Evaluation of Reproductive Capacity in Pond-Reared Penaeus monodon after Unilateral Eyestalk Ablation
Evaluation of Reproductive Capacity in Pond-Reared Penaeus monodon after Unilateral Eyestalk Ablation
Weigeng Wen1,2,3, Xu Chen1,2,3, Wang Zhao1,2,3, Qibin Yang1,2,3, Jian G. Qin4 and Zhenhua Ma1,2,3,*
1Tropical Aquaculture Research and Development Center, South China Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences, Sanya 572018, China
2Key Laboratory of South China Sea Fishery Resources Exploitation and Utilization, Ministry of Agriculture, Guangzhou 510300, China
3Sanya Tropical Fisheries Research Institute, Sanya 572018, China
4College of Sciences and Engineering, Flinders University, GPO Box 2100, Adelaide, SA 5001, Australia
Weigeng Wen and Xu Chen have contributed equally to this study.
ABSTRACT
This study investigated the reproductive capacity of pond-reared female Penaeus monodon after unilateral eyestalk ablation. A total of 240 females (57.0-143.3g) were used as broodstocks in a continuous three-year breeding experiment. After unilateral eyestalk ablation, the relationships of egg and nauplii at the first spawning event and female bodyweight were evaluated. At each spawning, a female could produce 120000-1250000 eggs which hatched into 80,000-950,000 nauplii. The egg hatching rate at each spawning varied from 27.4% to 86.3% without particular patterns of correlation with female body weight. The fecundity of pond-reared female P. monodon varied from 2 100 to 9 400 eggs per gram of bodyweight, and females with medium size of 85-120 g showed greater fecundity (around 9000 eggs/g) in comparison with smaller and larger counterparts. This study indicates that reproductive capacity of pond-reared female P. monodon is significantly related to bodyweight with a desirable size of 85-120 g as female broodstocks.
Article Information
Received 18 May 2019
Revised 30 July 2019
Accepted 27 August 2019
Available online 24 March 2020
Authors’ Contributions
WW, XC, JGQ, and ZM designed the study and conducted field test. XC, WZ and QY conducted data analysis. WW, JGQ and ZM drafted the manuscript.
Key words
Reproductive capacity, Body weight, Pond-reared, Penaeus monodon, Eyestalk ablation.
DOI: https://dx.doi.org/10.17582/journal.pjz/20190518040508
* Corresponding author: [email protected]
0030-9923/2020/0004-1283 $ 9.00/0
Copyright 2020 Zoological Society of Pakistan
Introduction
It has been generally acknowledged that Penaeus monodon is one of the most economic crustacean species in aquaculture. So far, most of the P. monodon seeds for aquaculture are sourced from the female shrimp collected from the wild (Palacios et al., 1999; Hall et al., 2003; Peixoto et al., 2003; Jiang et al., 2019). However, the wild source is an unreliable supply due to seasonal variation and technical constraint. Moreover, it is also possible that wild-caught females may carry pathogens and increase the risk of breeding failure (Yang et al., 2011). Therefore, it is necessary to use captive shrimp as broodstock in controlled conditions.
Up to today, gonad maturation and spawning performance of female shrimp from different sources (pond-reared vs. wild-caught) have been evaluated, and the use of pond-reared shrimp as broodstocks has been recommend as an effective operation for seed production (Menasveta et al., 1994; Cavalli et al., 1997; Coman et al., 2005, 2006; Arnold et al., 2013; Wen et al., 2015). In controlled conditions, domesticated P. monodon have shown superior growth and feed utilization (Glencross et al., 2013), ease harvest from commercial ponds (Preston et al., 2009, 2010), low viral load (Preston et al., 2010) and improved reproductive performance (Preston et al., 2009; Coman et al., 2013) as compared with wild-caught stock. However, fecundity and hatching rate of pond-reared broodstocks are not as good as those of wild-caught broodstocks (Emerencianoa et al., 2012; Arnold et al., 2013; Wen et al., 2015).
In pond-reared P. monodon, female fecundity is positively related to body size (Menasveta et al., 1994; Wen et al., 2015).In contrast, egg production in some specific pathogen free strains of P. monodon showed no particular pattern of relationship with body weight (Chotipuntu et al., 2013), and egg production rate and nauplii production rate of Penaeus semisulcatus are inversely correlated with female bodyweight (Maheswarudu et al., 2013).
