Evaluation of Trichoderma harzianum and Azadirachta indica in the Management of Meloidogyne incognita in Tomato
Evaluation of Trichoderma harzianum and Azadirachta indica in the Management of Meloidogyne incognita in Tomato
Wajahat Azeem1,*, Tariq Mukhtar1 and Tooba Hamid2
1Department of Plant Pathology, Pir Mehr Ali Shah Arid Agriculture University, Rawalpindi
2Department of Zoology, University of Gujrat, Gujrat
ABSTRACT
Root-knot nematodes of the genus Meloidogyne badly affect crop production worldwide and cause huge yield losses. Among the known root-knot species, Meloidogyne incognita is by far the most destructive, widely distributed and the most dominant and prevalent. In the present study, the efficacy of a biological control agent, Trichoderma harzianum and an antagonistic plant, Azadirachta indica was tested against M. incognita on tomato. The antagonistic fungus and plant caused significant hatching inhibition and larval mortality of M. incognita. The hatching inhibition and mortality was the maximum at 100% concentrations of both the agents while the minimum inhibition and mortality was obtained at 25% concentration. No statistical difference was observed between T. harzianum and A. indica in causing hatching inhibition and larval mortality. Numbers of galls, egg masses and reproductive factor were reduced significantly as a result of A. indica and T. harzianum applications. The reductions in these parameters were more pronounced where both the agents were integrated and resulted to the maximum where both the agents were mixed at the highest concentrations. The reductions in galls, egg masses and reproductive factor were found inversely proportional to the concentrations of A. indica and T. harzianum. Similarly, all the concentrations of A. indica when integrated with T. harzianum increased plant height and fresh shoot weight significantly over control. The increase in these parameters was directly proportional to the concentrations of A. indica leaves and T. harzianum. A. indica amendments proved at par with those of T. harzianum. The maximum increases in these parameters were obtained where A. indica leaves were mixed at the rate of 50 g with 6 g of T. harzianum. The amendments also showed significant effects on root weight. The maximum decrease in root weight was observed where both A. indica and T. harzianum were mixed at the highest concentrations. The root weight decreased with an increase in concentration and was found inversely proportional. It is therefore, recommended that the integration of antagonistic plants with the antagonistic fungi may be useful for the better control of plant parasitic nematodes.
Article Information
Received 05 September 2019
Revised 02 November 2019
Accepted 17 December 2019
Available online 11 December 2020
Authors’ Contribution
WA and TH designed the study, executed experimental work, analyzed the data and prepared the manuscript. TM designed the study and supervised the experimental work.
Key words
Root-knot nematode, Biocontrol, Trichoderma, Antagonistic plant, Neem, Reproduction.
DOI: https://dx.doi.org/10.17582/journal.pjz/20190905100940
* Corresponding author: [email protected]
0030-9923/2021/0001-0119 $ 9.00/0
Copyright 2021 Zoological Society of Pakistan
INTRODUCTION
Agriculture throughout the world and particularly in the developing countries is under constant threat by a multitude of biotic factors (Aslam et al., 2019a, b) including soil dwelling pathogens especially phytopathogenic nematodes (Mukhtar and Kayani, 2020). Among nematodes, root-knot nematodes of the genus Meloidogyne badly affect crop production worldwide and cause huge yield losses (Tariq-Khan et al., 2017, 2020). Root-knot nematodes are obligate plant endoparasites and rank first among the top ten most damaging and economic important plant parasitic nematodes. Root-knot nematodes are ubiquitous in distribution and have a very wide host range of cultivated and uncultivated plants (Chitwood and Perry, 2009; Jones et al., 2013; Saucet et al., 2015). Thus far more than one hundred root-knot species have been identified (Karssen and Moens, 2006). Among the known root-knot species, Meloidogyne incognita is by far the most destructive, widely distributed and the most dominant and prevalent in the tropical and subtropical regions of the world. The overall distribution and infestation of M. incognita in Pakistan is 52% (Maqbool, 1987). In another study, Kayani et al. (2013) reported that of all the root-knot species associated with cucumber, M. incognita constituted 78%. Similarly, in okra plantations, M. incognita was found prevailing in 73% of okra plantations in the Punjab province of Pakistan (Tariq-Khan et al., 2017, 2020).
