mg L-1. Day light was used as natural photoperiod (Lim et al., 2002). Water temperature, pH level, oxygen, and NH4 concentrations were measured using a YSI Professional Plus equipment. Color parameters of fish were measured using a Konica Minolta CR-400 color meter.
Preparation of diets
Fish meal, soybean meal, sunflower seed meal, semolina flour, maize gluten, fish oil, vitamin and mineral mixture were used as raw materials and additive materials in preparation of the diets used in fish feeding. While there was no purslane extract (whole plant) inclusion to the control feed, 3 experimental feeds were prepared with the addition of different amounts of purslane extract without changing the chemical property by compensating the amount of semolina flour in other feed groups. Total of 4 different feed groups were prepared to test in the experiment. The amount of purslane extract was determined based on the ratios experimented in the previous studies (Ramadan et al., 2017; Zhao et al., 2013). The purslane extract contains 4.9% crude protein (Hannan et al., 2014). The composition of extracts can vary depending on the method of analysis and the properties of the raw material. Therefore, the value of extracts was not calculated and the feed contents were assumed to be close to each other and T0 (control group) and T9 (the most ideal values obtained) were sent to analysis. T3 and T6 contents were calculated based on T0 and T9 analysis results. Analyzed compositions of experimental diets are presented in Table I. Proximate composition (dry matter-basis) and analyses were made by Bilim Laboratory in Turkey. Amino acid analyses in experiment (except lysine and methionine) diets were calculated based on INRA-CIRAD-AFZ Feed tables.
Table I. Feed ingredients and proximate composition of experimental diets used for goldfish in two-month experimental study (g.100g-1).
|
Quantity (%) |
|||||||
|
T0 |
T3 |
T6 |
T9 |
||||
|
Ingredients |
|||||||
|
Fish meal |
33 |
33 |
33 |
33 |
|||
|
Soybean meal |
20 |
20 |
20 |
20 |
|||
|
Sunflower seed meal |
10 |
10 |
10 |
10 |
|||
|
Semolina flour |
21 |
20.7 |
20.4 |
20.1 |
|||
|
Maize gluten |
4 |
4 |
4 |
4 |
|||
|
Wheat gluten |
7 |
7 |
7 |
7 |
|||
|
Fish oil |
3 |
3 |
3 |
3 |
|||
|
Mineral* |
1 |
1 |
1 |
1 |
|||
|
Vitamin* |
1 |
1 |
1 |
1 |
|||
|
Purslane extract |
- |
0.3 |
0.6 |
0.9 |
|||
|
Proximate composition |
|||||||
|
Moisture (%) |
13.11 |
11.43 |
9.75 |
8.07 |
|||
|
Crude protein (%) |
42.60 |
42.70 |
43.5 |
45.31 |
|||
|
Crude lipid (%) |
13.70 |
13.60 |
13.05 |
11.74 |
|||
|
Crude ash (%) |
9.35 |
9.46 |
9.56 |
9.97 |
|||
|
Vitamin A (IU kg-1) |
48354 |
51570 |
54787 |
67652 |
|||
|
Vitamin C (mg 100 g-1) |
3.65 |
3.66 |
3.67 |
3.7 |
|||
|
Vitamin E (mg kg-1) |
10.38 |
11.20 |
12.03 |
15.33 |
|||
|
Lysine (g 100g-1) |
2.394 |
2.38 |
2.38 |
2.36 |
|||
|
Methionine (g 100g-1) |
0.85 |
0.81 |
0.80 |
0.792 |