In shrimp hatcheries, egg production, nauplii production, and hatching rate vary among broodstocks (Peixoto et al., 2005; Coman et al., 2006, 2007; Pongtippatee et al., 2007; Arnold et al., 2012). To further understand the reproductive capacity of artificially reared shrimp broodstocks, the present study was designed to explore the correlation of egg production, nauplii production and female bodyweight of pond-reared P. monodon over three years. In the present study, egg production and nauplii production at each spawning, and the size of spawned females were used as key reproductive capacity indicators. Results from this study would provide a practical guide for selecting a proper size range as broodstocks in pond-reared P. monodon.
Materials and methods
The study was conducted at Tropical Aquaculture Research and Development Centre, South China Sea Fisheries Research Institute for continuous three years (2012-2014). In each year, post larvae (1.2 cm) were collected from a same commercial hatchery farm which used wild-caught P. monodon broodstocks, in the same cohort and reared with commercial feed (38% protein)in three grow-out earth ponds (1 hectare) over 8 months at a density of 15 individuals/m2 before harvest. For each experiment in a year, the female and male shrimps with initial body weight of 57.0-143.3 g and 38.7-46.8 g, respectively, were collected and transported to TARDC. Upon arrival, over 80 females and 40 males with intact appendages and some females with spermatophore were selected for each experiment. All animals were disinfected with 100×10-6 formalin for 5-10 min separately, and then females and males were acclimated separately into two indoor cement tanks with cement (5 × 6 × 0.5 m; length × width × height), filtered water with 5μm filter bag, for 5-days before ablation. The water temperature, salinity and pH were maintained at 29±0.5oC, 32‰ and 8.1. During acclimation, shrimps were fed with a fresh diet three times (0800, 1600 and 2000 h) a day at a ratio of 10% bodyweight. The fresh diet contained mussel, squid, blood clam and crab. The uneaten food and feces were cleaned daily after feeding for 3 h. Continuous aeration was provided to each tank and 50% of water in the tanks was exchanged daily.
After acclimation, the shrimps (80 females and 40 males) used for experiments were stocked in the indoor cement tank of same size at a density of 4 individuals/m2 in filtered seawater at a depth of 50 cm. Throughout the experiment, the tank was shaded with black cloth to control the light intensity at about 200 Lux. One eyestalk of all the females was ablated with pre-heated tweezers through the middle of the eyestalk. The amount of daily food ration and the time tank cleaning were the same as in the period of acclimation. The ablated females were the recently molted individuals. Artificial insemination was performed by extruding spermatophores from males and then inserted into the cavity underneath the female thelycum. Artificial insemination was only done within 8-10 h after molt when the shell remained in the semi-hardening. After insemination, females were transferred to the maturation tank (30 m2). The ablation experiment was conducted for a period of 35 days. The degree of ovarian development of females was assessed by macroscopic examination of the ovaries through the dorsal exoskeleton based on the daily change of ovary volume and color (Browdy et al., 1985) according to the following criteria: Stage I, the ovary is transparent with no distinguishable outline; Stage II, the ovary is visible as a thin opaque line along the dorsal central axis; Stage III, the ovary is visible as a thick and green band and Stage IV, the ovary is turgid, broad and dark green.
Ripe females (ovary stage III-IV) were transferred individually to spawning tanks (1 × 1× 1.2 m, length × width × depth) with water filtered through a 5-µm filter at 30°C. Moderate aeration was provided to the spawning tank and illumination was by natural daylight. Spawning tanks were monitored every 30 min. After each spawning, the females were undisturbed until spawning was over and then removed, weighed and transferred to another tank. Evenly homogenized in the water was performed prior to sampling. The volume of 50 mL water with eggs were taken randomly and all the eggs were counted carefully using optical observation or through a magnifying lens (5×-magnification). The average number of eggs (N) per sample was determined and egg production was estimated using the subsample volume for egg counting and the volume of the spawning tank. In the experiment, the egg production at each spawning event was estimated as 2N (×104) and the same method was used for the nauplii production number evaluation.
The following equations were used to calculate the parameters for reproductive performance of pond-reared P. monodon:
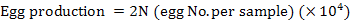
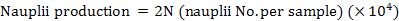
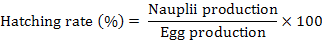

The number of eggs and nauplii and female bodyweight at each spawning in each year were obtained individually. Egg and nauplii production, hatching rate, and fecundity were compared between each spawning year through one-way ANOVA (SPSS 21.0). Our results indicate that egg and nauplii production, hatching rate, and fecundity were not significantly affected by spawning years (P > 0.05). Different relationships (egg number and bodyweight, nauplii number and bodyweight, fecundity and bodyweight, hatching rate and bodyweight of the female were established by regression analysis using Microsoft Excel 2010.
