Germination, Winter Survival and Plant Growth of Sophora secondiflora as Affected by Sowing Dates and Seed Scarification
Research Article
Germination, Winter Survival and Plant Growth of Sophora secondiflora as Affected by Sowing Dates and Seed Scarification
Muhammad Ihtisham1, Noor Amjad2, Muhammad Nauman3, Asghar Ali3, Khawar Riaz3, Muhammad Sajid3, Muhammad Owais Shahid*3
1Sichuan Agricultural University, Chengdu, PR China; 2Islamia College University, Peshawar, Khyber Pakhtunkhwa, Pakistan; 3The University of Agriculture, Peshawar, Khyber Pakhtunkhwa, Pakistan; 4China Agriculture University, Beijing, PR China.
Abstract | Seeds are the most common and cheap source of propagation. However, several plant species have water-impermeable hard seeds which take a long time to germinate. The hard seed coat can be scarified successfully through acid treatment. Sophora is a beautiful ornamental plant and its seeds have the same issue. In this experiment, seeds of Sophora secondiflora were treated with H2SO4 and then sown at different dates. Both H2SO4 scarification and sowing time had a significant effect on germination and seedling growth. The seeds scarified with sulfuric acid for 135 min gave the maximum plant height (25.42 cm), leaves plant-1 (34.45), leaf area (8.00 cm2), stem thickness (0.47 cm), fresh leaves weight (3.31 g), fresh stem weight (1.95 g), root length (22.10 cm) and winter survival (94.44%). However, the same treatment also gave minimum days to germination (2.96) and seedling emergence (12.96). The seeds scarified for 90 min gave maximum plant emergence percentage (97.78%). The seeds sown on 14th April gave minimum days to germination (9.03) and seedling emergence (18.38), but also gave maximum plant emergence percentage (85.83%), plant height (22.63 cm), number of leaves (28.34), leaf area (7.13 cm2), stem diameter (0.39 cm), fresh leaves weight (2.79 g), stem fresh weight (1.55 g), length of roots (20.99 cm) and winter survival of plants (93.03%). It was concluded from the results that scarification durations of 90 and 135 min and sowing on 4th-14th April were the most effective in improving the germination, growth attributes and winter survival of Sophora.
Received | March 18, 2019; Accepted | February 03, 2021; Published | April 19, 2021
*Correspondence | Muhammad Owais Shahid, China Agriculture University, Beijing, PR China; Email: [email protected]
Citation | Ihtisham, M., N. Amjad, M. Nauman, A. Ali, K. Riaz, M. Sajid and M.O. Shahid. 2021. Germination, winter survival and plant growth of Sophora secondiflora as affected by sowing dates and seed scarification. Sarhad Journal of Agriculture, 37(2): 456-467.
DOI | https://dx.doi.org/10.17582/journal.sja/2021/37.2.456.467
Keywords | Sophora secondiflora, Germination, Scarification, Sulfuric acid, Sowing dates
Introduction
There are various sources of plant propagation such as seeds, cuttings, bulbs, etc. These sources depend upon the species and their effectiveness in that species. Seed are the most common and cheapest way of propagation for a lot of plant species. However, many of them have hard seed coat that doesn’t allow proper germination. Some plant species have hard seed coat which is impermeable to water and/or gases, such as plants of Leguminaceae family (Argel and Paton, 1999; Egley, 1989; Cavanagh, 1987; Tran and Cavanagh, 1984; Ballard, 1973). However, the seed coat-imposed dormancy is a delaying mechanism of some plant species. It doesn’t allow germination under unfavorable conditions. These seeds have the ability to remain dormant for a long time. This mechanism is found in those species grown under unpredictable climates, especially arid (dry) areas. Most of the leguminous species also have this ability. Nonetheless, this dormancy can be broken in order to obtain rapid, uniform and high germination (Teketay, 1996a). There are various methods to overcome dormancy imposed by hard seed-coat (Baskin and Baskin, 1998; Bewley and Black, 1994; Bradbeer, 1988). However, these methods vary from species to species. There is a need to formulate a species-specific method.
Sophora secundiflora (or mescal bean mountain laurel) is a small evergreen flowering tree of the Fabaceae/Leguminoseae family. It is believed to be native to southern America. It is a beautiful element in most of the landscape gardens (Crosswhite and Randall, 1985; Little, 1979), especially in alkaline and drought areas (Ruter and Ingram, 1991). The genus Sophora has various synonyms, such as Dermatophyllum, Calia, Broussonetia and Virgilia (Turner, 2012; Gandhi et al., 2011). It is propagated primarily through seed. Besides propagation, its red-colored hard seeds have other various uses such as in ornaments, jewelry and medicine (Murakoshi et al., 1986; Kingsbury, 1964; Vines, 1960). Because of its extremely hard seed coat, germination takes a long time (Froberg, 1985) as it is water impermeable. Germination gets more difficult when the seeds are stored for long (Smith and Pittock, 1989). The long period of seed dormancy can be shortened to a few days by subjecting to various treatments. Unfortunately, there is limited information concerning the potential seed dormancy problems of Sophora secondiflora. Several endogenous and exogenous factors can determine seed dormancy and, as a result, the methods vary.