|||
|
Arginine (%) |
3.78 |
3.67 |
3.22 |
3.19 |
|||
|
Treonine (%) |
3.73 |
3.66 |
3.55 |
3.47 |
|||
|
Leucine (%) |
5.71 |
5.69 |
5.54 |
5.48 |
|||
|
Isoleucine (%) |
3.55 |
3.42 |
3.33 |
3.24 |
|||
|
Valine (%) |
4.03 |
3.95 |
3.88 |
3.71 |
|||
|
Histitine (%) |
1.84 |
1.75 |
1.69 |
1.54 |
|||
|
Potassium (K, ppm) |
9189 |
9001 |
8948 |
846 |
|||
|
Phosphorus (P, %) |
3.02 |
3.10 |
3.22 |
3.35 |
|||
|
Calcium (C, mg/kg) |
17430 |
17143 |
16514 |
16280 |
|||
|
Magnesium (Mg, ppm) |
1729 |
1727 |
1725 |
1723 |
|||
|
Iron (Fe, ppm) |
147.9 |
151.65 |
155.4 |
170.4 |
|||
|
Manganese (Mn, ppm) |
149.5 |
154.55 |
159.6 |
179.8 |
|||
|
Zinc (Zn, ppm) |
1359 |
1249.5 |
1140 |
1030 |
|||
|
Copper (Cu, ppm) |
61.14 |
70.16 |
79.39 |
115.9 |
|||
* Per 100 g feed: Vit. A 2200 IU; Vit. B1-Thiamin 85 mg, Vit. B2-Riboflavin, 77 mg, Vit. B3-Niacin 350 mg, Vit. B6-Pyridoxine 88 mg, Vit. B12-cobalamin 0.2 mg, Vit. D 200 mg, Vit. K 20 mg, Ca 1000mg, Mg 600 mg, K 450 mg, Zn 90 mg, Mn 12 mg, Cu 5 mg. T0, 0% plant extract; T3, 3% plant extract; T6,6% plant extract; T9, 9% plant extract.
Assessment of the results
The growth, survival rate and coloring parameters were calculated using the following equations;
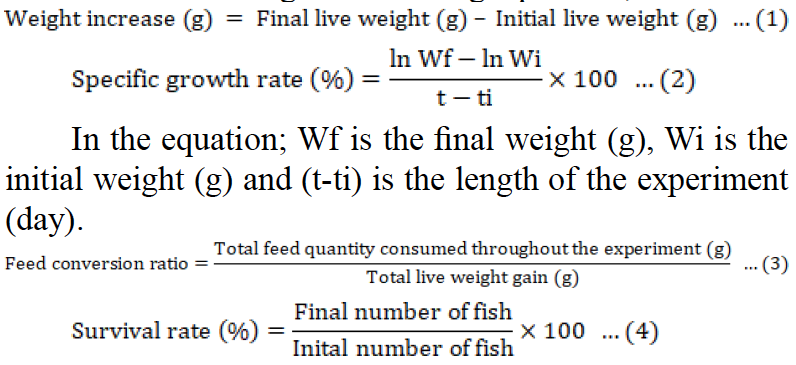
Color parameters were determined using physical color (instrumental) identification method. A colorimeter (Konica Minolta CR 400) was used to measure the skin L*, a* and b* values from around the dorsal section. The C* and Habº values were calculated using the a* and b* values. Color parameters determined were L*(+) lightness, (-) darkness, a*: (+) redness, (-) greenness, b*: (+) yellowness, (-) blueness (Nickell and Bromage 1998).
Chroma (Cab*) indicates the saturation, density or brightness of a color and calculated using the following equation;
Cab*= (a*²+ b*²)½ …(5)
Hue indicates the relationships among yellowness, greenness and blueness of skin color. If a*>0, then Habº= tan-1 (b*/a*) or if a*<0, then Habº= 180+tan-1 (b*/a*) equation is used to calculate the hue angle (Hunt, 1977). The hue indicates a red color tone at 0º, a yellow color tone at 90º, a green color tone at 180º and a blue color tone at 270º (Nickell and Bromage, 1998; Yeşilayer and Erdem, 2011).
Statistical analyses
Statistical analyses were carried out using “Minitab Release 17 for Windows” software at 0.05 level of significance. The data shown as mean ± standard error were subjected to one-way variance analysis (ANOVA). When ANOVA indicated significant difference between the treatments for a given parameter, then the means were compared with Fisher’s test.
Ethics and legal aspects
The study protocol was approved by ethics committee of Sinop University and experiments were carried out in accordance with the ethical guidelines and regulations declared by the Sinop University and the international principles of laboratory animal use and care.