The root-knot nematodes have been reported to incur annual losses to the tune of 22% in tropical environments (Sasser, 1979). Bhatti and Jain (1977) reported yield losses to the extent of 99% in India. Likewise, yield losses up to 100% in important staple crops and vegetables have been reported by M. incognita (Onkendi et al., 2014). Losses in Pakistan due to nematodes to crops have been found more serious and complex as compared to the developed countries owing to numerous causes. Firstly, the country is positioned in the tropical region where the environmental conditions are encouraging for infectivity, growth, and reproduction of these nematodes all the year round. Secondly, the arid zone of the country being sandy in nature is favorable for the activities of these nematodes. Lastly, the cultivation of perennial crops or susceptible crops year after year in the same piece of land in the irrigated plains permits rapid multiplication of nematodes which results in severe infections and damage (Tariq-Khan et al., 2017, 2020). On the other hand, entomopathogenic nematodes can reduce the incidence and severity of root-knot nematodes (Gulzar et al., 2020; Iqbal and Mukhtar, 2020; Rahoo et al., 2017). Root-knot nematodes have also been found associated with fungal and bacterial pathogens resulting in disease complexes and aggravate the severity of the latter (Mukhtar and Kayani, 2020).
Root-knot nematodes can be controlled by employing different strategies such as regulatory, physical, chemical, cultural and biological but there is one or the other limitations associated with these methods (Javed et al., 2019a, b; Mukhtar and Kayani, 2019; ). Although nematicides is the commonest approach for the management of root-knot nematodes but due to unfavorable cost-benefit ratio, non-availability of conventional nematicides, growing awareness of global community against pollution and possible health hazards associated with the use of these hazardous chemicals, search for suitable and feasible alternatives has been emphasized. There have been some efforts towards the search for cheaper and safer alternatives to the chemical nematicides which involve the use of antagonistic plants and fungi exhibiting strong nematicidal and nematostatic properties. In the present study, the efficacy of a biological control agent, Trichoderma harzianum and an antagonistic plant, Azadirachta indica was tested against M. incognita on tomato.
Materials and methods
Preparation of Trichoderma harzianum
The biocontrol fungus, T. harzianum, was mass produced on chopped wheat grains. The wheat grains were immersed in water for 12 h, surface dried using a paper towel, 250 g was added to 500 ml capacity flasks and were autoclaved at 15 psi for about 50 min. The sterilized wheat grains in flasks were inoculated with pure culture of T. harzianum and incubated at 25±1°C for 15 days. The flasks were shaken on alternate days for uniform colonization of the fungus. The number of spores per gram of the grains were counted using haemocytometer after making spore suspensions in distilled water.
For obtaining culture filtrates, T. harzianum was grown on potato dextrose broth. The medium (100 ml in 250 ml flasks) was autoclaved at 15 psi for 15 min. Each flask was then inoculated with four scoops of 5 mm diameter of the fungus from an actively growing culture of the fungus on potato dextrose agar under sterilized conditions and incubated at 25°C for 15 days. Some flasks were inoculated with the scoops of the medium only. At the end of incubation period, the cultures were passed through Whatman filter paper No. 1 to remove the mycelial mats. Filtrates thus obtained were designated as 100% concentration. Further concentrations (50% and 25%) were made by adding requisite amount of sterilized distilled water. The filtrates were stored at 4°C until use.
Preparation of Azadirachta indica extracts
Aqueous extracts of A. indica were prepared by grinding 25 g of fresh leaves of the plant in 75 ml of distilled water with the help of a sterilized pestle and mortar. The ground leaves were first passed through 4 ply muslin cloth and then filtered through Whatman filter paper No. 1. The extract thus obtained was arbitrarily termed as 100% and subsequent dilutions viz. 50% and 25% were prepared by the addition of requisite amount of distilled water. For pot assay, the leaves were washed, dried under shade and powdered.