There are various methods for breaking seed dormancy, such as seed scarification and heat treatment by fire (Aran et al., 2013; Gresta et al., 2011). However, choosing the intensity and duration of fire for breaking seed dormancy is very difficult (Saharjo and Watanabe, 1997) and using fire is more hazardous for experimental procedures. Seed scarification has been one of the methods traditionally used to break the hard seed coat. The most widely used chemical substance to scarify seeds having a hard coat is sulfuric acid at high concentrations (Lulanda, 1981). Sophora seed’s germination had been significantly improved with sulfuric acid scarification. Treating Sophora seeds with concentrated sulfuric acid from 10 to 120 mins not only increased the germination rate but also reduced the time to germination. It also resulted in better plant growth and development (Ghadiri and Niazi, 2005; Wang, 1991; Ruter and Ingram, 1991; Everitt, 1983a; Scowcroft, 1981). Sulfuric acid scarification has been found very effective in decreasing germination time, increasing germination rate and eventually improving plant growth attributes in hard seed coat plants. Those include plants from Leguminaceae family and others such as Turgenia Latifolia, Cuscuta sp., Sabal palmetto, Thrinax morrisii, Cycas revolota and Monotheca buxifolia (Huma et al., 2013; Li et al., 2013; Soliman and Abbas, 2013; Wang et al., 2013; Asl et al., 2011; Dewir et al., 2011; Merou et al., 2011; Pipinis et al., 2011; Zarchini et al., 2011; Contreras and Ruter, 2009; Olmez et al., 2007; Travlos et al., 2007a, b; Ghadiri and Niazi, 2005; Karaboon et al., 2005; Karaguzel et al., 2004; Uzun and Aydin, 2004; Rincon et al., 2003; Teketay, 1996a; Rehman et al., 1999).
Like other physiological phenomena, environmental conditions (temperature, light, humidity) have a great role in seed germination. There is a great variation in optimal germination temperature among plant species (Teketay, 1996b). Therefore, it is a need to have knowledge of sub and supra-optimal limits of temperature for seed germination. Little work has been done regarding the seed sowing time. Sophora sp. and other leguminous seeds when sown earlier (in winter or spring) had more time in soil, which resulted in higher germination percentage and better plant growth and performance (Yucedag and Gultihen, 2011; Nu et al., 2009; Mackay et al., 2002; Scowcroft, 1981). Germination of Sophora sp. is helped by temperatures higher than 20oC (Everitt, 1983a). There is a need to study the interaction between seed scarification treatments and sowing time in hard to germinate seeds.
The aims of the present study were to formulate effective acid scarification treatment to enhance seed germination of Sophora secundiflora, as well as to find its optimal sowing time. For this purpose, seeds of S. secundiflora were scarified with concentrated sulfuric acid and then sown at different dates. Results showed that there was a highly significant effect of acid scarification and sowing time on germination and seedling growth of Sophora. The sulfuric acid scarification treatment for 90 and 135 mins was the most effective in improving the attributes of Sophora. Moreover, sowing the seeds at high-temperature dates such as 4th and 14th April germinated more quickly and the seedlings performed very well. Hence in order to quickly germinate the hard seeds such as Sophora, seed scarification should be done for more time with concentrated sulfuric acid. Those seeds should be sown on higher temperature days. This experiment not only revealed that the hard seed coat and temperature are the main barriers inducing dormancy to the plant species such as Sophora; but also formulated methods to break such dormancy.
Materials and Methods
The current study was undertaken to investigate the effect of scarification and sowing times on germination and growth of Sophora’ at the ornamental horticulture nursery, department of horticulture, the university of agriculture, Peshawar-Pakistan. The experiment was laid out in Randomized Complete Block Design (RCBD) with two factors (duration of scarification and sowing dates) factorial arrangement having three replications. Mature seeds of Sophora secondiflora were carefully scarified in the laboratory with sulfuric acid for 0, 45, 90 and 135 min, followed by sowing the seeds at different dates with 10 days interval, i.e. 25th March, 4th April and 14th April. Intensive care was taken during handling and treatment of sulfuric acid. It is highly corrosive to human tissues and laboratory containers.
Seed scarification and sowing
For each treatment, seeds were gently and periodically agitated at the laboratory in 18N H2SO4 followed by a two-hour wash with distilled water at room temperature. After treatment, 10 seeds per treatment were rolled in moist paper towels. Then they were placed in polythene bags filled with canal silt for germination. The bags were irrigated each day and no kind of fertilizer or growth enhancer was applied.
Germination and growth analysis
The experiment was observed regularly to record data on germination rate and growth response. The days to 50% germination were counted when half of the seeds were germinated. A seed was considered germinated if the radical could be seen protruding through the seed coat (Ruter and Ingram, 1991). After that, the number of days taken by the radicals to emerge from the soil was recorded as days to 50% emergence. Then the total number of seedlings that emerged were counted and emergence percentage was calculated by using the following formula:

The percentage of plants that survived through winter was calculated by counting the number of plants survived from each treatment by the following formula:
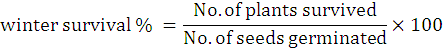
The plant height of five randomly selected plants was measured for each treatment with the help of measuring tape. The total number of leaves were counted for each treatment in five randomly selected plants. Leaf area was noted of randomly selected five leaves from five plants for each treatment with the help of leaf area meter. The stem thickness of randomly selected plants from each treatment were measured with the help of Vernier Caliper. The fresh weight of leaves and stems plant-1 was also calculated for each treatment in randomly selected plants. The length of primary roots of randomly selected plants in each treatment was measured with the help of measuring tape.
Statistical procedures
The data recorded were subjected to Analysis of Variance (ANOVA) technique appropriate for RCB design with two factors factorial arrangement. Means were compared by using Least Significance Differences (LSD) using STATIX version 8.1 (Jan et al., 2009).