RESULTS
Water parameters
Mean temperature, pH, dissolved oxygen level and NH4 content of water at the beginning of the experiments were 24.00±0.03 ºC, 8.57±0.02, 8.17±0.04 mg L-1 and 0.8±0.01 mg L-1, respectively. The differences in the values of parameter recorded at the beginning and end of the experiment were not significantly different (p>0.05).
Growth and color parameters
Initial live weights of juvenile goldfish were determined individually and mean live weights of T0, T3, T6 and T9 were 2.550±0.08, 2.552 ±0.08, 2.551 ±0.08 and 2.552 ±0.08g, respectively. The difference in initial live weights determined for different groups was not significantly different (p>0.05). The experiments lasted for 2 months (60 days) and the growth parameters were determined at the end of the experiments. Mean individual live weight gains, specific growth rates, feed conversion rates and survival rates of the experimental groups determined at the end of the experiment were given in Table II. When the weight gain and SGR values were examined, it was determined that the difference between T0 and T3 was insignificant (p>0.05), but there was difference between T6 and T9 (p<0.05). When the survival rate data were examined, it was determined that the difference between T0, T3, T6 and T9 was insignificant (p>0.05). FCR in the treatment groups decreased with increasing extract dose and especially, T9 groups had significantly lower FCR than the other groups.
Table II. Growth parameters, feed conversion ratio (FCR) and survival rate of goldfish (Carassius auratus) fed different levels of purslane (Portulaca sp.) extract for 60 days (n= 39) (mean± standard error).
|
Parameters |
Treatment groups |
|||
|
T0 |
T3 |
T6 |
T9 |
|
|
Initial weight (g) |
2.55±0.08 |
2.55±0.08 |
2.55±0.08 |
2.55±0.08 |
|
Final weight (g) |
2.63±0.05a |
2.71±0.06a |
3.00±0.08b |
3.37±0.09c |
|
Weight gain (g) |
0.078±0.01a |
0.162±0.01a |
0.452±0.01b |
0.815±0.01c |
|
Spesific growth rate (%) |
0.050±0.00a |
0.103±0.01a |
0.272±0.01b |
0.462±0.01c |
|
Feed conversation ratio |
2.68±0.13a |
1.89±0.06b |
1.14±0.00bc |
0.86±0.01c |
|
Survival rate (%) |
100.00±0.00 |
100.00±0.00 |
100.00±0.00 |
100.00±0.00 |
Values (mean ± standard error of means for triplicate) with different superscripts in a row are significantly different.
For details of treatment, see Table I.
Table III. Color parameters (L, a, b) on skin of goldfish (Carassius auratus) (± standard error of means) fed different levels of purslane (Portulaca sp.) extract for 60 days (n= 39).
|
Colour parameters |
Groups (End of the treatments) |
||||
|
Beginning of treatment |
Control (T0) |
T3 |
T6 |
T9 |
|
|
L* |
77.78±0.37b |
79.76±1.56ab |
83.18±0.14a |
82.00±0.00ab |
83.53±0.25a |
|
a* |
1.38±0.13b |
4.28±0.18a |
3.49±0.03a |
4.53±0.13a |
3.76±0.11a |
|
b* |
25.04±1.15b |
29.82±0.40a |
21.05±1.56c |
23.65±0.55bc |
21.11±1.92c |
|
Hue (Habº) |
86.73±0.32a |
81.29±0.52b |
79.44±0.73bc |
77.64±0.47c |
78.81±0.75c |
|
Chroma (C*) |
25.12±1.14b |
30.22±0.37a |
21.44±1.53b |
24.25±0.53b |
21.53±1.90b |
Values (mean ± standard error of means for triplicate) with different superscripts in a row are significantly different.
For details of treatment, see Table I.
Skin color parameters of the experimental groups were determined at the beginning and end of the experiments. The L*, a*, b*, Hue (Habº) and Chroma (C*) values were presented in Table III.