Preparation of nematode inoculum
The nematode Meloidogyne incognita, raised from a single egg mass, was used in the experiment. For nematode reproduction, the most susceptible variety of tomato (money maker) was used as the host plant. Three-week-old tomato plants were transplanted into pots containing 2.5 kg formalin sterilized sandy loam soil. One week after transplantation, the plants were inoculated with approximately 5,000 freshly hatched second stage juveniles (J2s) of M. incognita. The plants were kept in a green house at 25±2°C and watered as needed.
Evaluation of T. harzianum culture filtrates and A. indica extracts on egg hatching and larval mortality of M. incognita
To study the effect of different concentrations of the culture filtrates of T. harzianum and A. indica extracts on larval hatching of M. incognita, 5 ml of culture filtrate/plant extract from each concentration was pipetted into 5-cm-dia Petri dishes. One uniformed-sized egg mass of M. incognita was handpicked and placed into each dish. Egg masses placed in only medium and distilled water served as control. Each treatment was replicated five times. All the dishes were maintained at 25°C. Data on egg hatching were recorded after 24, 48, and 72 h and percentage egg inhibitions were calculated. Fresh fungal filtrate was replaced after each data. Similarly, to evaluate the effect of different concentrations of the culture filtrate and plant extract on larval mortality, 5 ml of the culture filtrate or plant extract from each concentration was poured into each Petri dish and about 80 freshly hatched second stage juveniles of M. incognita in 0.2 ml of distilled water were added to each Petri dish. Juveniles added in medium or distilled water served as control. Each treatment was replicated five times. Number of dead (unmoved) larvae in each Petri dish were counted after 24, 48 and 72 h and their percentages were calculated.
Corrected mortality (CM) or hatching inhibition (HI) was calculated by Abbott’s (1925) formula as follows:
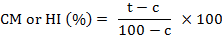
Where, t is percent hatching inhibition or mortality in culture filtrate/plant extract and c is percent hatching inhibition or mortality in control.
Effect of T. harzianum and A. indica amendments against M. incognita
The ground leaves of A. indica (0, 10, 25 and 50 g/kg of soil) and T. harzianum colonized grains (0, 2, 4 and 6 g/kg of soil) were mixed thoroughly with formalin sterilized soil singly and in combinations in plastic pots. The pots were watered daily to facilitate decomposition of plant materials and colonization of fungus. Ten days after amendments, one month old seedlings of tomato cv. Money maker were transplanted to each pot. Each pot was then inoculated with 3000 freshly hatched J2s of M. incognita one week after transplantation. Each treatment was replicated five times. The pots were arranged in a completely randomized design in the glass house at 25±2°C for seven weeks. The pots were watered when needed. After stipulated period, the plants were carefully removed, the roots were excised from the shoot, carefully washed under tap water and blotted dry. The data were recorded on shoot lengths, shoot and root weights, number of galls, egg masses, and reproduction factor. The percent increases and reductions in these variables were calculated over control (Mukhtar and Kayani, 2020).
Statistical analysis
Completely randomized design was used in the lab and pot experiments. All the data were subjected to analysis of variance using GenStat Package 2009 (12th edition) version 12.1.0.3278 (www.vsni.co.uk). Means were compared by Fisher’s Protected Least Significant Difference Test at 5%.
Results
Egg hatching and larval mortality of M. incognita
The analysis of variance regarding effect of culture filtrates and plant extracts on hatching imbibition and larval mortality of M. incognita juveniles showed significant effects (P < 0.01). The antagonistic fungus and plant caused significant hatching inhibition and larval mortality of M. incognita. The hatching inhibition and mortality was the maximum at 100% concentrations of both the treatments while the minimum inhibition and mortality was obtained at 25% concentration. No statistical difference was observed between T. harzianum and A. indica in causing hatching inhibition and larval mortality (Fig. 1).