Results and Discussion
The results of the studied attributes are presented and discussed in the following paragraphs:
Germination and seedling emergence
Data analysis showed that germination and seedling emergence of Sophora was significantly affected by sulfuric acid scarification and sowing dates. The results regarding the days to 50% seed germination (Figure 1a) revealed that the seeds left untreated (control) germinated in a maximum (32.02) number of days. The seeds treated with H2SO4 for 135 min germinated in minimum (2.96) days, which was at par with the value (3.07) given by the seeds scarified for 90 min. In the same parameter, the mean data related to sowing dates showed that the maximum number of days (12.44) to 50% germination was recorded in the seeds sown on 25th march. The minimum days (9.03) were taken by the seeds sown on 14th April. A similar trend was observed in the data about days to 50% seedling emergence (Figure 1b). The seedling emergence percentage (Figure 1c) showed that the
maximum seedling emergence (97.8%) was observed in 90 min scarification treatment, followed by 45 min and 135 min treatment, while the minimum (56.7%) was recorded in the control treatment. In regard to sowing dates, the maximum seedling emergence (85.8%) was observed in the seeds sown on 14th April, which was at par with the emergence of seedlings (83.3%) given by the seed sown on 4th April. The minimum (76.7%) was recorded in the plants of the seeds sown on 25th March.
The most limiting factor in plant propagation is seed dormancy. There are many causes of seed dormancy, in which the hard seed coat is the most common. This hard seed coat blocks the water from entering the seed that delays the germination (Ballard, 1973). Germination is the imbibition of water and breaking of the hard seed coat. Hence breaking of the hard seed coat is very important in the early and effective germination of a seed and the emergence of its seedling. The acid scarification (especially sulfuric acid) is highly effective for softening the hard seed coat in many leguminous species, like Cassia (Al-Menaie et al., 2010). Hence, the seeds scarified for more time eventually germinated and emerged in less time. Moreover, the seeds of Sophora are temperature sensitive. They require high temperatures for effective and early germination, as well as for seedling emergence. The decrease in days to germination and emergence of Sophora might be due to the fact that with ascending sowing dates, the temperature also increased in the area. Everitt (1983a, b) stated that Sophora seeds usually germinate in temperatures around 20 to 30oC. These results are in close similarity with the findings of Soliman and Abbas (2013) who observed that Cassia fistula seeds germinated quickly when subjected to sulfuric acid scarification and hot water treatment. Similarly, Vivekmitter et al. (1993) found quick germination of Psoralea corylifolia seeds when treated with concentrated sulfuric acid and heat application. The reason for results regarding seedling emergence percentage might be that the seeds that germinated quickly and didn’t stay for longer in the soil had maximum percent emergence. On the other hand, the ones that stayed for months in the soil were damaged. However, the acid pre-treatment could also cause damage to the seeds and the embryo inside. That is why the acid treatment at the highest duration (135 min) caused damage and thus the emergence percentage was lower.
Winter survival
The plants that survived through winter were observed (Figure 1d), which showed that the highest number of plants (94.4%) were survived in the seed scarification treatment for 135 min, which was in harmony with the other two levels. The lowest survival (75%) was observed in the control treatment. Winter survival rate as affected by sowing dates showed that the maximum survival (93.1%) was recorded in the plants of the seeds sown on 14th April, which was at par with the subsequent treatment. The minimum winter survival (83.0%) was given by the plants of the seeds sown on 25th March.
According to the results, the winter survival percentage was enhanced in the plants of the seeds treated with sulfuric acid and sown on later dates. It might be because these plants were stronger and healthier as compared to the others. The plants were stronger because they had more root growth (as given in Figure 2c), more height (Figure 2a) and more number of leaves (Figure 2b), that enabled the plants to perform well to fight the harsh winter. The root growth helped the plants to absorb more minerals and moisture. It gave better quality to the plants to perform well in winter. Photosynthesis rate might also be higher in these plants because of more number and size of leaves. Photosynthesis enhances the energy production, which might have made the plants stronger to withstand harsh weather like winter. Another reason for such results might be that these plants emerged very early (Figure 2b). It provided the plants with a greater number of days of summer to grow and develop so that they can survive in the low winter temperatures. These results can be confirmed with the observations of Zoghi et al. (2011) and El-Juhany et al. (2009) who found that the seeds treated with sulfuric acid gave maximum plant survival through winter. Zhang et al. (2009) found that early plant emergence of Sophora flavescens enhanced the root to grow thicker and went deeper into the soil. Thus, more water and nutrients were absorbed that resulted in good quality plants to withstand winter.