DISCUSSION
This is the first study investigating the effects of purslane extract supplemented to the goldfish diet on growth and pigmentation. In this study, purslane extract in three different doses (3, 6 and 9 g kg-1) was added to the diets of goldfish to evaluate its growth and pigmentation parameters. A dose dependent increase in the growth and survival is a noteworthy suggestive of its acceptance and palatability, and a promising additive in ornamental fish feed. Furthermore, the highest weight gain and SGR were determined in T9 (P<0.05).
Similar studies were conducted with Cyprinus carpio Wu et al. (2007) administered with a product called “Qompsell” consisting of a mixture of plant extracts, such Astragalus sp., Portulaca sp., Flavescent sp. and Andrographis sp. Wu et al. (2007) used Qompsell extract mixture at 0.3% level and reported crude protein, crude lipid, ash, calcium and phosphorus contents as 32, 5.3, 10, 1.2 and 0.4%, respectively. In the present study, the best growth performance yielded with 9 g kg-1 purslane extract supplementation in goldfish diets.
The highest growth ratio (3.5±0.1%) of Cyprinus carpio fry (with a mean initial live weight of 3.11±0.05 g) were obtained with the fish fed with Portulaca oleracea seed oil and coconut oil-supplemented feeds for 60 days instead of fish oil (Hildayanti et al., 2016). Similarly, the highest growth ratio (0.462±0.01%) was recorded in the T9 group which had the highest purslane extract ratio. Similarly, Ruiz (2017) investigated the effects of purslane plants on immune systems of sea breams and reported the SGR value for purslane supplemented and control groups as 0.56 and 0.64%, respectively. The effects of purslane plants on immune system of O. niloticus species have been studied by Razek et al. (2019). The SGR, FCR and survival rate were reported as 1.487±0.022, 1.44±0.032, 97.8%±2.22 for the control group and 1.328±0.051, 1.42±0.049, 100%±0.0 for the highest purslane supplemented group. An increase in weight was determined with the increase in protein in the diet of fish. The goldfish used in this study shows omnivorous feeding characteristics and needs high protein values during their fry (Kim et al., 2016; Ahmed and Maqbool, 2017).
However, this study focuses on the effects of purslane extract on growth and pigmentation; therefore, significantly differs from other studies. In addition, the effects of fresh, frozen and dried purslane and purslane extracts in diets on growth, reproduction and immune systems of poultry (hens and roosters), experimental animals (rats and rabbits) and birds, the effects of fresh, frozen, dried purslane and purslane extracts on growth, reproduction and immune systems were investigated. Similar to our study, in these studies with animals other than fish, it has been reported that the addition of purslane to the feed has a positive effect on growth parameters and disease (Aydın and Doğan, 2010; Ezekwe et al., 2011; Hozayen et al., 2011; Moneim et al., 2012; Zhao et al., 2013; Hanan et al., 2014; Kartikasari et al., 2017; Ramadan et al., 2017).
The effects of purslane extract on pigmentation of fish have not been studied by others. Therefore, the coloration data recorded in this study were discussed with the results of the studies carried out on different fish species using different coloration materials. The effects of red pepper feed on growth and skin coloration of yellow cichlids with an average initial live weight of 1.07±0.02 g have been investigated by Yılmaz and Ergün (2011). The SGR and FCR values of 3 experimental groups (control, 20 g kg-1 and 50 g kg-1 red pepper feed) were 0.62±0.02, 0.59±0.05, 0.68±0.02 and 4.31±0.19, 4.38±0.36, 3.80±0.04, respectively. Positive b* values reported by Yılmaz and Ergün (2011) were in accordance with our findings. Increasing doses of extract intensified the yellow coloration.
The effects of different carotenoid sources (anthocyanin, canthaxanthin, red pepper extract, gammarus) on growth performance and skin coloration of Carassius auratus species were studied (Yeşilayer et al., 2011b). The differences in growth parameters were not significantly different. The a* values in experimental groups increased depending on the carotenoid sources and the highest red color formation was recorded in the experimental groups compared to the control group. The results showed that the a* values of T6 thus, the red color formation was higher compared to the initial values and the control group, however, the differences in a* values between control and group T6 was statistically significant.