Nematode infestations
The numbers of galls, egg masses and reproductive factor were reduced significantly as a result of A. indica and T. harzianum applications. The reductions in these parameters were more pronounced where both the agents were integrated and resulted to the maximum where both the agents were mixed at the highest concentrations. The reductions in galls, egg masses and reproductive factor were found inversely proportional to the concentration of A. indica and T. harzianum (Table I).
Table I.- Effect of T. harzianum and A. indica on number of galls and number of egg masses on tomato by M. incognita, on reproduction factor of M. incognita, percent increase in height, fresh shoot weight of tomato and percent decrease in root weight of tomato.
|
Azadirachta indica (g) |
Trichoderma harzianum (g) |
||||
|
0 |
2 |
4 |
6 |
||
|
Number of galls on tomato |
0 |
306.8 a |
250.4 c |
171.6 h |
69.8 jkl |
|
10 |
263.2 b |
221.6 d |
90.4 ij |
72.4 jkl |
|
|
25 |
226.4 d |
151.8 i |
70.4 jkl |
40.2 mn |
|
|
50 |
91.6 m |
54.8 o |
37.2 o |
24.2 op |
|
|
Number of egg masses on tomato |
0 |
289.8 a |
224.8 c |
159.2 f |
46.6 gh |
|
10 |
234.2 b |
208.2 d |
69.2 e |
51.8 gh |
|
|
25 |
190.6 d |
116.4 g |
68.2 i |
12.8 jk |
|
|
50 |
71.2 f |
22.2 m |
16.6 o |
7.6 jk |
|
|
Reproduction factor of M. incognita |
0 |
22.08 a |
18.09 c |
11.46 f |
8.23 ij |
|
10 |
19.53 b |
11.95 e |
6.96 jkl |
5.25 klm |
|
|
25 |
16.34 d |
10.72 i |
5.90 klm |
2.01 o |
|
|
50 |
8.59 j |
3.45 op |
2.15 p |
1.45 nop |
|
|
% increase in height of tomato |
0 |
0.00 m |
27.9 ij |
27.9 ij |
29.4 i |
|
10 |
25.2 kl |
34.4 ef |
35.8 e |
51.7 b |
|
|
25 |
27.6 ij |
36.3 e |
44.3 d |
52.0 ab |
|
|
50 |
27.8 ij |
42.2 d |
49.4 c |
53.4 a |
|
|
% increase in fresh shoot weight of tomato |
0 |
0.0 m |
26.8 l |
27.9 ij |
31.4 g |
|
10 |
26.5 jk |
36.2 f |
42.0 e |
42.4 c |
|
|
25 |
28.2 i |
36.6 f |
43.2 d |
47.8 b |
|
|
50 |
39.2 h |
42.2 f |
44.2 c |
48.6 a |
|
|
% decrease in fresh root weight of tomato |
0 |
0.00 n |
19.1i |
21.4 ij |
31.1 g |
|
10 |
17.2 lm |
31.2 g |
32.4 g |
36.5 d |
|
|
25 |
29.4 h |
34.8 e |
35.2 d |
42.8 b |
|
|
50 |
31.1 g |
36.2 d |
40.2 c |
43.4 a |
|
Data are means of five replicates. Means sharing common letters do not differ significantly (P < 0.05).
Growth variables
All the concentrations of A. indica when integrated with T. harzianum increased plant height and fresh shoot weight significantly over control. The increase in these parameters was directly proportional to the concentration of A. indica leaves and T. harzianum. A. indica amendments proved comparatively better than those of T. harzianum. The maximum increases in these parameters were obtained where A. indica leaves were mixed at the rate of 50 g with 6 g of T. harzianum (Table I). The amendments also showed significant effects on the root weight. The maximum decrease in root weight was observed where both A. indica and T. harzianum were mixed at the highest concentrations. The root weight decreased with an increase in concentration and was found inversely proportional as shown in Table I.