Plant growth
Analysis of the data regarding plant growth attributes revealed that plant height, number of leaves, leaf area, stem thickness, fresh plant weight and root length were significantly influenced by sulfuric acid scarification and different sowing dates. The data analysis for plant height (Figure 2a) suggested that the highest plants (25.42 cm) were recorded in the treatment having seed scarification for 135 min, which was at par with the value (24.03 cm) given by scarification treatment of 90 min. The minimum plant height (15.47 cm) was given by control treatment. The observations for the effect of sowing dates on Sophora showed that the maximum plant height (22.63 cm) was recorded in seeds sown on 14th April, which was in harmony with the value (21.68 cm) given by the plants grown from the seeds sown on 4th April, whereas the minimum (19.39 cm) was observed in the seeds sown on 25th march. Almost a similar trend was observed in the data analyzed for leaves quantity plant-1 (Figure 2b) and root length (Figure 2c). The data analyzed for leaf area (Figure 2d) proved that the highest value (8.00 cm2) was given by the plants raised from the seeds scarified for 135 min, which was almost similar to the leaf area given by 90 min treatment. However, it was at par with 45 min treatment. The minimum leaf area (4.74 cm2) was recorded in the plants grown from the seed without any scarification treatment. The data for the same parameter regarding sowing dates showed that the maximum leaf area (7.13 cm2) was observed in the plants of the seeds sown on 14th April. The minimum (6.05 cm2) was given by the plants of the seeds sown on 25th March. However, the leaf area of the seeds sown on 4th April was at par with that of both 14th April and 25th March. Figure 3a shows data analyzed for stem thickness, which revealed that the thickest stems (0.47 cm) were recorded in the treatment involving seed soaked in sulfuric acid for 135 min. The thinnest stems (0.22 cm) were observed in the plants raised from the seeds without sulfuric acid treatment. Different sowing dates showed that maximum stem thickness (0.39 cm) was given by the plants of the seeds sown on 14th April. The minimum stem thickness (0.33 cm) was recorded in plants of the seeds sown on 25th March. The data analysis for fresh plant weight (Figure 3b, c) proved that the maximum fresh weight of leaves (3.31 g) and stems (1.95 g) were given by the plants raised from the seeds treated for 135 min with sulfuric acid, followed by the 90 and 45 min treatment. The minimum fresh weight of leaves (1.76 g) and stems (0.77 g) was observed in the control treatment. Seeds sown on 14th April gave the maximum fresh weight of leaves (2.79 g) and stems (1.55 g) while the minimum values (2.49 g and 1.35 g, respectively) were given by the control treatment.
It is obvious from these results that the seeds that were scarified for a longer duration and sown on later dates (in which temperature was higher) had better plant growth attributes, as compared to un-scarified seeds and the seeds that were sown on early dates. The reason for such results is that the plants that had better growth, emerged and germinated earlier as compared to the suppressed plants. In other words, the seeds that germinated quickly enhanced earlier seedling emergence. In this way, more time was there for the increment of vegetative growth parameters of these plants, such as plant height, leaves quantity, leaf area, stem thickness, fresh plant weight and root length. All these attributes have inter-correlative roles. The shoot and root growth are positively correlated to each other. The bigger leaf area and number of leaves
had more photosynthesis which enhanced the plants to have longer and thicker stems as well as more root growth. Moreover, due to comparatively higher temperatures on later sowing dates, the respiration rate might also be higher due to which the plants attained more water and nutrients from the soil and hence plant growth was improved. Hence, early seed germination, early seedling emergence and optimum temperature are essential for better plant growth.
The influence of acid pre-treatments on plant growth has been reported by several scientists. Olatunji (2013) observed that the seeds treated with acid had longer plants, more quantity of leaves and thicker stems. Soliman and Abbas (2013) found improvement in leaf area and root length of the plants due to seed scarification with acid and hot water. Increment in plant height after seed scarification with acid has been reported by El-Juhany et al. (2009), Oloridnrnaiye et al. (2004) and Khan (2001). The number of leaves per plant was also increased due to pre-sowing treatment with acid (Offiong et al., 2010; Hossain et al., 2001). Similarly, Mabundza et al. (2010) and Anim-Kuapong and Teklehaimanot (2001) recorded the maximum root length of plants when seeds were treated with sulfuric acid. Improvement in leaf area has been found by Joshi and Pant (2010), Mehta and Sen (1990) and Bhuyar et al. (2000) when seeds were treated with acid before sowing. Fresh weight of the leaves and stem was also improved in the treatments involving acid scarification of seeds (Habibpour et al., 2013; Takhti and Shekafandeh, 2012; Nath et al., 2007). On the other hand, the positive impact of different sowing dates on plant growth attributes has
Table 1: Correlation matrix among parameters showing different correlation trends.
| Days to 50% germ-ina-tion | Days to 50 % emer-gence | Emerge-nce % | Survi-val % | Plant height |
Leaves plant-1 |
Root length | Leaf area | Stem thick-ness | Lea-ves fresh wt. | Stem fresh wt. | |
|
Days to 50% germination |
1 | 0.99 | -0.94 | -0.90 | -0.76 | -0.81 | -0.75 | -0.80 | -0.84 | -0.89 | -0.89 |
|
Days to 50% emergence |
0.99 | 1 | -0.95 | -0.92 | -0.83 | -0.87 | -0.82 | -0.85 | -0.89 | -0.93 | -0.92 |
| Emergence % | -0.94 | -0.95 | 1 | 0.90 | 0.79 | 0.77 | 0.79 | 0.76 | 0.78 | 0.84 | 0.83 |
| Survival % | -0.90 | -0.92 | 0.90 | 1 | 0.76 | 0.79 | 0.82 | 0.76 | 0.80 | 0.85 | 0.84 |
| Plant height | -0.76 | -0.83 | 0.79 | 0.76 | 1 | 0.94 | 0.96 | 0.94 | 0.95 | 0.92 | 0.91 |
|
Leaves plant-1 |
-0.81 | -0.87 | 0.77 | 0.79 | 0.94 | 1 | 0.92 | 0.90 | 0.95 | 0.98 | 0.98 |
| Root length | -0.75 | -0.82 | 0.79 | 0.82 | 0.96 | 0.92 | 1 | 0.93 | 0.92 | 0.92 | 0.91 |
| Leaf area | -0.80 | -0.85 | 0.76 | 0.76 | 0.94 | 0.90 | 0.93 | 1 | 0.97 | 0.92 | 0.91 |
| Stem thickness | -0.84 | -0.89 | 0.78 | 0.80 | 0.95 | 0.95 | 0.92 | 0.97 | 1 | 0.96 | 0.95 |
| Leaves fresh wt. | -0.89 | -0.93 | 0.84 | 0.85 | 0.92 | 0.98 | 0.92 | 0.92 | 0.96 | 1 | 1 |
| Stem fresh wt. | -0.89 | -0.92 | 0.83 | 0.84 | 0.91 | 0.98 | 0.91 | 0.91 | 0.95 | 1 | 1 |
also been recorded. Kakaraparthi et al. (2011) observed increased plant height, leaf quantity and fresh leaves weight when the seeds were sown on different dates with ascending temperature. Moosavi (2012) and Suna et al. (2007) found improved stem diameter and fresh plant weight when the plant seeds were sown on different dates. Enhancement in plant height, root length, stem thickness and fresh leaf weight due to various sowing dates with ascending temperature has been reported by Seghatoleslami et al. (2013), Jafari (2010), Hussein (2008), Bharath (2008), Amiri (2003) and Morin and Dormency (1993), respectively.
