Evaluation of Resistance of Capsicum annum against Meloidogyne incognita and Sclerotium rolfsii and their Integrated Management
Evaluation of Resistance of Capsicum annum against Meloidogyne incognita and Sclerotium rolfsii and their Integrated Management
Adnan Yousaf1, Jia Wu1, Qaiser Shakeel2, Yasir Iftikhar3, Muhammad Irfan Ullah4, Uzma Tahira5, Mustansar Mubeen1 and Wubei Dong1,*
1State Key Laboratory of Agricultural Microbiology and Key Laboratory of Plant Pathology of Hubei Province, Huazhong Agricultural University, Wuhan 430070, China
2Department of Plant Pathology, University College of Agriculture and Environmental Sciences, The Islamia University of Bahawalpur, Bahawalpur
3Department of Plant Pathology, University of Sargodha, Sargodha, Pakistan
4Department of Agri-Entomology, University College of Agriculture, University of Sargodha, Sargodha, Pakistan
5In-Service Agriculture Training Institute, Sargodha, Punjab, Pakistan
ABSTRACT
Experiments were carried out to screen resistance of chili cultivars against Meloidogyne incognita and Sclerotium rolfsii and their integrated management by using derosol, cadusafos and Trichoderma harzianum in the green house at 25 ± 4ºC. Primarily, 15 chilies cultivars viz., 28-2010, C-72, Sanam, C-73, Gola Peshawari, 27-2010, Tata Puri, C-302, C-33, C-19, 5-2010, C-68, 11-2010, 18-2010 and 15-2010 were evaluated against M. incognita and S. rolfsii. Two week old chilies plants were inoculated with M. incognita, S. rolfsii separately and in combination as well. At harvesting, roots of C-302 contained significantly fewer galls (30) and egg-masses (51) compared to the other fourteen cultivars. Seven cultivars including C-33, Gola Peshawari, 11-2010, 18-2010, 15-2010, 27-2010 and C-68 had 10 root galling and egg-masses indices (from a scale of 0-10). Hence, present study shows that none of the available chili cultivars are resistant to attack from M. incognita and S. rolfsii. The moderate tolerance was found in C-302 followed by 5-2010 and 28-2010. The most susceptible cultivar (C-33) was selected for further evaluation of management sources. All three management sources were applied individually as well as in combination. After six weeks, all treatments showed better results but there was no significantly difference in set of plants treated with combination of all three (Derosal + Cadusafos + T. harzianum) and T. harzianum alone. Moreover, it was observed that the ability of T. harzianum to manage root rot and knot pathogen enhanced when it was integrated with bio-products. It was hypothesized that direct antagonism and defense by bio-product enhance the resistance in chili cultivars. So, T. harzianum is an efficient biological control for integrated management of chili plants under controlled condition cultivation. To explore the nature of resistance response of C-33 to M. incognita and S. rolfsii further research is needed.
Article Information
Received 04 March 2017
Revised 24 April 2017
Accepted 13 May 2017
Available online 25 August 2017
Authors’ Contribution
DW and YI designed the experiments. AY, UT and MM performed the experiments and wrote the manuscript. WJ, QS and MIU analyzed data and helped in preparation of the manuscript.
Key words
Chilies, Meloidogyne incognita, Sclerotium rolfsii, Synergetic effect, Biological control.
DOI: http://dx.doi.org/10.17582/journal.pjz/2017.49.5.1671.1682
* Corresponding author: dwb@mail.hzau.edu.cn
0030-9923/2017/0005-1671 $ 9.00/0
Copyright 2017 Zoological Society of Pakistan
Introduction
The consumption of Chilies (Capsicum annum) as fresh vegetables or as spice are very popular in Subcontinent as nutritional and medicinal sources. While extract is used in pharmaceutical and cosmetic products (Abbas, 2007; Iqbal, 2009). Chilies are attacked by several plant pathogens depending upon the stage of growth. Both pre- and post-emergence infection is reported as a result of infection by pathogens. It starts from seed sowing goes to seedling and then upto flowering stage of growth. Among the many plant pathogens that cause the reduction in yield and quality of crops, soil borne plant pathogens are one of the major problems in agricultural production throughout the world. The diseases that are significantly affecting the world production are damping-off, root rot and wilt of vegetables (Hibar et al., 2006). Worldwide, Plant-parasitic nematodes (PPN) cause deleterious economic losses to agricultural crops by causing severe damage to a wide range of important crops (Sasser and Freckman, 1987). Globally, US$ 500 million, with average yield losses of 25%, is spent on nematode control. The yield reduction up to 60% is also reported in individual crop (Keren-Zur et al., 2000; Sasser and Carter, 1982). Different nematode makes the crops unproductive resulting in huge economic losses. Among them Meloidogyne incognita is one of the major pests of vegetables in the world as well as in Pakistan (Williamson and Hussey, 1996; Anwar et al., 2007). Moreover, nematode-fungi and nematode-bacteria association makes the situation even more alarming to agricultural crops in connection with economic losses (Maqbool et al., 1987).
Sclerotium rolfsii is a soil-borne pathogen that causes root, stem, foot rot and wilt, in almost all the agricultural and horticultural crops (Aycock, 1966; Domsch et al., 1980). It is often found that S. rolfsii and Meloidogyne spp. remain closely associated with plants in soil and cause disease complex (Powel, 1971). Individual effect as well as combine effect of these organisms in disease complex is not yet thoroughly studied in Pakistan although this kind of studies has already been done in other countries. In order to reduce the incidence of disease as result of this disease complex, several control measures are being adopted including cultural practices, use of nematicide and fungicides as well as biological control methods.
The use of biocontrol agents (BCAs) with the economic and ecological advantages is one of the best method to manage the Meloidogyne spp. and S. rolfsii (Stirling and Smith, 1998). Among BCAs examined against the plant pathogens, Trichoderma spp. have been widely studied for their biocontrol ability (Abd El-Khair et al., 2010; Mohiddin et al., 2010). Trichoderma species are useful, avirulent plant symbionts that act as biocontrol agents against phytopathogens via mechanisms of competition, rhizosphere competence, mycoparasitism, antibiotic and enzyme production, induced resistance, and promoting plant growth (Harman et al., 2004; Howell, 2003). The majorities of Trichoderma species is antagonist of phytopathogenic fungi and have been broadly used as the most important biocontrol agent (Tjamos et al., 1992). Nowadays, several kinds of commercial products are formulated based on Trichoderma strains. Hence, current research was undertaken to compare and evaluate the bio-efficacy of T. harzianum, derosal and cadusafos individually as well as in combination for the management of M. incognita and S. rolfsii infecting chilies.