Results
In this study, a total of 240 females were ablated in continuous three years resulting in 153 spawning females and 58 dead females, and 29 females failed to maturation. The percentage of spawned females was 63.75%, and accumulative mortality was 24.17%. The reproductive performance varied among individual females and the egg production and nauplius production of female in each spawning event varied from 120000 to 1250000 and from 80000 to 950000, respectively. The egg and nauplius production of pond-reared female P. monodon in each spawning event had a close relationship with female body weight. Relationship between egg production (y, ten thousands/g) and female body weight (x) from three continually years can be expressed as: y2012 = 1.3173x - 42.83 (R² = 0.90666), y2013 = 1.3196x - 43.145 (R² = 0.90761), and y2014 = 1.353x - 45.878 (R² = 0.92295, Fig. 1). The relationship between nauplius production (y, ten thousands/g) and female body weight (x) can be expressed as: y2012 = 1.0169x - 36.694 (R² = 0.91713), y2013 = 1.0348x - 37.629 (R² = 0.91168), and y2014 = 1.0504x - 39.183 (R² = 0.91803, Fig. 2).
The fecundity of pond-reared female P. monodon was also varied among individuals, ranging from 2 100 to 9 400 (eggs/g). The lowest fecundity observed in a female with a bodyweight of 57.0g, while the highest fecundity occurred in a female weighting 117.6g (Fig. 3). Throughout the 3-year experiment, the best fecundity (about 9000 eggs/g) occurred in females with body weight range from 85-120g and then gradually decreased with a further increase of bodyweight. The pattern of relationship between fecundity (y, ten thousand eggs/g) and female body weight (x) could be expressed in a logarithmic equations: y2012 = 0.6137ln(x) - 1.9413 (R² = 0.64515), y2013 = 0.5954ln(x) - 1.8621 (R² = 0.65601), and y2014 = 0.006ln(x) + 0.2848 (R² = 0.61649, Fig. 3). Through three years, fecundity was higher in females weighing 85-120 g than in other weights (Fig. 3).
The hatching rate in each spawning event also varied among individuals. Throughout the 3-year experiment, the lowest hatching rate of 27.4% and highest (85.2%) occurred in a female of 59.3g and 86.2g, respectively. Also, the hatching rates of 71.3% and 71.4% were observed in females weighting 76.9g and 125.0g, respectively (Fig. 4).The egg hatching rate was shown not to be related to female body weight. Through three years’ analysis, the relationship between hatching rate (y, %) and female body weight (x) can be expressed as: y2012 = 0.2001x + 54.713 (R² = 0.18908), y2013 = 0.1051x + 63.885 (R² = 0.11974), and y2014 = 0.2236x + 52.642 (R² = 0.22851, Fig. 4).
Discussion
In the present study, egg production and nauplius production were correlated with female bodyweight. It agrees with Menasveta et al. (1994) that total egg produced in large shrimp (>120 g) was significantly more than those in small shrimp (<110 g). Moreover, Wen et al. (2015) found that pond-reared females in a large size (120 g) produced 2-times more eggs than those in a small size (80g). In the present study, the egg production and nauplii production of pond-reared female P. monodon show a particular pattern of relationship with body weight. However, Chotipuntu et al. (2013) reported that egg production of pond reared P. monodon showed no particular pattern in relationship with body weight (n = 15), and Maheswarudu et al. (2013) found that the egg and nauplii production rates of Penaeus semisulcatus were inversely correlated with female bodyweight (n = 9) (Maheswarudu et al. 2013). The reason caused this difference may be due to low number (n = 9-15) of female samples in their studies and high number (n = 153) of females used in the present study. The production of eggs and nauplii is influenced by the stage of ovarian maturation (Hall et al., 2003; Peixoto et al., 2005; Coman et al., 2006), egg quality (Palacios et al., 1998; Coman et al., 2007) and sperm quality and quantity (Pongtippatee et al., 2007; Arnold et al., 2013).The present study focused on the relationship between reproductive output and female bodyweight. The results suggest that the greater fecundity (9000 eggs/g) would be obtained from a female of reared P. monodon broodstocks with body weight of 85-120g.