Correlation among the studied parameters showed varying trends in the variables (Table 1). Days to 50% germination and emergence were positively correlated with each other, whereas negatively correlated with all other variables. On the other hand, emergence %, survival %, plant height, leaves plant-1, root length, leaf area, stem thickness, fresh wt. of leaves and stem were all positively correlated with each other while negatively correlated with days to 50% germination and emergence.
Conclusions and Recommendations
The influence of sulfuric acid scarification and sowing dates on the germination and growth of Sophora was studied in an experiment carried out at Ornamental Horticulture nursery, Department of Horticulture, The University of Agriculture, Peshawar-Pakistan. It included treatment (scarification) of the seeds of Sophora secondiflora with 18N sulfuric acid for different duration, i.e. 0, 45, 90 and 135 min and then sown on different dates of spring, i.e. 25th March, 4th April and 14th April. Various attributes regarding growth and germination of Sophora plants were studied. From the obtained results, it can be concluded that most of the parameters of Sophora seeds and plants were significantly affected by sulfuric acid scarification and sowing dates. The scarification or soaking duration of 135 min was the most effective in improving the attributes of Sophora. However, its effect was statistically at par with the 90 min duration in almost all the parameters. Moreover, sowing the Sophora seeds on 4th and 14th April gave plants with early germination and emergence and other growth attributes. As there was a quick seed germination and seedling emergence due to acid scarification and warmer sowing dates, the Sophora plant growth was enhanced that made it survive through the winter. From these results, it could be recommended that Sophora seeds should be treated with 18 N sulfuric acid for the duration of 90 minutes and sown in late spring or early summer for enhanced germination, seedling emergence, plant growth and winter survival.
Acknowledgements
We are thankful to Prof. Dr. Abdul Mateen Khattak, Prof. Dr. Abdur Rab and Prof. Dr. Raziuddin for their valuable feedback and assistance in the experiment designing and layout; and all the working staff of the nursery who helped during this research. Special thanks and prayers for Prof. Dr. Muhammad Zubair (L).
Novelty Statement
This research affirmed that the hard seed coat and temperature are the major hurdles inducing dormancy to the plant species like Sophora secondiflora. Meanwhile, it also helped us to develop techniques to break such dormancy.
Author’s Contribution
MI and NA designed the experiments and wrote the first draft. AA, MOS, KR and MI did data collection and interpretation. MN and NA analyzed the data and proofread the manuscript. MOS and MS made the final draft. All the authors contributed equally to this research work, article, read and approved the final manuscript.
Conflict of interest
The authors have declared no conflict of interest.
References
Al-Menaie, H.S., O. Al-Ragam, A. Al-Shatti, M. Mathew and N. Suresh. 2010. The effects of different treatments on seed germination of the Cassia fistula L. and Cassia nodosa Buch.-Ham. Ex Roxb. in Kuwait. Afr. J. Agri. Res. 5(3): 230-235.
Amiri, H., 2003. Effect planting date and plant density on forage and seed of corn in Gorgan. Thesis on Agriculture, Islamic Azad University, Birjand Branch, Iran, pp. 98.
Anim-Kuapong, G.J. and Z. Teklehaimanot. 2001. Albizia zygia (DC) Macbride, a shade tree or cocoa. The effects of duration of acid scarification and substrate acidity on the germination of seeds. For. Trees Livehoods, 11: 47-55. https://doi.org/10.1080/14728028.2001.9752370
Arán, D., J. García-Duro, O. Reyes and M. Casal. 2013. Fire and invasive species modifications in the germination potential of Acacia melanoxylon, Conyza canadensis and Eucalyptus globulus. For. Ecol. Manage. 302: 7-13. https://doi.org/10.1016/j.foreco.2013.02.030
Argel, P.J. and C.J. Paton. 1999. Overcoming legume hardseedness. In: Loch, D.S. and J.E. Ferguson (eds), Forage seed production, Volume 2: Tropical and Subtropical Species. CAB Intern, UK. pp. 247-259.