Material and Methods
Fungal strains, media and chemicals
Trichoderma harzianum (TH) (accession number 1277) isolated from rhizosphere soil of Lahore, Pakistan was purchase from Institute of Agricultural Sciences, University of Punjab, Lahore, Pakistan. Potato dextrose agar (PDA) was used for mass culturing of Trichoderma 9.0 cm sterile petri dishes. Agar plugs (5 mm in diameter) from the advancing margins of 5 day old culture was transferred to new PDA containing plates and incubated for 14 days at 25°C. The pathogen S. rolfsii was isolated from root of infected tomato plants. The infected portion of roots were cut in to small pieces of about 3-5 mm thick and sterilized with 1 % sodium hypochlorite solution for two minutes, rinsed thrice in sterilized distilled water (SDW) and dried on sterilized filter paper at room temperature. The tissue sections were then placed on PDA and incubated at 25°C for seven days. Ultimately, the pure culture of the pathogen was isolated and subsequently maintained on PDA. The inoculated dishes of S. rolfsii were incubated for one month for the production and maturation of sclerotia. While M. incognita was isolated from infected soil and root sample of chilies field in University of Agriculture, Faisalabad. PDA was prepared from fresh peeled potato and fungi were cultured on PDA. Both the chemical (cadusafos and derosal) were purchased from the FMC Corporation, Pakista
Dual culture test
Mycelial disks (5 mm diameter) of S. rolfsii were placed on one edge of petri dishes containing PDA and incubated at 25ºC. 48 h later, mycelial disks (5 mm diameter) of Trichoderma harzianum were placed on the opposite side of S. rolfsii in previous. Petri dishes with Trichoderma alone served as control. They were incubated in the same thermal conditions. Interactions between Trichoderma and S. rolfsii was evaluated based on radial growth of pathogen, overgrowth speed of Trichoderma on pathogen colony, production of yellow pigment in overlapped area of two colonies and hyperparasitism (mycelial coiling) (Dennis and Webester, 1971; Kucuk and Kivance, 2003). There were five replicates and the experiments were repeated three times.
Culture filtrate (non-volatile metabolites) and early volatile metabolites tests
Mycelial disks (5 mm diameter) from 10-day old colony of Trichoderma were inoculated into potato dextrose broth and incubated at 25oC and 200 rpm in rotary shaker incubator for 10 days. The fungal biomass was removed by centrifugation at 10,000 rpm for 30 min and vacuum filtered through sterile Whatman No.1 filter paper (0.2 µm) at least twice to ensure that there was no fungal growth in the microwell assays (You et al., 2016). The filtrates were designated as 100% concentrated (Standard dilution). The filtrate was further diluted with ddH2O to three different dilutions (25%, 50% and 75%). Inhibitory effect of plant extracts was assessed using poisoned food technique. Different concentrations of filtrate at 2% were added into PDA and subsequently poured into plates (20 mL). After medium solidifying, mycelial disk of S. rolfsii derived from actively growing colonies were places in the center of the plates and were incubated at 25oC (Dennis and Webester, 1971; Kucuk and Kivance, 2003). For early volatile metabolites test, pathogen and Trichoderma actively growing colonies were sub-cultured on PDA and incubated in dark condition at 25°C. Then, opened Petri dishes containing 24 h old colony of S. rolfsii were placed on 48 h old colony of Trichoderma and were airtight using paraphylm. Control was Petri dishes containing PDA medium only. Five replicates were used per treatment and the experiments were repeated three times.The Petri dishes were incubated in the same temperature and dark conditions (Dennis and Webester, 1971; Fiddman and Rossall, 1993). Radial growth on pathogen was measured daily in both tests. Inhibitory percentages were calculated.
Screening of cultivars of chilies against M. incognita and S. rolfsii
The reproduction of the selected populations was performed on susceptible plants of tomato (Lycopersicon esculenlum cv. Rio-grande) with two true leaves, approximately 20 days post germination, which were transplanted to pots containing 300 cm3 of sterile sandy soil. One week after transplant, pots were inoculated with one egg mass from original field population, achieving an inoculums level of about 130 M. incognita juveniles (second stage juveniles, J2) per 100 g sterile sandy soil. Tomato plants were kept in a growth chamber (Humidity 60%) at 25 ± 1oC, 16 h light, for 45 days.
Nursery of different chili cultivars viz., 28-2010, C-72, Sanam, C-73, Gola Peshawari, 27-2010, Tata Puri, C-302, C-33, C-19, 5-2010, C-68, 11-2010, 18-2010 and 15-2010 was raised in sterilized soil in trays. Three week old chili seedlings were transplanted into 13 cm diameter clay pots containing formalin sterilized sandy loam soil (70% sand, 22% silt, and 8% clay). Seven days after transplanting all the pots except control were inoculated with 2000 M. incognita J2 per pot (NA), S. rolfsii (SR) at 1 sclerotia g-1 individually and one set of plants were inoculated with both M. incognita J2 and S. rolfsii (NA+SR) in the rhizosphere of each plant by making 3-4 holes of about half-pot depth in the sand around root system (Campos and Campos, 2005) and then filled with soil. The temperature during the growth period ranged from 25-30 oC. The plants were fertilized twice with ammonium nitrate at 2g per pot without sterilization. Each treatment was replicated five time and the experiment was repeated 3 times. Plants were uprooted after 60 days of inoculation.