In the current study, the egg and nauplii productions of pond-reared female P. monodon varied among individual shrimp. Due to significant size differences among female shrimp, the relative number of spawned eggs per gram of female (i.e., fecundity) was used in this study. This method has been used in domesticated P. monodon (Arnold et al., 2013) and Macrobrachium rosenbergii (Habashy, 2013). The fecundity ranged from 2 100 to 9 400 (eggs/g) with the highest in a 117.6g female. The fecundity was observed to increase from small to large females and then decrease with a further increase of female size. This result is similar to the report by Habashy (2013) who demonstrated that the number of eggs of female M. rosenbergii was directly proportional to body weight. Moreover, the result of this study is consistent with that of Jee and Kok (2008) who reported that the number of eggs per body weight increased with female size in M. rosenbergii. Therefore, the number of eggs produced per female body weight may be size dependent and is generally assumed to increase with female size. The similar trend was found in M. lamarrei (Sharma and Subba, 2005). In contrast, Rao (1991) reported that in wild M. rosenbergii smaller females produced more eggs per body weight than larger ones. In the current study, however, medium size P. monodon females were more efficient in terms of egg production per body weight. In a hatchery, more and more females (for example 80 females) are used, egg production by per body weight is crucial estimated. The present study suggests that hatchery operators select a medium size range of female shrimp as boodstocks. The variation of fecundity may be attributed to the condition of female maintenance in the laboratory, female size and conditions.
In this study, hatching rate varied from 27.4% to 85.2% with the respective female weight from 59.3g to 86.2g. A similar result was reported by Chotipuntu et al. (2013) that hatching rate of P. monodon varied from nil to 85%. In the present study, the hatching rate (71.3-71.4%) was observed in females of 76.9g and 125.0g, indicating a steady hatching rate in females of different body size. No pattern of relationship with the female size and hatching rate was found in this study, which is in agreement with Babu (2013) and Wen et al. (2015) who reported that hatching rate was similar in ablated P. Monodon females and, the hatching rates in wild caught or pond-reared females were not significantly affected by female bodyweight. Hatching rate normally depends on the quality of eggs and sperm and the egg to sperm ratio. Culture conditions especially food availability and quality play a significant role in sperm quality of shrimp. Age and size of male shrimp broodstocks have been proven to significantly affect sperm quality (Hoang et al., 2002; Ceballos-Vazquez et al., 2003). Wongprasert et al. (2016) reported that hatching rate was higher in the 5-hydroxytryptamine-injected shrimp than eyestalk-ablated shrimp. The reason of this difference maybe that the quality of eggs is better maintained in the 5HT-injected shrimp than in the eyestalk-ablated shrimp.
In summary, reproductive performance in P. monodon is a highly complex process that requires a precise condition of external and internal factors. Reproductive performance is constrained by inferior ovary maturation, spawning, egg production in domesticated females. The study suggests that a female with medium size 85-120g could produce a better reproductive performance.
Acknowledgements
This study was supported by Applied Technology Research, Development and Demonstration Projects of Hainan Province (ZDXM 2015056).
Statement of conflict of interest
The authors declare no conflict of interest.
References
Arnold, S.J., Coman, G.J., Burridge, C. and Rao, M., 2012. A novel approach to evaluate the relationship between measures of male fertility and egg fertilization in Penaeus monodon. Aquaculture, 338–341: 181-189. https://doi.org/10.1016/j.aquaculture.2012.01.013
Arnold, S.J., Coman, G.J. and Emerenciano, M., 2013. Constraints on seed stock production in eighth generation domesticated Penaeus monodon broodstock. Aquaculture, 410-411: 95-100. https://doi.org/10.1016/j.aquaculture.2013.06.023
Babu, K.N., Pallavi, P.N., Reddy, D.C. and Nanda-Kumar, N.V., 2013. Ovarian maturation and spawning in the tiger shrimp, Penaeus monodon by serotonin and dopamine injection. Int. J. Pharm. Life Sci., 4: 2785-2793.
Babu, K.R., 2013. Improved maturation of wild and pond-reared black tiger shrimp Penaeus monodon (Fabricius) using different combinations of live and wet feeds. Asian J. exp. Sci., 27: 37-42. https://doi.org/10.17257/hufslr.2013.37.2.27
Browdy, C.L. and Samocha, T.M., 1985. The effect of eyestalk ablation on spawning, moulting and mating of Penaeus semisulcatus De Haan. Aquaculture, 49: 19-29. https://doi.org/10.1016/0044-8486(85)90187-5
Cahu, C.L., Cuzon, G. and Quazuguel, P., 1995. Effect of highly unsaturated fatty acids, α-tocopherol and ascorbic acid in broodstock diet on egg composition and development of Penaeus indicus. Comp. Biochem. Physiol., 112A: 417-424. https://doi.org/10.1016/0300-9629(95)02009-8
Cavalli, R.O., Scardua, M.P. and Wasielesky, W.J., 1997. Reproductive performance of different sized wild and pond-reared Penaeus paulensis females. J. World Aquacult. Soc., 28: 260-267. https://doi.org/10.1111/j.1749-7345.1997.tb00641.x
Ceballos-Vazquez, B.P., Rosas, C. and Racotta, I.S., 2003. Sperm quality in relation to age and weight of white shrimp Litopenaeus vannamei. Aquaculture, 228: 141-151. https://doi.org/10.1016/S0044-8486(03)00322-3
Chotipuntu, P., Wuthisuthimethavee, S., Direkbusrakom, S. and Songtuay, S., 2013. Reproductive aspects of SPF Penaeus monodon grown in closed culture captivity. Agric. Tech. biol. Sci., 10: 227-236.