Hancz et al. (2003) were investigated body color intensity enhanced by paprika as feed additive in goldfish and koi carp using computer-assisted image analysis. In study, red, green and blue values of images were recorded and grayscale values of these colors were analyzed. The red values had a tendency to increase due to paprika feeding, but significant differences can be observed only after four weeks at the feeding rate and carotenoid concentration. Similarly, in our study increasing red color detected at the end of the experiment.
The best growth parameters (mean live weight gain, specific growth ratio, feed conversion ratio) at the end of the experiment were recorded in T9 group. The results revealed that purslane plant, which is highly nutritious and available in all around the country, could reliably be used as feed a feed additive in aquaculture activities.
Promising results were obtained on growth and color parameters. Vitamin A and E and phosphorus contents of T9 group were significantly higher than the control group (T0) (Table III). Previous studies concluded that purslane is especially rich in omega 3 fatty acids (Simopoulos et al., 1992; Petropoulus et al., 2019). Positive effects of purslane on fish growth and pigmentation may be attributed to the high omega 3 fatty acid composition of purslane. High reproductive vitamins, especially vitamin E of purslane indicates the importance of studies on reproduction. Therefore, further research is recommended to investigate the effects of purslane extracts on water quality, growth, skin coloration and reproduction of other fish species.
CONCLUSIONS
The best parameters (mean live weight gain, specific growth ratio, and survival rate) were recorded in the groups fed with purslane extract supplemented feeds. The purslane extract was supplemented for the first time into goldfish diets, and the effects of purslane extract on growth and pigmentation of goldfish were determined. Therefore, the results reported in this study are of special importance in aquaculture and may guide to the studies that will be carried out in the future.
ACKNOWLEDGMENTS
The authors are grateful to Sinop University Scientific Research Projects Coordinatorship for gently providing all things for this research (Project No. MYO 1901-15-01).
Statement of conflict of interest
The authors have declared no conflicts of interest.
REFERENCES
Ahmed, I. and Maqbool, A., 2017. Effects of dietary protein levels on the growth, feed utilization and haematobiochemical parameters of freshwater fish, Cyprinus carpio Var. specularis. Fish. Aquacul. J., 8: 1-12. https://doi.org/10.4172/2150-3508.1000187
Aydın, R. and Doğan, I., 2010. Fatty acid profile and cholesterol content of egg yolk from chickens fed diets supplemented with purslane (Portulaca oleracea L.). J. Sci. Fd. Agric., 90: 1759–1763. https://doi.org/10.1002/jsfa.4018
Devi, A., Surendran, A. and Thatheyus, A.J., 2019. Effect of chosen artificial fish feeds on the growth rate of the black molly, Poecilia sphenops. J. Vet. Med. Anim. Sci., 2: 1011-1016.
Ezekwe, M.O., Nyoka, Q.E., Besong, S.A. and Igbokwe, P.E., 2011. Dietary supplements of freeze-dried purslane leaves lower serum cholesterol in growing pigs. Res. J. Anim. Sci., 5: 27-33. https://doi.org/10.3923/rjnasci.2011.27.33
Hanan, A.A.E.A., Sobhy, M.H., Kawkab, A.A., Azza, K.A.E., Zeinab, A.R. and Wedad, A.H., 2014. Chemical and remedial effects of purslane (Portulaca oleracea) plant. Life Sci. J., 11: 31-42.
Hancz, C., Magyary, I., Molnar, T., Sato, S., Horn, P. and Taniguchi, N., 2003. Evaluation of color intensity enhanced by parika as feed additive in goldfish and koi carp using computer-assisted image analysis. Fish. Sci., 69: 1156-1159. https://doi.org/10.1111/j.0919-9268.2003.00740.x
Hildayanti, W., Setiawati, M. and Jusadi, D., 2016. Pemanfaatan minyak biji krokot Portulaca oleracea sebagai sumber asam lemak esensial pada pakan ikan mas, Cyprinus carpio Linnaeus 1758. J. Iktiol. Indonesia, 16: 145-157. https://doi.org/10.32491/jii.v16i2.37
Hozayen, W., Bastawy, M. and Elshafeey, H., 2011. Effects of aqueous purslane (Portulaca oleracea) extract and fish oil on gentamicin nephrotoxicity in albino rats. Nat. Sci., 9: 47-62.