Discussion
Trichoderma harzianum is a ubiquitous soil fungus which colonizes root surfaces and root cortices (Sharon et al., 2009). Several species of Trichoderma including T. harzianum, T. viride, T. atroviride, and T. asperellum, have provided excellent control of root-knot nematodes in previous studies (Sharon et al., 2007). Application of Trichoderma species resulted in reduced nematode galling and improved plant growth and tolerance. The highly branched conidiophores of Trichoderma produce conidia that can attach to different nematode stages. Conidial attachment and parasitism varies among fungal species and strains (Sharon et al., 2007). This process was often associated with the formation of fungal coiling and appressorium-like structures. T. harzianum colonizes isolated eggs and J2s of M. javanica (Sharon et al., 2007). Successful parasitism of the nematode by Trichoderma requires mechanisms to facilitate penetration of the nematode cuticles or eggshells. The involvement of lytic enzymes has long been suggested and demonstrated in Meloidogyne parasitism (Spiegel et al., 2005). Besides direct antagonism, other mechanisms involved in Meloidogyne control by Trichoderma spp. include production of fungal metabolites and induced resistance (Freitas et al., 1995; Goswami et al., 2008). In general, Trichoderma should be applied before planting to achieve maximum nematode control as good establishment of the fungus in plant rhizospheres seems to be important for nematode control. The reason for increased plant growth, yield and other parameters observed here could be attributed to the release of growth promoting substances by bio-agents or by producing toxic metabolites which inhibit nematodes and exclude other deleterious microorganisms. The result obtained in current investigation uphold the results observed by Goswami et al. (2008) who observed increased growth and yield of tomato, soybean, tobacco and capsicum in pot and field experiments by the inoculation of Trichoderma spp. Reduction in nematode galls and egg masses might be due to high rhizosphere competency of bio-agents as they can easily colonize roots and may reduce feeding sites for nematodes. The reduction in root gall number may be due to the failure of majority of the juveniles to penetrate the host root.
Antagonistic plants have also been reported to reduce galls and egg masses which might be attributed to water soluble compounds in different plant parts which are highly toxic to nematodes (Miller et al., 1973; Siddiqui and Alam, 1988) and/or to the increased host resistance (Alam et al., 1980). The commonly occurring chemicals in A. indica are nimbin, nimbidin, nimbidic acid, azadirachtin, kampfaeral, quercetin, desacetyllimbin and thionemone, and all are found to be highly deleterious to root-knot and other nematodes (Khan et al., 1974; Siddiqui and Alam, 1993). These toxic chemicals may cause immobilization, mortality, poor penetration and later retardation in different activities of second stage juveniles. Even if some of the juveniles penetrate the plant, they may not cause economic damage to the plant due to their smaller number. The unaffected females may develop normally but their egg laying potential may be reduced by the application of leaf extracts. Reduction in egg masses would lead to a decrease in secondary inoculum of the nematode. It is possible that some chemicals are either absorbed by the roots or there might have been some chain reaction which was triggered due to some factor (elicitor/activator) present in the extracts (Giebel, 1982; Siddiqui and Alam, 1988). Better growth of plants in A. indica applied pots appears to be due to their manurial effects. Application of extracts may change the physical structure and the fertility of soil resulting in increased tolerance of the plants to nematode attack (Mahmood and Saxena, 1992). It is suggested that the integration of antagonistic plants with the antagonistic fungi may be useful for the better control of plant parasitic nematodes.
Statement of conflict of interest
The authors have declared no conflict of interest.
References
Abbott, W.S., 1925. A method of computing the effectiveness of an insecticide. J. econ. Ent., 18: 165-167. https://doi.org/10.1093/jee/18.2.265a
Alam, M.M., Ahmad, M. and Khan, A.M., 1980. Effect of organic amendments on the growth and chemical composition of tomato, eggplant and chili and their susceptibility to attack by Meloidogyne incognita. Pl. Soil, 57: 231-236. https://doi.org/10.1007/BF02211683
Aslam, M.A., Javed, K., Javed, H., Mukhtar, T. and Bashir, M.S., 2019a. Infestation of Helicoverpa armigera Hübner (Noctuidae: Lepidoptera) on soybean cultivars in Pothwar region and relationship with physico-morphic characters. Pak. J. agric. Sci., 56: 401-405.