Galling indices were determined by visual estimation of galling percentages on roots, the characteristic symptom of the genera, where an index of 5 equates to 50% of nematode affected roots, including main roots. Study of host status of chilli began when the galling index was higher than 5 on a 0–10 scale (Bridge and Page, 1980) where, 0 = healthy root system, 1 = very few galls only detected upon close examination, 2 = small galls easy to detect, 3 = numerous small galls, 4 = numerous small galls and a few big ones, 5 = 25% of the root system severely galled, 6 = 50% of the root system severely galled, 7 = 75% of the root system severely galled, 8 = no healthy root but plant still green, 9 = completely galled root system and plant dying, 10 = plants and roots dead. Plants with scores ranging from 0-3 were rated as resistant while those with scores ranging from 4-6 and from 7-10 were rated as moderately resistant and susceptible, respectively and brown (mature) egg masses were observed. Egg masses were stained using phloxine B (Holbrook et al., 1983) and quantified using a scale of 1-9 where 1=no egg masses, 2=1-5, 3=6-10, 4= 11-20, 5=21-30, 6=31-50, 7=51-70, 8=71-100, 9= >100 egg masses per root system (Sharma and Saxena, 1992). Data of the parameters including root weight, shoot weight, root length, galls, galling index, no. of females, egg masses, egg masses index and lateral roots, were recorded. Plant resistance to nematodes is characterized by failure of the nematodes to produce functional feeding sites in the host after invasion and to develop subsequently as reproducing females, including hypersensitive responses (Williamson and Kumar, 2006). Therefore, it was considered that host plants were resistant (R) when the root galling index was 0 and no egg masses were present, and susceptible (S) when this value was higher than 0 and brown (mature) egg masses were present.
Isolation of S. rolfsii from root
Roots all the five plants were washed in running tap water to remove soil particles. Seven randomly selected 1cm long root pieces from each plant were surface sterilized with 1% NaOCI solution for 5 min and transferred onto plates (5 plate per root piece) containing Penicillin (at 100,000 units L-1) and Streptomycin (at 0.2 g L-1). After incubation for 5 days at room temperature, colonization of roots by S. rolfsii was recorded using the following formula:
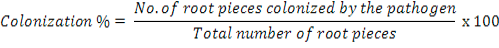
Data on root colonization were converted into roots colonization index (RCI) according to a 0-5 scale of Shahzad and Ghaffar (1995) where 0=0, l=l-10, 2=11-25, 3=26-50, 4=51-75 and 5=75-100% root pieces colonized by the pathogen.
Management practices of M. incognita and S. rolfsii
Most susceptible cultivar was selected on the basis of results of previous experiments. It was grown in green house. Plants were inoculated after two weeks with S. rolfsii and M. incognita alone and in combination. After one week, inoculated plants were treated with derosal, cadusafos, TH, TH+derosal, TH+cadusafos, derosal+cadusafos and TH+derosal+cadusafos. The concentration of 1000 ppm was used in case of cadusafos and derosal while 100% cultural filtrate of T. harzianum was used. After six weeks, data was recorded with the parameters including No. of galls/root system, No. of females/root system, No. of egg masses/root system, Shoot weight, No. of lateral roots, Fresh roots weight and RCI for S. rolfsii. Disease severity index (DSI) was calculated by using following formula:
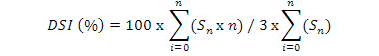
Where, n represents the galling scale (0 to 10) and Sn represents the number of seedlings corresponding to the galling scale n, number “3” represents the highest rating scale. The experiment was repeated three times.
Control efficacy (E) was calculated using the following formula:
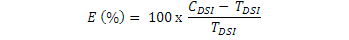
Where, CDSI and TDSI represent the DSI values for the treatments of control and management practices (T. Harzianum, Cadusafos and derosal) of isolate 3-10, respectively.
Data analysis
Analysis of variance (ANOVA) was conducted to assess the impact of S. rolfsii and M. incognita on chili cultivars and also different management strategies using SAS (version 9.1, SAS Institute Inc.) and significant difference among the cultivars was separated by Duncan’s multiple range test (DMRT) at probability levels of P=0.05.
Results
Antifungal abilities of Trichoderma harzianum in vitro
In dual culture test, Trichoderma limited the colony growth of the S. rolfsii. Overgrow speed, unnatural production of yellow pigment and mycoparasitism are shown in Table I. In culture filtrate test, S. rolfsii colony growth was reduced except when filtrates were used after 96 H which enhanced the pathogen growth. The inhibitory effects of filtrated metabolites reduced as the incubation period increased (Table I). In early volatile metabolites test, significantly reduction in pathogen colony growth while the maximum growth reduction was observed about 50% (Table I).
Table I.- Reaction and antifungal activity of Trichoderma harzianum on S. rolfsii.
| Control | T. harzianum | |
| Hyphal interaction in dual culture test | ||
|
Overgrowth1 |
- | - |
|
Yellow pigment2 |
- | Nil |
|
Hyperparasitism3 |
- | No |
|
Inhibitory effect of cultural filtrate (%)4 |
||
| 25% | 0% |
-3%5 |
| 50% | 0% | 49% |
| 75% | 0% | 79% |
| 100% | 0% | 0% |
| Inhibitory effect of early volatile metabolites (%) | ||
| 0% |
50% |
|
1The growth of Trichoderma isolates on pathogen colony. -, not overgrowth; +, complete with slow speed; ++, complete with rapid speed. 2Production of yellow pigment more than growth normal condition. 3Observation of hyphal coiling of Trichoderma isolates around pathogen hypha. 4Inhibitory percentages of Trichoderma isolates on mycelial growth of S. rolfsii in different concentration of culture filtrate trial. 5Negative symbols mean that this treatment increased 3 % mycelial growth.
Evaluation of chili cultivars against M. incognita
The pathogenic effects of M. incognita was variable to chili cultivars. It caused significant reduction in plant growth at varying degree of infestation. An increase in number of galls, egg masses, and number of eggs was observed. The rate of built-up in nematode population was initially at maximum level. Root weight was increased due to feeding of M. incognita and it varied among 15 cultivars of chilies (Fig. 1A).
Root-shoot weights
Root weight had direct relationship with the inoculums level. The increased root weight exhibited negative effect on shoot weight and caused reduction in foliage growth at increased inoculums (Fig. 1A, B). Maximum shoot weight (4.86 g) was recorded in C-302 cultivar while the least was in C-33 as compared to control. While C-33 showed the maximum root weight (2.52 g) and least (1.23 g) was observed in C-302 as compared to control (Fig. 1A, B).
Root-shoot lengths
The root and shoot lengths were inversely proportional to nematode build up. The maximum root length (9.23 cm) was observed in C-302 cultivar while the least root length (1.61 cm) was observed in C-33. While the root length in case of 5-2010, 28-2010, sanam, C-68 and C-19 was significantly higher than C-33 but much less than C-302 which show the development of M. incognita on these cultivars as well (Fig. 2A).