Asl, M.B., R. Sharivivash and A. Rahbari. 2011. Effect of different treatments on seed germination of honey locust (Gleditschia triacanthos). Modern App. Sci. 5(1): ISSN 1923-1844. https://doi.org/10.5539/mas.v5n1p200
Ballard, L.A.T., 1973. Physical barriers to germination. Seed Sci. Technology, 1: 285-303.
Baskin, C.C. and J.M. Baskin. 1998. Seeds: Ecology, biogeography and evolution of dormancy and germination. Academic Press, San Diego.
Bewley, J.D. and M. Black. 1994. Seeds: Physiology of development and germination, 2nd Edn. Plenum press, New York. https://doi.org/10.1007/978-1-4899-1002-8
Bharath, I.K., 2008. Standardization of seed testing procedures and storage studies in selected medicinal crops. M.Sc. thesis. College of Agriculture, Dharwad, Uni. of Agricultural Sciences, Dharwad.
Bhuyar, S.A., S.G. Wankhade, J.T. Paturde and P.T. Khode. 2000. Seed germination studies in Sarpagandha (Rauvolfia serpentina Benth). Res. Crops, 1(2): 189-191.
Bradbeer, J.W., 1988. Seed dormancy and germination. Blackie and Son Ltd, London. https://doi.org/10.1007/978-94-011-6574-7
Cavanagh, T., 1987. Germination of hard-seeded species (order Fables). In: Langkamp (Editor), Germination of Australian Native Plant Seed. AMIRA, Melbourne/Sydney, pp. 58-70.
Contreras, R.N. and J.M. Ruter. 2009. Sulfuric acid scarification of Callicarpa americana L. (Lamiaceae) seeds improves germination. Native Plants J., 10(3): 283-286. https://doi.org/10.2979/NPJ.2009.10.3.283
Crosswhite, C.D. and C. Randall. 1985. Damage to mescal bean (Sophora secundiflora) by a Pyralid moth (Uresiphita reversalis). Desert Plants, 7(1): 32.
Dewir, Y.H., M.E. El-Mahrouk and Y. Naidoo. 2011. Effects of some mechanical and chemical treatments on seed germination of Sabal palmetto and Thrinax morrisii palms. Aust. J. Crop Sci., 5(3): 248-253.
Egley, G.H., 1989. Water-impermeable seed coverings as barriers to germination. In: R.B. Taylorson (Ed.), Recent Advances in the development and Germination of Seeds. Plenum Press, New York, pp. 207-223. https://doi.org/10.1007/978-1-4613-0617-7_16
El-Juhany, L.I., I.M. Aref and M.A. Al-Ghamdi. 2009. Effects of different pretreatments on seed germination and early establishment of the seedling of Juniperus procera trees. World Appl. Sci. J., 7(5): 616-624.
Everitt, J.H., 1983a. Germination of Mescal bean (Sophora secundiflora) seeds. The Southwestern Naturalist, 28(4): 437-443. https://doi.org/10.2307/3670824
Everitt, J.H., 1983b. Seed germination characteristics of three woody plant species from south Texas. J. Range Manage., 36(2): 246-249. https://doi.org/10.2307/3898175
Froberg, C.A., 1985. Tissue culture propagation of Sophora secundiflora. Proc. Intl. Plant Prop. Soc., 35: 750-754.
Gandhi, K.N., M.A. Vincent and J.L. Reveal. 2011. Dermatophyllum, the correct name for Calia (Fabaceae). Phytoneuron, 57: 1-4.
Ghadiri, H. and M. Niazi. 2005. Effects of scarification and stratification on seed germination and dormancy of Turgenia Latifolia, Cuscuta sp. and Sophora Alopecuroides in different temperatures Regimes. Iran Agric. Res., 23(2).
Gresta, F., G. Avola, R. Tuttobene, A. Onofri, V. Barrile, A. Cristaudo and V. Abbate. 2011. The effect of fire on the dormancy break of annual legume seeds. Ital. J. Agron., 6(3): e23. https://doi.org/10.4081/ija.2011.e23
Habibpour, S., M. Shamili and M.A. Soltanipour. 2013. Effect of chemical and physical treaments on seed germination and seedling growth of Cymbopogon olivieri (boiss) Bar. Adv. Crop Sci., 3(9): 599-604.
Hossain, M.K., B.M. Khan and B. Koirala. 2001. Effects of pre-owing treatments on Tectona grandis. Silviculture. Collage of Forestry and Natural Resources, University of Philippines. pp. 23-30.
Huma, Z., A. Rashid, M. Ibrar, Barkatullah and I. Hameed. 2013. Effects of chemical and mechanical scarification treatments on germination rate of Monotheca buxifolia (Flac) seeds. PJLS. 1(1): 17-27.
Hussein, M.M.M., 2008. Studies on the rooting and the consequent plant growth of stem cuttings of Thunbergia grandiflora, (Roxb cx Rottl.) Roxb effect of different planting dates. World J. Agric. Sci., 4(2): 125-132.
Jafari, M., 2010. Effect of planting date, irrigation intervals and nitrogen rates on morphological and yield of forage millet. M.Sc. thesis on Agri. Islamic Azad University, Birjand Branch, Iran, pp. 113.
Jan, M.T., P. Shah, P.A. Hollington, M.J. Khan and Q. Shohail. 2009. Agriculture research: Design and analysis. 1st ed. Dept. of Agronomy, KPK Agric. Univ. Peshawar, Pakistan.
Joshi, S.C. and S.C. Pant. 2010. Effect of H2SO4 on seed germination and viability of Canna indica L. a medicinal plant. J. Am. Sci., 6(6): 24-25.