Hunt, R.W.G., 1977. The spesification of colour appearance 1 consept and terms. Colour Res. Appl., 2: 55-68. https://doi.org/10.1002/col.5080020202
Karsli, Z., Aral, O. and Yeşilayer, N., 2016. The effects of different proportions of the 17 β -estradiol and 17 α-methyltestosterone on growth, sex reversal and skin coloration of the electric blue hap (Sciaenochromis ahli Trewavas, 1935). Aquacul. Res., 47: 640–648. https://doi.org/10.1111/are.12524
Karsli, Z., Sahin, D., Oz, M. and Aral, O., 2018. The effect of hormone (17α-methyltestosterone, 17β-estradiol) usage on development, sex inversıon and pigmentation of electric Blue streak hap (Labidochromis caeruleus Fryer, 1956). Appl. Ecol. environ. Res., 16: 8093-8103. https://doi.org/10.15666/aeer/1606_80938103
Kartikasari, L.R., Hertanto, B.S., Pranoto, D., Salim W.N. and Nuhriawangsa, A.M.P., 2017. Exterior and interior physical quality of egg of laying hens fed diets containing different dietary purslane levels. IOP Conf. Ser. Mater. Sci. Eng., 193: 012027. https://doi.org/10.1088/1757-899X/193/1/012027
Kim, K., Moniruzzaman, M., Kim, K., Han, H.S., Yun, H., Lee, S. and Bai, S.C., 2016. Effects of dietary protein levels on growth performance and body composition of juvenile parrot fish, Oplegnathus fasciatus. Int. Aquat. Res., 8: 239–245. https://doi.org/10.1007/s40071-016-0139-9
Lim, L.C., Dhert, P., Chew, W.Y., Dermaux, V., Nelis, H. and Sorgeloos, P., 2002. Enhancement of stress resistance of the guppy Poecilia reticulata through feeding with vitamin C supplement. J. World Aquacul. Soc., 33: 32-40. https://doi.org/10.1111/j.1749-7345.2002.tb00475.x
Moniem, A.A.E., Al-Nasr, I., Dkhil, M.A. and Al-Quraishy, S., 2012. Neuronal activities of Portulaca oleracea in adult rats. J. med. Pl. Res., 6: 3162-3168. https://doi.org/10.5897/JMPR11.1616
Nickell, D.C. and Bromage, N.R., 1998. The effect of dietary lipid level on variation of flesh pigmentation in rainbow trout (Oncorhynchus mykiss). Aquaculture, 161: 237-251. https://doi.org/10.1016/S0044-8486(97)00273-1
NRC (National Research Council), 1993. Nutrients requirements of fish. Washington. D.C., pp. 115.
Petropoulos, S.A., Fernandes, A., Dias, M.I., Vasılakoglou, I.B., Konstantınos, P., Barros, L. and Ferreira, I.C.F.R., 2019. Nutritional value, chemical composition and cytotoxic properties of common purslane (Portulaca oleracea L.) in relation to harvesting stage and plant part. Antioxidants, 8: 293. https://doi.org/10.3390/antiox8080293
Ramadan, B.K., Schaalan, M.F. and Tolba, A.M., 2017. Hypoglycemic and pancreatic protective effects of Portulaca oleracea extract in alloxan induced diabetic rats. BMC Complemen. Altern. Med., 17: 37. https://doi.org/10.1186/s12906-016-1530-1
Razek, A.N., Awad, S.M. and Abdel-Tawwab, M., 2019. Effect of dietary purslane (Portulaca oleracea L.) leavespowder on growth, immunostimulation, and protectionof Nile tilapia, Oreochromis niloticus against Aeromonas hydrophila infection. Fish Physiol. Biochem., 45:1907-1917. https://doi.org/10.1007/s10695-019-00685-8
Ruiz, M.C., 2017. Effects of purslane (Portulaca oleracea L.) and Shewanella putrefaciens probiotic enriched diet on gilthead seabream (Sparus aurata L.). Unıversıdade do Algarve Faculdade de Ciências e Tecnologia, pp. 75. https://core.ac.uk/download/pdf/154852743.pdf.