Number of galls, egg masses and female population
There was increase in number of galls as nematode population increases. The minimum numbers of galls were observed in C-302 while the highest in case of C-33 cultivars (Fig. 3A). A significant increase in number of female nematode population was found in all the cultivars. The highest female population was found in cultivar C-33 whereas the minimum numbers of females were found in the C-302. A significant lower production of egg masses per plant was observed in C-302. The egg masses indices were almost same in all the cultivars (Table II). Production of egg masses clearly demonstrated the reproduction potential of M. incognita on different chilies cultivars. C-302 was found resistant according to galls and egg mass indices while the cultivars 28-2010, C-302, C-73, and C-19 showed moderate resistance against M. incognita (Fig. 3A). Reduction in lateral root was found in all the fifteen cultivars of chilies. Significantly higher number of lateral roots was found in the cultivar C-33 (Fig. 2B). C-33 was found most susceptible that’s why higher number of lateral roots was found in this cultivar as nematode feeding induces lateral root production.
Evaluation of chili cultivars against S. rolfsii
Similar trend of susceptibility to S. rolfsii was found as was in case of M. incognita infection. Soil infestation with S. rolfsii showed significantly reduction in germination of maximum chilies cultivars i.e., C-33, C-72, C-72 and gola peshawari as compared to control. While C-302, 5-2010, 28-2010 and sanam germination was slightly reduced. All the plant growth parameters were affected by infection of S. rolfsii. Cultivar C-33 exhibited minimum shoot and root weight as compared to other 14 cultivars of chili (Fig. 1A, B). Highest shoot and root weight was found in cultivar C-302. Root length and lateral roots also followed the same pattern (Fig. 2A, B). C-302 showed no effect of S. rolfsii on plant growth followed by 5-2010 and 28-2010 (Figs. 1, 2). Root colonization was observed in all tested plants growing in artificially infested soil. Similarly, RCI was low in case of C-302 which range from 0 to 1 on scale (Fig. 4). However, maximum RCI 5 was found in 9 different chilies cultivars (C-72, C-73, Gola peshawari, 27-2010, C-33, 11-2010, 18-2010 and 15-2010) which show that these cultivars were susceptible to S. rolfsii.
Synergistic effect of M. incognita and S. rolfsii on chili development
The effect of simultaneous inoculation of M. incognita and S. rolfsii on the growth of chilies cultivars was additive however there was reduction in final nematode population and root knot index when both pathogens were inoculated simultaneous.
Symptomatology
The pathogens severely infected the root and shoot of chili plants and produced dark brown lesion in collar region while root knot were found on roots causing wilt and ultimately plants get dried.
Table II.- Comparison of galling index and egg mass index between M. incognita alone and in combination with S. rolfsii in 15 chili cultivars.
|
M. incognita |
M. incognita + S. rolfsii |
|||
|
GI* |
EMI* |
GI |
EMI |
|
| 28-2010 |
5 |
9 |
4 |
6 |
| C-72 |
9 |
9 |
10 |
9 |
| Sanam |
5 |
9 |
5 |
8 |
| C-73 |
9 |
9 |
10 |
9 |
| Gola Peshawari |
10 |
9 |
10 |
9 |
| 27-2010 |
10 |
9 |
9 |
9 |
| Tata Puri |
8 |
9 |
8 |
9 |
| C-302 |
3 |
6 |
2 |
4 |
| C-33 |
10 |
9 |
10 |
9 |
| C-19 |
5 |
9 |
4 |
8 |
| 5-2010 |
7 |
7 |
4 |
6 |
| C-68 |
10 |
9 |
7 |
9 |
| 11-2010 |
10 |
9 |
8 |
9 |
| 18-2010 |
10 |
9 |
10 |
9 |
| 15-2010 |
10 |
9 |
9 |
9 |
GI, galling index; EMI, egg mass index.
Root and shoot weight
The increased root weight had negative effect on shoot weight and caused reduction in foliage growth. Maximum shoot weight was recorded in C-302 cultivar while lowest in C-33 as compared to control (Fig. 1B). While in case of root weight, difference was observed in inverse order as maximum root weight was observed in C-33 cultivar whereas minimum root weight was observed C-302 as compared to control (Fig. 1A). The root and shoot length of test plants was significantly (P=0.05) reduced as a result of feeding of both pathogens compared with control plants (Fig. 2A). The shoot and root lengths were normal in the beginning of experiment but as the pathogens development increased, the shoot and root lengths were decreased significantly. The control plants showed maximum shoot and root lengths.
Number of galls, egg masses and female population
The increase in number of root galls, females and egg masses was recorded when nematode and fungus was inoculated collectively (Fig. 3B).
Lateral root
Reduction in lateral root was found in all the fifteen cultivars of chilies. C-33 was found to be most susceptible as least number of lateral roots was found in this cultivar.
RCI
Root colonization was observed in all plants growing in artificially infested soil. However C-72, C-73, Gola peshawari, 27-2010, C-33, 11-2010, 18-2010 and 15-2010 showed greater RCI as compared to 28-2010, Sanam, Tata puri, C-302, C-19, 5-2010 and C-68 (Fig. 4). C-33 was found most susceptible as there was maximum root colonization while C-302 was found slightly resistant as development of pathogen was less.
The host status of available chilli cultivars for the Meloidogyne populations and S. rolfsii was determined. C-302 was resistant to both M. incognita and S. rolfsii when they were applied individually and both at same time. Response of 5-2010, 28-2010 and sanam to infestation of both pathogens was from susceptible to immune as these cultivars perform batter against S. rolfsii while against M. incognita they were not found up to the make. C-33 was found most susceptible among all other chilli cultivars followed by C-72, C-73, 27-2010, 11-2010, 15-2010 and 18-2010.
Management of M. incognita and S. rolfsii
The objective of current experiment was to find out most suitable method to manage both S. rolfsii and M. incognita collectively. Chili cultivar C-33, being most susceptible in the previous experiments, was used to check the effect of different treatments for the management of pathogens.