Aslam, M.N., Mukhtar, T., Jamil, M. and Nafees, M., 2019b. Analysis of aubergine germplasm for resistance sources to bacterial wilt incited by Ralstonia solanacearum. Pak. J. agric. Sci., 56: 119-122.
Bhatti, D.S. and Jain, R.K., 1977. Estimation of losses in okra, tomato and eggplant yield due to Meloidogyne incognita. Ind. J. Nematol., 7: 37-41.
Chitwood, D.J. and Perry, R.N., 2009. Reproduction, physiology and biochemistry. In: Root-knot nematodes (eds. R.N. Perry, M. Moens and J.L. Starr). London, UK, pp. 182-200. https://doi.org/10.1079/9781845934927.0182
Freitas, L.G., Ferraz, S. and Muchovey, J.J., 1995. Effectiveness of different isolates of Paecilomyces lilacinus and an isolate of Cylindorcarpon destructans on the control of Meloidogyne javanica. Nematropica, 25: 109-115.
Giebel, J., 1982. Mechanism of resistance to plant nematodes. Annu. Rev. Phytopathol., 20: 257-279. https://doi.org/10.1146/annurev.py.20.090182.001353
Goswami, J., Pandey, R.K., Tewari, J.P. and Goswami, B.K., 2008. Management of root- knot nematode on tomato through application of fungal antagonists, Acremonium strictum and Trichoderma harzianum. J. environ. Sci. Hlth., 43: 237-240. https://doi.org/10.1080/03601230701771164
Gulzar, A., Mukhtar, T. and Wright, D.J., 2020. Effects of entomopathogenic nematodes Steinernema carpocapsae and Heterorhabditis bacteriophora on the fitness of a Vip3A resistant subpopulation of Heliothis virescens (Noctuidae: Lepidoptera). Bragantia, 79: 281-292. https://doi.org/10.1590/1678-4499.20190501
Iqbal, U. and Mukhtar, T., 2020. Evaluation of biocontrol potential of seven indigenous Trichoderma species against charcoal rot causing fungus, Macrophomina phaseolina. Gesunde Pflanz., 72: 195–202. https://doi.org/10.1007/s10343-020-00501-x
Javed, K., Javed, H., Mukhtar, T. and Qiu, D., 2019a. Efficacy of Beauveria bassiana and Verticillium lecanii for the management of whitefly and aphid. Pak. J. agric. Sci., 56: 669-674.
Javed, K., Javed, H., Mukhtar, T. and Qiu, D., 2019b. Pathogenicity of some entomopathogenic fungal strains to green peach aphid, Myzus persicae Sulzer (Homoptera: Aphididae) Myzus persicae (Homoptera: Aphididae). Egypt. J. Biol. Pest Contr., 19: 92. https://doi.org/10.1186/s41938-019-0183-z
Jones, J.T., Haegeman, A., Danchin, E.G.J., Gaur, H.S., Helder, J., Jones, M.G.K., Kikuchi, T., Manzanilla-López, R., Palomares-Rius, J.E., Wesemael, W.M.L. and Perry, R.N., 2013. Top 10 plant-parasitic nematodes in molecular plant pathology. Mol. Pl. Pathol., 14: 946-961. https://doi.org/10.1111/mpp.12057
Karssen, G. and Moens, M., 2006. Root-knot nematodes. In: Plant nematology (eds. R.N. Perry and M. Moens). CABI International, Wallingford, UK, pp. 59-90. https://doi.org/10.1079/9781845930561.0059
Kayani, M.Z., Mukhtar, T., Hussain, M.A. and Haque, M.I., 2013. Infestation assessment of root-knot nematodes (Meloidogyne spp.) associated with cucumber in the Pothowar region of Pakistan. Crop Prot., 47: 49-54. https://doi.org/10.1016/j.cropro.2013.01.005
Khan, M.W., Alam, M.M., Khan, A.M. and Saxena, S.K., 1974. Effect of water soluble fractions of oil-cakes and bitter principles of neem on some fungi and nematodes. Acta Bot. Ind., 2: 120-128.