Kakaraparthi, P.S., D.K. Rajput, K. Komaraiah, N. Kumarand and R.R. Kumar. 2011. Effect of sowing dates on morphological characteristics. CIMAP Res. Centre, Boduppal, India.
Karaboon, S., S. Ripona, S. Thanapornpoonpong, E. Pawelzik and S. Vearasilp. 2005. Breaking dormancy and optimum temperature of golden shower (Cassia fistula) seeds. Conf. Int. Agric. Res. Dev.,
Karaguzel, O., S. Cakmakcl, V. Ortacesme and B. Aydinoglu. 2004. Influence of seed coat treatment on germination and early seedling growth of Lupinus Varius. Pak. J. Bot., 36(1): 65-74.
Khan, M.S., 2001. Floral diversity of Bangladesh and its conservation. Bangladesh National Herbarium, Dhaka pp. 135.
Kingsbury, J.M., 1964. Poisonous plants of the United States and Canada. Englewood Cliffs, NJ: Prentice-Hall, Inc. pp. 626. https://doi.org/10.1097/00010694-196411000-00022
Li, S., T. Shi, F. Kong, Q. Ma, Y. Mao and T. Yin. 2013. Methods for breaking the dormancy of eastern redbud (Cercis canadensis) seeds. Seed Sci. Tech., 41(1): 27-35. https://doi.org/10.15258/sst.2013.41.1.03
Little, E.L., 1979. Checklist of United States trees (native and naturalized). Agric. Handbook. 541. Washington, DC: USDA Forest Service. pp. 375.
Lulanda, L., 1981. Seed viability, germination and pretreatment of Leucaena leucephala. Leucaena Res. Report. 2: 59-62.
Mabundza, R.M., P.K. Wahome and M.T. Masarirambi. 2010. Effects of different pre-germination treatment methods on the germination of passion (Passiflora edulis) Seeds. J. Agric. Soc. Sci., 6(3).
Mackay, A.C., C.R. Mcgilla, D.W. Fountaina and R.C. Southwarda. 2002. Seed dormancy and germination of a panel of New Zealand plants suitable for re-vegetation. N. Z. J. Bot., 40(3): 373-382. https://doi.org/10.1080/0028825X.2002.9512798
Mehta, M. and D.M. Sen. 1990. Effect of different photoperiod and light of different wave length on seed germination of Withania somnifera (Linn.) Dunal. Sci. Cult., 56(7): 300-301.
Merou, T., I. Takos, E. Konstantinidou, S. Galatsidas and G. Varsamis. 2011. Effect of different pretreatment methods on germination of Albiziz julibrissin seeds. Seed Sci. Tech., 39(1): 248-252. https://doi.org/10.15258/sst.2011.39.1.26
Moosavi, S.G., 2012. Effect of planting date and plant density on morphological traits and LAI of forage corn (Sc. 370) yield in second cultivation. Int. Res. J. Appl. Basic Sci., 3(1): 57-63.
Morin, C.D. and H. Dormency. 1993. Limits of a simple model to predict yield losses in maize. Weed Res., 33: 248-261. https://doi.org/10.1111/j.1365-3180.1993.tb01940.x
Murakoshi, I., H. Kubo, M. Ikram, M. Israr, N. Shafi, S. Ohmiya and H. Otomasu. 1986. (+)-11-oxocytisine, a lupin alkaloid from leaves of Sophora secundiflora. Phytochemistry. 25(8): 2000-2002. [Horticultural Abstracts 56: 9115; 1986]. https://doi.org/10.1016/S0031-9422(00)81198-X
Nath, D.D., M.M. Rahman, G.M.M. Rahman, K.K. Islam and M.A. Mondal. 2007. Effect of pre-treatment of seeds of Kalo koroi [Albizia lebbeck (L.) Benth.] on germination and seedling growth. J. Agrofor. Environ., 1(2): 43-46.
Nu, Z., L. Hai-ming and J.I. Ying. 2009. Effects of sowing dates on growth and winter survival rate of Sophora flavescens. J. Gansu Agric. Univ., 44(4): 77-80.
Offiong, M.O., S.I. Udofia and I.N. Ufot. 2010. Comparative study of pre-germination treatments and their effects on the growth of Tectona grandis (Linn. F) seedlings. Afr. Res. Rev., 4(3b): 368-378. https://doi.org/10.4314/afrrev.v4i3.60275
Olatunji, D., J.O. Maku and O.P. Odumefun. 2013. The effect of pre-treatments on the germination and early seedlings growth of Acacia auriculiformis Cunn. Ex. Benth. Afr. J. Plant Sci., 7(8): 325-330.
Olmez, Z., A. Gokturk and F. Temel. 2007. Effects of cold stratification, sulfuric acid, submersion in hot and tap water pretreatments on germination of bladder-senna (Colutea armena Boiss. and Huet.) seeds. Seed Sci. Tech., 35(2): 266-271. https://doi.org/10.15258/sst.2007.35.2.02
Oloridnrnaiye, K.S., P.O. Faloba and I. Nathaniel. 2004. Effect of seed coat removal on seed germination and seedling. NISEB J., 4(1): 5-9.
Pipinis, E., E. Milios, P. Smiris and C. Gioumousidis. 2011. Effect of acid scarification and cold moist stratification on the germination of Cercis siliquastrum L. seeds. Turk. J. Agric., 35: 259-264.