No evidence of phytotoxicity in chilli plants treated with the biocontrol agent was found during trails. All the treatments had positive effect on plant growth compared to uninoculated control (Table III). Inoculation of plant with M. incognita and S. rolfsii produced typical symptoms as roots were swollen and there were few or no lateral roots. But when plant were inoculated with different management treatment alone or in combination, there were less root galls and some healthy roots were present as compared to pathogens alone. The root system of plant with Trichoderma alone or combination of all three treatments looked similar or even better than the root system of blank control. Plant co-inoculated with management component and pathogens had larger shoot and leaves than those inoculated with pathogens alone as plant were stunted. The highest plant height or other parameters were found after treating TH + derosal + cadusafos per plant followed by TH + derosal at the same time, blank control and plants inoculated with Trichoderma had more branches than the plants inoculated with either M. incognita or S. rolfsii alone.
| Treatments |
Shoot weight (g) |
Root weight (g) |
Root length (cm) |
No. lateral roots |
Galls / Root |
Females/ Root |
Egg masses / Root |
| T. harzianum |
3.1 ± 0.04e* |
2.1 ± 0.02b |
10.7 ± 0.2g |
27 ± 3.2e |
11± 2.9gh |
14 ± 4.3g |
9 ± 2.9fg |
| Derosal |
3.1 ± 0.01e |
1.6 ± 0.07d |
11.7 ± 0.2cd |
21 ± 1.4f |
78 ± 12.3d |
143 ± 11.1d |
96 ± 8.2d |
| Cadusafos |
3.9 ± 0.03d |
1.4 ± 0.08e |
11.2 ± 0.1ef |
36 ± 3.7d |
26 ± 7.9ef |
48 ± 15.0f |
37 ± 7.6e |
| T. harzianum + Derosal |
4.1 ± 0.02c |
1.6 ± 0.05d |
11.5 ± 0.2de |
42 ± 2.3c |
32 ± 4.1e |
86 ± 10.2e |
45 ± 5.9e |
| T. harzianum + Cadusafos |
3.9 ± 0.06d |
1.3 ± 0.01f |
11.1 ± 0.02f |
32 ± 1.6d |
14 ± 2.6fg |
18 ± 4.3g |
13 ± 3.3f |
| Derosal + Cadusafos |
3.1 ± 0.04e |
1.6 ± 0.04d |
7.5 ± 0.7i |
21 ± 2.2f |
145 ± 15.4c |
188 ± 10.9c |
134 ± 2.7c |
| T. harzianum + Derosal + Cadusafos |
4.8 ± 0.05a |
1.6 ± 0.01d |
13.1 ± 0.1b |
62 ± 5.2a |
8 ± 1.8gh |
13 ± 4.8g |
7 ± 2.8fg |
| M. incognita |
2.8 ± 0.04f |
2.3 ± 0.05a |
13.3 ± 0.1b |
42 ± 6.3c |
208 ± 10.9a |
243 ± 9.1a |
186 ± 7.8a |
| S. rolfsii |
2.1 ± 0.03g |
1.4 ± 0.02ef |
11.8 ± 0.1c |
43 ± 1.4c |
0.0h |
0.0h |
0.0g |
| S. rolfsii + M. incognita |
1.7 ± 0.05h |
2.0 ± 0.04bc |
8.1 ± 0.1h |
24 ± 1.4ef |
186 ± 11.9b |
208 ± 9.0b |
158 ± 9.3b |
| Healthy |
4.4 ± 0.03b |
1.98 ± 0.02c |
15.9 ± 0.1a |
56 ± 3.7b |
0.0h |
0.0h |
0.0h |
*Means within each column followed by same letters do not significantly (P = 0.05) different from each other according to Duncan’s multiple range test.
Similarly, the maximum number of lateral roots was found after treatment were applied at the same time (Table III). However, the lowest numbers of lateral roots were found in the plants treated with cadusafos and derosal alone or in combination when compared with the effects of pathogens individually or blank control. Root weight was higher when plant was inoculated with M. incognita alone as compare to blank control (Table III). Minimum root weight (1.3 g) was observed when plants were co-inoculated with TH + cadusafos while highest root weight was observed when plant were inoculated with T. harzianum (2.1 g) after M. incognita alone. This also resulted in higher root indices. Root weight data was further investigated by separating the galled root and non-galled roots which revealed that greater portion of root weight was represented by non-galled root when plant were inoculated with Trichoderma and combination of all treatments (TH + derosal + cadusafos).
All the management treatment significantly reduced infection rate of both pathogens as revealed by data of galls, females and number of egg masses. As results of this significantly reduction in the severity of disease was observed (Table III). All the three replicates of the growth cabinet trails showed that the severity was moderate to high (mean DSI < 100) in the treatments. In addition to reducing the gall size, combination of all three treatments was highly effective in reducing DSI by 83.3% (P = 0.05) relative to the treatment of pathogens alone. The treatments of Trichoderma with cadusafos and Trichoderma alone also reduced DSI by 76.67% and 73.33%, respectively. However, biocides alone had very little effect had little or no effect on reduction of DSI. The lowest effective treatment was found when both biocides were used in combination was used.
Highest root length (13.1 cm) was found in which all the management measures were applied in combination. The lowest root length (7.5 cm) was found in the treatment which was treated with cadusafos + derosal treatment as compared to the effect of Fungi and root knot nematode (RKN) alone (Table III). Development of lateral root was also affected by the application of different treatments. The highest number of lateral roots was found in the plant treated with TH+derosal+cadusafos (Table III).
Discussion
The response of all cultivars to M. incognita and S. rolfsii infestation was variable which might be due to presence of a nematode resistance gene (Hadisoeganda and Sasser, 1982; Roberts and Thomson, 1986). These genes made the plant less attractive for attacking nematode. Different plant responses to nematode infection were observed. Compatible and incompatible reactions may be due to the presence of resistant genes that are activated as a result of nematode invasion and some visible reactions can be observed in the plant cells (Williamson, 1999; Davis et al., 2000; Williamson and Kumar, 2006). In the resistant plants nematodes fail to produce functional feeding sites in the host after invasion due to hypersensitive responses that leads in failure to develop subsequently as reproducing females (Williamson and Kumar, 2006). Roberts and May (1986) found greater number of females, galls and eggs per plant in susceptible cultivars inoculated with M. incognita as compared to moderately resistant cultivars. While in case of S. rolfsii it may prevent cell wall degrading enzymes which will prevent tissue decay and ultimately development of sclerotia by S. rolfsii.