Mahmood, I. and Saxena, S.K., 1992. Effect of green manuring with certain legumes on the control of plant parasitic nematodes. Pak. J. Nematol., 10: 139-142.
Maqbool, M.A., 1987. Distribution and host association of plant parasitic nematodes in Pakistan. Pak. J. Nematol., 5: 15-17.
Miller, P.M., Turner, N.C. and Tomlinson, H., 1973. Toxicity of leaf and stem extracts to Tylenchorhynchus dubius. J. Nematol., 5: 173-177.
Mukhtar, T. and Kayani, M.Z., 2019. Growth and yield responses of fifteen cucumber cultivars to root-knot nematode (Meloidogyne incognita). Acta Sci. Pol. Hortorum Cultus, 18: 45-52. https://doi.org/10.24326/asphc.2019.3.5
Onkendi, E.M., Kariuki, G.M., Marais, M. and Moleleki, L.N., 2014. The threat of root-knot nematodes (Meloidogyne spp.) in Africa: A review. Pl. Pathol., 63: 727–737. https://doi.org/10.1111/ppa.12202
Rahoo, A.M., Mukhtar, T., Bughio, B.A., Gowen, S.R. and Rahoo, R.K., 2017. Infection of Galleria mellonella larvae by Steinernema affine and production of infective juveniles. Pak. J. Nematol., 35: 65-71.
Sasser, J.N., 1979. Economic importance of Meloidogyne in tropical countries. In: Root-knot nematodes (Meloidogyne spp.): Systematics, biology and control (eds. F. Lamberti and C.E. Taylor). Academic Publishers, New York, pp. 359-374.
Saucet, S.B., Ma, Y., Sarris, P.F., Furzer, O.J., Sohn, K.H. and Jones, J.D.G., 2015. Two linked pairs of Arabidopsis TNL resistance genes independently confer recognition of bacterial effector AvrRps4. Nat. Commun., 6: 1-12. https://doi.org/10.1038/ncomms7338
Sharon, E., Chet, I. and Spiegel, Y., 2009. Improved attachment and parasitism of Trichoderma on Meloidogyne javanica in vitro. Eur. J. Pl. Pathol., 123: 291-299. https://doi.org/10.1007/s10658-008-9366-2
Sharon, E., Chet, I., Viterbo, A., Bar-Eyal, M., Nagan, H., Samuels, G.J. and Spiegel, Y., 2007. Parasitism of Trichoderma on Meloidogyne javanica and role of the gelatinous matrix. Eur. J. Pl. Pathol., 118: 247-258. https://doi.org/10.1007/s10658-007-9140-x
Siddiqui, M.A. and Alam, M.M., 1988. Control of rootknot and reniform nematodes by bare root dip in leaf extracts of margosa and Persian lilac. Z. Pflanzenkrank. Pflanzensch., 95: 138-142.
Siddiqui, M.A. and Alam, M.M., 1993. Evaluation of nematicidal potential in neem allelochemicals. World Neem Conference, 24th to 28th Feb. 1993, Bangalore, India, pp. 39.
Spiegel, Y., Sharon, E. and Chet, I., 2005. Mechanisms and improved biocontrol of the root-knot nematodes by Trichoderma spp. Acta Hort., 698: 225-228. https://doi.org/10.17660/ActaHortic.2005.698.30
Tariq-Khan, M., Mukhtar, T., Munir, A., Hallmann, J. and Heuer, H., 2020. Comprehensive report on the prevalence of root-knot nematodes in the Poonch division of Azad Jammu and Kashmir, Pakistan. J. Phytopathol., 168: 322–336. https://doi.org/10.1111/jph.12895
Tariq-Khan, M., Munir, A., Mukhtar, T., Hallmann, J. and Heuer, H., 2017. Distribution of root-knot nematode species and their virulence on vegetables in northern temperate agro-ecosystems of the Pakistani-administered territories of Azad Jammu and Kashmir. J. Pl. Dis. Protect., 124: 201-212. https://doi.org/10.1007/s41348-016-0045-9
To share on other social networks, click on any share button. What are these?