Rehman, S., R.N.J. Loescher and P.J.C. Harris. 1999. Dormancy breaking and germination of Acacia salicina Lindl. seeds. Seed Sci. Technol., 27(2): 553–557.
Rincon, R.R., N.R.C. Espinosa, F.A.G. Micceli and Dendooven. 2003. Scarification of seeds of Acacia angustissima (Mill.) ‘kuntz’ and its effect on germination. Seed Sci. Tech., 31(2): 301-307. https://doi.org/10.15258/sst.2003.31.2.07
Ruter, J.M. and D.L. Ingram. 1991. Germination and morphology of Sophora secundiflora seeds following scarification. Hortic. Sci., 26(3): 256-257. https://doi.org/10.21273/HORTSCI.26.3.256
Saharjo, B.H. and H. Watanabe. 1997. The effect of fire on the germination of Acacia mangium in a plantation in south Sumatra, Indonesia. Common Wealth For. Rev., 76: 128-131.
Scowcroft, P.G., 1981. Regeneration of mamane: Effects of seed coat treatment and sowing depth. For. Sci., 27(4): 771-779.
Seghatoleslami, M.J., S.G. Mousavi and T. Barzgaran. 2013. Effect of irrigation and planting date on morpho-physiological J. Anim. Plant Sci., 23(1): 256-260.
Smith, G.C. and K. Pittcock. 1989. The collector’s quest. Am. Nurseryman, 169(1):56-65.
Soliman, A.S. and M.S. Abbas. 2013. Effects of sulfuric acid and hot water pre-treatments on seed germination and seedling growth of Cassia fistula L. Am. Eurasium J. Agric. Environ. Sci., 13(1): 7-15.
Suna, H., X. Zhang, S. Chena, D. Pei and Ch. Liu. 2007. Effects of harvest and sowing time on the performance of the rotation of winter wheat–summer maize in the North China plain. Ind. Crops Prod., 25: 239-247. https://doi.org/10.1016/j.indcrop.2006.12.003
Takhti, S. and A. Shekafandeh. 2012. Effect of different seed priming on seed germination rate and seedling growth of Zizphus spina-chresti. Adv. Environ. Biol., 6(1): 159-164.
Teketay, D., 1996a. Germination ecology of twelve indigenous and eight exotic multipurpose leguminous species from Ethiopia. For. Ecol. Manage. 80(1-3): 209-223. https://doi.org/10.1016/0378-1127(95)03616-4
Teketay, D., 1996b. Seed ecology and regeneration in dry Afromontane forests of Ethiopia. Doctoral thesis, Swedish University of Agricultural Sciences, Umea.
Tran, V.N. and A.K. Cavanagh. 1984. Structural aspects of dormancy. In: Murray, D.R. (eds), Seed Physiology, Vol. 2, Germination and Reserve Mobilization. Academic Press, Sydney. pp. 1-44. https://doi.org/10.1016/B978-0-12-511902-3.50006-3
Travlos, I.S., G. Economou and A.J. karamanous. 2007a. Seed germination and seedling emergence of Spartium junceum in response to heat and other pre-sowing treatments. Agron. J., 6(1): 152-156. https://doi.org/10.3923/ja.2007.152.156
Travlos, I.S., G. Economou and A.I. Karamanos. 2007b. Germination and emergence of the hard seed coated Tylosema esculentum (Bruch) A. Schreib in response to different pre-sowing seed treatments. J. Arid Environ., 68(3): 501-507. https://doi.org/10.1016/j.jaridenv.2006.07.001
Turner, B.L., 2012. New names in Dermatophyllum (Fabaceae). Phytoneuron, 3: 1–4.
Uzun, F. and I. Aydin. 2004. Improving Germination Rate of Medicago and Trifolium species. Asian J. Plant Sci., 3(6): 714-717. https://doi.org/10.3923/ajps.2004.714.717
Vines, R.A. 1960. Trees, shrubs and woody vines of the Southwest. Austin, TX: University of Texas Press. pp. 1104.
Vivekmitter, K., Srinivasan and B.M. Singh. 1993. Overcoming hard seededness on Psorlea carylifolia. Seed Res., 21(1): 31-34.
Wang, Y.R., J. Hanson and Y.W. Mariam. 2013. Effects of sulfuric acid pretreatments on breaking hard seed dormancy in diverse accessions of five wild Vigna species. Seed Sci. Tech., 35(3): 550-559. https://doi.org/10.15258/sst.2007.35.3.03
Wang, Y.T., 1991. Enhanced germination of Sophora secundiflora seeds. Subtrop. Plant Sci., 44: 37-39. [Seed Abstracts 15: 3304; 1992].
Yucedag, C. and H.C. Gultekin. 2011. The effect of sowing time on germination of twenty-two leguminosae species. Afr. J. Agric. Res., 6(16): 3809-3816.
Zarchini, M., D. Hashemabad, B. Kaviani, P.R. Fallahabad and N. Negahdar. 2011. Improved germination condition in Cycas revolute L. by using sulfuric acid and hot water. POJ. 4(7): 350-353.
Zhang, N.L., Hai-ming and J.I Ying. 2009. Effects of sowing dates on growth and winter survival rate of Sophora flavescens. J. Gansu Agric. Univ.,
Zoghi, Z., D. Azadfar and Y. Kooch. 2011. Effect of different trearments on seed dormancy breaking and germnination of caspian Locusta (Gleditschia caspica) tree. Ann. Biol. Res., 2(5): 400-406.
To share on other social networks, click on any share button. What are these?