Two types of mechanisms, Pre-infection and post-infection resistance, have been reported. Pre-infection resistance is result of toxic or antagonistic chemicals present in root tissue which prevent the entry of pathogens in roots (Haynes and Jones, 1976; Bendezu and Starr, 2003) while in post infection resistance, in which pathogens penetrate but fail to develop. Upon infection, death of cells around infection occur which is termed as hypersensitive reaction (HR) and it inhibit the development of feeding sites by pathogen which leads to resistance. Boiteux and Charechar (1996) reported that gene for resistance are present in gene pool of resistant cultivars that confers resistance against M. incognita. In the resistant roots, catalase activities are decreased as a result of RKN attack. There is a possible role of alkaloids or phenolics that may inhibit the synthesis of these enzymes and act as an elicitor of resistance in plant attacked by Meloidogyne species.
Development and reproduction of pathogens is reflected by resistance and susceptibility of the plant (Cook and Evans, 1987; Khan et al., 2004; Sajid et al., 2011) as our results indicated on cultivars C-302, 5-2010, 28-2010, sanam and C-19 development of both pathogens were lowered as compared to C-33.
The idea of a sustainable agricultural practice and environmental protection enhances the importance of biocontrol. The adoption of a sustainable agricultural practice, using strategies that are environmentally friendly, less dependent on agricultural chemicals is gaining worldwide recognition. One of the key elements of such sustainable agriculture is the application of biocontrol agents. Trichoderma species are antagonistic by nature with rich resource and a broad action scope.
There are many nematophagous fungi, but not all of them are used as biocontrol agents under field conditions due to their complex ecology and adverse effects on soil microbiota. However, such fungi play a vital role in the natural decline of pathogens in heavily infested soils (Khan and Saxena, 1997; Eapen et al., 2005). Appreciably, the results of the present study, confirmed clearly that Trichoderma species inhibited the growth of pathogens remarkably well. The competence shown by Trichoderma species to inhibit the growth of the tested pathogens both in vitro and in vivo suggests that the phenomenon of hyphal interaction described by Dennis and Webster (1971) in case of S. rolfsii. Trichoderma spp. produces different lytic enzymes such as polysaccharide lyases, proteases and lipases (Cherif and Benhamou, 1990; Harman et al., 1993). These enzymes play an important role in penetration of nematode egg surface, resulting in death of juveniles inside the hard and protective egg barriers. The J2 cuticle is composed mainly of proteins (Blaxter and Robertson, 1998) and the proteolytic enzymes produced by Trichoderma spp. (Harman et al., 1993), decrease egg hatching and J2 activity (Abid et al., 2005; Pathak and Kumar, 1995). Eggs play a key role in nematode perpetuation and survival under adverse environmental conditions. Egg shell protects the nematode larvae from environmental hazards. T. harzianum has been reported as an egg parasite of M. incognita race 3, killing 53% eggs in vitro (Dos Santos et al., 1992). Saifullah and Thomas (1996) reported the ability of T. harzianum to grow on the egg surface of potato cyst nematodes. Most plant-parasitic nematode eggs have a second layer as a thick chitin layer of egg shell and Trichoderma spp. produce chitinases, a-gluconase and cellulase, which helps in parasitizing the eggs of M. javanica (Chet and Baker, 1981). The eggs of M. javanica were colonized by the antagonistic strains of T. harzianum as observed by Sharon et al. (2001). Sankaranarayanan et al. (1999) reported that three isolates of T. harzianum reduced egg masses on root system of chilli plants. Sharon et al. (2001) reported that certain strains of T. harzianum could not grow on egg masses or penetrate the eggs inside gelatinous matrix. Goswami et al. (2006) found significant reduction in the egg masses of M. incognita on tomato treated with certain fungi alone and in different combinations. These results are in agreement with our study results.
Root weight of chilli plants was found to be reduced in the T. harzianurn-treated pots as compared to the control. This might be due to the accumulation of synthesized food in the galled roots of RKN infected. In the present studies, T. harzianurn showed significant increase in chilli root and shoot length. Different strains of T. harzianum have been reported to suppress pathogen populations thereby, leading to increased root and shoot length (Bjorkman et al., 1994; Inbar et al., 1994; Altomare et al., 1999; Harman, 2000; Yang et al., 2000). The present findings confirmed results of previous research conducted in Pakistan and elsewhere on the use of T. harzianum for management of root pathogens especially RKN and S. rolfsii. The fungus can be developed as a biopesticide for use under green house and field conditions. Trichoderma and its relatives are known as mycoparasites of many organisms causing diseases (Hadar et al., 1984; Lifshitz et al., 1986; Sivan et al., 1984). T. harzianum has also been reported to cause necrosis on the roots of cotton (Yang et al., 2000) and tobacco plants (Powel, 1971) in association with M. incognita. However, it is not at all pathogenic in the absence of Meloidogyne spp. (Windham et al., 1986). In general, Trichoderma sp. are mycoparasites and not plant pathogenic organism, but rather plant growth stimulators (Windham et al., 1989). Overall, this study contributed information on the reaction of various chilli cultivars to M. incognita and S. rolfsii. C-302 could be recommended for the large scale cultivation and also this study provides sufficient evidences of the effective use of T. harzianum for management of RKN and S. rolfsii.
Acknowledgements
We are thankful to Department of Plant Pathology, University of Agriculture, Faisalabad for financial and intellectual input in this work. We also wish to thank Institute of Agricultural Sciences, University of Punjab, Lahore for providing us Trichoderma harzianum culture. Kind appreciations are due to Ayyub Agricultural Research Institute, Faisalabad, Pakistan for providing us chilli germplasm.
Statement of conflict of interest
Authors have declared no conflict of interest.
References
Abd-El-Khair, H., Khalifa, R.K.M. and Haggag, K.H.E., 2010. Effect of Trichoderma species on damping off diseases incidence, some plant enzymes activity and nutritional status of bean plants. J. Am. Sci., 6: 122–134.
Abid, M., Zaki, M.J., Khan, M.Q. and Sattar, A., 2005. Use of marine algae for the management of root-knot nematode (Meloidogyne javanica) in okra and tomato plants. Int. J. Phycol. Phycochem., 1: 187–192.
Altomare, C., Norvell, W.A., Bjorkman, T. and Harman, G.E., 1999. Solubilization of phosphates and micronutrients by the plant-growth promoting and biocontrol fungus Trichodrma harzianum Rifai strain 1295-22. Appl. environ. Microbiol., 65: 2926–2933.