Sales, J. and Janssens, G.P.J., 2003. Nutrient requirements of ornamental fish. Aqua. Living Res., 16: 533-540. https://doi.org/10.1016/j.aquliv.2003.06.001
Simopoulos, A.P., Norman, H.A., Gillaspy, J.E. and Duke, J.A., 1992. Common purslane: a source of omega-3 fatty acids and antioxidants. J. Am. Coll. Nutr., 11: 374-382. https://doi.org/10.1080/07315724.1992.10718240
Uddin, M.K., Juraimi, A.S., Hossain, M.S., Nahar, M.A.U., Ali, M.E. and Rahman, M.M., 2014. Purslane weed (Portulaca oleracea): A prospective plant source of nutrition, omega-3 fatty acid and antioxidant attributes. Scient. World J., Article ID 951019. https://doi.org/10.1155/2014/951019
Wu, G., Yuan, C., Shen, M., Tang, J., Gong, Y., Li, D., Sun, F., Huang, C. and Han, X., 2007. Immunological and biochemical parameters in carp (Cyprinus carpio) after Qompsell feed ingredients for long-term administration. Aquacul. Res., 38: 246-255. https://doi.org/10.1111/j.1365-2109.2007.01660.x
Yeşilayer, N. and Erdem, M., 2011. Effects of oleoresin paprika (Capsicum annum) and synthetic carotenoids (canthaxantin and astaxanthin) on pigmentation levels and growth in rainbow trout Oncorhynchus mykiss W. J. Anim. Vet. Adv., 10: 1875-1882. https://doi.org/10.3923/javaa.2011.1875.1882
Yeşilayer, N., Aral, O., Karslı, Z., Öz, M., Karaçuha, A. and Yağcı, F., 2011. The effect of different carotenoid sources on skin pigmentation of goldfish (Carassius auratus). Israel. J. Aquacil. Bamidgeh, 63, 9 pages. http://hdl.handle.net/10524/36293
Yeşilayer, N., Kaymak, İ.E., Gören, H.M. and Karslı, Z., 2013. Balık yemlerinde balık ununa alternatif bitkisel protein kaynaklarının kullanım olanakları. Gaziosmanpaşa Üniversitesi Fen Bilimleri Enstitüsü Bilimsel Araştırma Dergisi, 4: 12-30 (in Turkish).
Yeşilayer, N., Mutlu, G. and Yıldırım, A., 2020. Effect of nettle (Urtica spp.), marigold (Tagees erecta), alfalfa (Medicago savita) extracts and synthetic xanthophyll (zeaxanthin) carotenoid supplementations into diets on skin pigmentation and growth parameters of electric Blue streak hap (Labidochromis caeruleus). Aquaculture, pp. 520. https://doi.org/10.1016/j.aquaculture.2020.734964
Yeşilayer, N., Öz, M., Karslı, Z., Aral, O., Karaçuha, A. and Öz, Ü., 2011a. Growth performance and feed utilization of koi carp (Cyprinus carpio, L., 1758) fed partial or total replacement of fish feed with hazelnut meal and soybean meal. J. Anim. Vet. Adv., 10: 1956-1961. https://doi.org/10.3923/javaa.2011.1956.1961
Yılmaz, S. and Ergün, S., 2011. Effect of red pepper (Capsicum annuum) on pigmentation of Blue Streak Hap (Labidochromis caeruleus). Israel. J. Aquacu;. Bamidgeh, IIC: 63.633, 6 pages.
Zhao, X.H., He, X., Yang, X.F. and Zhong, X.H., 2013. Effect of Portulaca oleracea extracts on growth performance and microbial populations in ceca of broilers. Poult. Sci., 92:1343–1347. https://doi.org/10.3382/ps.2012-02434
To share on other social networks, click on any share button. What are these?