Anwar, S.A., Zia, A., Hussain, M. and Kamran, M., 2007. Host suitability of selected plants to Meloidogyne incognita in the Punjab, Pakistan. Int. J. Nematol., 17: 144–150.
Aycock, R., 1966. Stem rot and other diseases caused by Sclerotium rolfsii. N. Carol. agric. Exp. Station Techn. Bull., 174: 202.
Bendezu, I.F. and Starr, J., 2003. Mechanism of resistance to Meloidogyne arenaria in the peanut cultivar Coan. J. Nematol., 35: 115–118.
Bjorkman, T., Hugh, P. and Gary, H., 1994. Improving performance of sweet corn using Trichoderma as a bioprotectant and growth enhances. State Sweet Corn Research Association, New York, USA.
Blaxter, M.L. and Robertson, W.M., 1998. The cuticle. In: The physiology and biochemistry of free-living and plant-parasitic nematodes (eds. R.N. Perry and D.J. Wright). CAB International, New York, pp. 25–48.
Boiteux, L.S. and Charechar, J.M., 1996. Genetic resistance to root-knot nematode (Meloidogyne javanica) in eggplant (Solanum melongena). Pl. Breed., 115: 198–200. https://doi.org/10.1111/j.1439-0523.1996.tb00902.x
Bridge, J. and Page, S.L.J., 1980. Estimation of root knot-nematode infection levels on roots using a rating chart. Crop Pest Manage., 26: 296–298. https://doi.org/10.1080/09670878009414416
Cherif, M. and Benhamou, N., 1990. Cytochemical aspects of chitin breakdown during the parasitic action of Trichoderma spp. on Fusarium oxysporum f. sp. radicis-Iycopersici. Phytopathology, 80: 1406–1414. https://doi.org/10.1094/Phyto-80-1406
Chet, I. and Baker, R., 1981. Isolation and biocontrol potential of Trichoderma hamatum from soil naturally suppressive to Rhizoctonia solani in the soil. Phytopathology, 71: 286–290. https://doi.org/10.1094/Phyto-71-286
Davis, E.L., Hussey, R.S., Baum, T.J., Bakker, J., Schots, A., Rosso, M.N. and Abad, P., 2000. Nematode parasitism genes. Annu. Rev. Phytopath., 38: 365–396. https://doi.org/10.1146/annurev.phyto.38.1.365
Dennis, C. and Webester, J., 1971. Antagonistic properties of species of groups of Trichoderma I. Production of non-volatile antibiotics. Trans. Br. Mycol. Soc., 57: 25–29. https://doi.org/10.1016/S0007-1536(71)80050-5
Domsch. K.H., Gams W. and Anderson, T.H., 1980. Compendium of soil fungi. Academic Press, New York.
Dos Santos, M.A., Ferraz S. and Muchovez J.J., 1992. Evaluation of 20 species of fungi from Brazil for biocontrol of Meloidogyne incognita race-3. Nematropica, 22: 183–192.
Eapen, V., Ghubash, R., Salem, M.O. and Sabri, S., 2005. Familial predictors of childhood shyness: a study of the United Arab Emirates population. Commun. Genet., 8: 61–64. https://doi.org/10.1159/000083342
Fiddman, P.J. and Rossall, S., 1993. The production of antifungal volatile by Bacillus subtilis. J. appl. Bact., 74: 119–126. https://doi.org/10.1111/j.1365-2672.1993.tb03004.x
Goswami, B.K., Pandey, R.K., Rathour, K.S., Bhattacharya, C. and Singh, L., 2006. Integrated application of some compatible biocontrol agents along with mustard oil seed cake and furadan on Meloidogyne incognita infecting tomato plants. J. Zhejiang Univ. Sci. B, 7: 873–875. https://doi.org/10.1631/jzus.2006.B0873
Hadar, Y., Harman, G.E. and Taylor, A.G., 1984. Evaluation of Trichoderra koningii and T. harzianum from New York soils for biological control of seed rot caused by Pythium spp. Phytopathology, 74: 106-110. https://doi.org/10.1094/Phyto-74-106
Hadisoeganda, W.W. and Sasser, J.N., 1982. Resistance of tomato, bean, southern pea, and garden pea cultivars to root-knot nematodes based on host suitability. Pl. Dis., 66: 145–150. https://doi.org/10.1094/PD-66-145
Harman, G.E., 2000. Myths and dogmas of biocontrol. Pl. Dis., 84: 377–393. https://doi.org/10.1094/PDIS.2000.84.4.377
Harman, G.E., Hayes, C.K., Lorito, M., Broadway, R.M., Dipierro, A., Peterbauer C. and Tronsmo, A., 1993. Chitionlytic enzymes of Trichoderm harzianum, purification chitobiosidase and endochitinae. Phytopathology, 83: 313–318. https://doi.org/10.1094/Phyto-83-313
Harman, G.E., Howell, C.R., Viterbo, A., Chet, I. and Lorito, M., 2004. Trichoderma species – opportunistic, avirulent plant symbionts. Nature Rev. Microbiol., 2: 43–56. https://doi.org/10.1038/nrmicro797
Hibar, K., Daami-Remadi, M., Jabnoun-Khiareddine, H. and El-Mahjoub, M., 2006. Temperature effect on mycelial growth and on disease incidence of Fusarium oxysporum f. sp. radicis-lycopersici. Pl. Pathol. J., 5: 233–238. https://doi.org/10.3923/ppj.2006.233.238
Holbrook, C.C., Knauft, D.A. and Dickson, D.W., 1983. A technique for screening peanut for resistance to Meloidogyne incognita. Pl. Dis., 57: 957–958. https://doi.org/10.1094/PD-67-957
Howell, C.R., 2003. Mechanisms employed by Trichoderma species in the biological control of plant diseases: The history and evolution of current concepts. Pl. Dis., 87: 4–10. https://doi.org/10.1094/PDIS.2003.87.1.4
Inbar, J., Abramsky, M., Cohen, D. and Chet, I., 1994. Plant growth enhancement and disease control by Trichoderma harzianum in vegetable seedlings grown under commercial conditions. Eur. J. Pl. Pathol., 100: 337–346. https://doi.org/10.1007/BF01876444
Keren-Zur, M., Antonov, J., Bercovitz, A., Feldman, K., Husid, A., Kenan, G., Markov, N. and Rebhun, M., 2000. Bacillus firmus formulations for the safe control of root-knot nematodes. In: Proceedings of the brighten crop protection conference on pests and diseases, Vol. 2A. UK, pp. 47–52.
Khan, T.A., Nasir, S. and Ashraf, M.S., 2004. Effect of population levels of Meloidogyne javanica on plant growth and nematode multiplication on cucurbits. Pak. J. Nematol., 22: 83–87.
Khan, T.A. and Saxena, S.K., 1997. Effect of root-dip treatment with fungal filtrates on root penetration, development and reproduction of M. javanica on tomato. Int. J. Nematol., 7: 85–88.
Kucuk, C. and Kivanc, M., 2003. Isolation of Trichoderma spp. and determination of their antifungal, biochemical and physiological features. Turk. J. Biol., 27: 247–253.
Lifshitz, R., Windham, M.T. and Baker, R., 1986. Mechanism of biological control of pre-emergence damping off of pea by seed treatment with Trichoderma spp. Phytopathology, 76: 720-725. https://doi.org/10.1094/Phyto-76-720
Mohiddin, F.A., Khan, M.R. and Khan, S.M., 2010. Why Trichoderma is considered super hero (super fungus) against the evil parasites? Pl. Pathol. J., 9: 92–102. https://doi.org/10.3923/ppj.2010.92.102
Powel, N.T., 1971. Interaction between nematodes and fungi in disease complexes. Annu. Rev. Phytopathol., 9: 253–274. https://doi.org/10.1146/annurev.py.09.090171.001345
Roberts, P.A. and Thomason, I.J., 1986. Variability in reproduction of isolates of Meloidogyne incognita and M. javanica in resistant tomato genotypes. Pl. Dis., 70: 547–557. https://doi.org/10.1094/PD-70-547
Saifullah, and Thomas, B.J., 1996. Studies on the paratism of Globodera rostochiensis by Trichoderma harzianum using low temperature scanning electron microscopy. Afro-Asian J. Nematol., 6: 117–122.
Sajid, A.K., Javed, N., Kamran, M., Haq, I.U. and Haq, M.A., 2011. Invasion and development of Meloidogyne incognita race 1 in different tomato cultivars. Pak. J. Nematol., 29: 63–70.
Sankaranarayanan, C., Hussaini, S.S., Kumar, P.S., Rangeshwaran, R., Dhawan, S.C. and Khushal, K.K., 1999. Antagonistic effect of Trichoderma and Gliocladiuin spp. against the root-knot nematode (Meloidogyne incognita) on sunflower. In: Proceeding of National symposium on Rational Approaches in Nematode Management for Sustainable Agriculture, India, pp. 23–25.
Sasser, J.N. and Carter, C.C., 1982. Overview of the International Meloidogyne project-Rationale, Goals, Implementaiton and progress to date. Proc. 3rd Res. Plan. Conf. on root-knot nematodes Meloidogyne spp. Panama, pp. l–7.
Sasser, J.N. and Freckman, D.W., 1987. A world perspective on Nematology: The role of society. In: Vistas on nematology (ed. J.A. Veech and D.W. Dickson). A Commemoration of the 25th Anniversary of the Society of Nematologists, Lakeland, FL, pp. 7-14.
Shahzad, S. and Ghaffar, A., 1995. New records of soilborne root infecting fungi in Pakistan. Pak. J. Bot., 17: 209–216.
Sharma, M. and Saxena, S.K., 1992. Effect of culture filtrates of the Rhizoctonia solani and Trichoderma viride on hatching of larvae of root-knot nematode (Meloidogyne incognita). Curr. Nematol., 3: 61-63.
Sharon, E., Bar, E.M., Chet, I., Herrera, E.A., Kleifeld, O. and Spiegel, Y., 2001. Biological control of the root-knot nematode Meloidogyne javanica by Trichoderma harzianum. Phytopathology, 91: 687–693. https://doi.org/10.1094/PHYTO.2001.91.7.687
Sivan, A., Elad, Y. and Chet, I., 1984. Biological control effects of new isolates of Trichoderma harzianum on Pythiurn aphanidermatum. Phytopathology, 74: 489-501. https://doi.org/10.1094/Phyto-74-498
Stirling, G.R. and Smith, L.J., 1998. Field test of formulated products containing either Verticillium chlamydosporium or Arthrobotrys dactyloides for biological control of root-knot nematodes. Biol. Contr., 11: 231–239. https://doi.org/10.1006/bcon.1997.0604
Tjamos, E.C. and Papavizas, G.C. and Cook, R.J., 1992. Biological control of plant diseases, progress and challenges for the future. Plenum Press, New York. https://doi.org/10.1007/978-1-4757-9468-7
Williamson, V.M., 1999. Plant nematode resistance genes. Curr. Opin. Pl. Biol., 2: 327–331. https://doi.org/10.1016/S1369-5266(99)80057-0
Williamson, V.M. and Hussey, R.S., 1996. Nematode pathogenesis and resistance in plants. Pl. Cell, 8: 1735–1745. https://doi.org/10.2307/3870226
Williamson, V.M. and Kumar, A., 2006. Nematode resistance in plants: the battle underground. Trends Genet., 22: 396–403. https://doi.org/10.1016/j.tig.2006.05.003
Windham, G.L., Windham, M.T. and Williams, W.P., 1989. Effects of Trichoderma spp. on maize growth and Meloidogyne arenaria reproduction. Pl. Dis., 73: 493–495. https://doi.org/10.1094/PD-73-0493
Windham, M.T., Elad, Y. and Baker, A.R., 1986. A mechanism for increased plant growth induced by Trichoderma spp. Phytopathology, 76: 518–521. https://doi.org/10.1094/Phyto-76-518
Yang, X.J., He, Y.X., Chen, F.R. and Zheng, L., 2000. Isolation and selection of egg-nuiss fungi cif Muloirlogyne sp. in Fujian Province. Fujian J. agric. Sci., 15: 12–15.
You, J.Q., Zhang, J., Wu, M., Yang, L., Chen, W.D. and Li, G.Q., 2016. Multiple criteria-based screening of Trichoderma isolates for biological control of Botrytis cinerea on tomato. Biol. Contr., 101: 31-38. https://doi.org/10.1016/j.biocontrol.2016.06.006
To share on other social networks, click on any share button. What are these?