Coman, G.J., Crocos, P.J., Arnold, S.J., Keys, S.J., Murphy, B. and Preston, N.P., 2005. Growth, survival and reproductive performance of domesticated Australian stocks of giant tiger prawn, Penaeus monodon, reared in tanks and raceways. J. World Aquacult. Soc., 36: 464-479. https://doi.org/10.1111/j.1749-7345.2005.tb00394.x
Coman, G.J., Arnold, S.J., Peixoto, S., Crocos, P.J., Coman, F.E. and Preston, N.P., 2006. Reproductive performance of reciprocally crossed wild-caught and tank-reared Penaeus monodon broodstock. Aquaculture, 252: 372-384. https://doi.org/10.1016/j.aquaculture.2005.07.028
Coman, G.J., Arnold, S.J., Callaghan, T.R. and Preston, N.P., 2007. Effect of two maturation diet combinations on reproductive performance of domesticated Penaeus monodon. Aquaculture, 263: 75-83. https://doi.org/10.1016/j.aquaculture.2006.10.016
Coman, G.J., Arnold, S.J., Wood, A.T. and Preston, N.P., 2013. Evaluation of egg and nauplii production parameters of a single stock of domesticated Penaeus monodon (giant tiger shrimp) across generations. Aquaculture, 400-401: 125-128. https://doi.org/10.1016/j.aquaculture.2013.03.015
Cristiane, H.C. and Lídia, M.Y.O., 2010. The influence of eyestalk ablation on the reproduction of the fresh water Macrobrachium acanthurus shrimp in captivity. Acta Scient. biol. Sci., 32: 217-221.
Emerencianoa, M., Cuzon, G., Mascaro, M., Arevalo, M., Norena-Barroso, E., Jeronimo, G., Ilie, S. Racotta, S. and Gaxiola, G., 2012. Reproductive performance, biochemical composition and fatty acid profile of wild-caught and 2nd generation domesticated Farfantepenaeus duorarum (Burkenroad, 1939) broodstock. Aquaculture, 344-349: 194-204. https://doi.org/10.1016/j.aquaculture.2012.03.014
Glencross, B., Tabrett, S., Irvin, S., Wade, N., Anderson, M., Blyth, D., Smith, D., Coman, G. and Preston, N., 2013. An analysis of the effect of diet and genotype on protein and energy utilization by the black tiger shrimp, Penaeus monodon- why do genetically selected shrimp grow faster? Aquacult. Nutr., 19: 128-138. https://doi.org/10.1111/j.1365-2095.2012.00941.x
Habashy, M.M., 2013. On the breeding behavior and reproduction of the freshwater prawn, Macrobrachium rosenbergii (de Man 1879) (Decapoda-Crustacea) under laboratory conditions. Aquacult. Res., 44: 395-403. https://doi.org/10.1016/j.micron.2012.09.005
Hall, M.R., Mastro, R., Young, N., Fraser, C., Strugnell, J. and Kenway, M., 2003. High quality eggs and nauplii for the Australian prawn industry. FRDC Final Report 1995/166, Fisheries Research and Development Corporation, pp. 142.
Hoang, T., Lee, S.Y., Keenan, C.P. and Marsden, G.E., 2002. Observations on growth, sexual maturity and spawning performance of pond-reared Penaeus merguiensis. Aquacult. Res., 33: 863-873. https://doi.org/10.1046/j.1365-2109.2002.00726.x
Huang, J.H., Yang, Q.B., Ma, Z.M., Chen, X., Zhou, F.L., Wen, W.G. and Jiang, S.G., 2013. The growth, development and sexual maturity of pond-reared Penaeus monodon. J. Fish. China, 37: 397-406. https://doi.org/10.3724/SP.J.1231.2013.38174
Jee, A.K. and Kok, L.Y., 2008. Fecundity changes in Macrobrachium rosenbergii (De Man) during egg incubation. Aquacult. Res., 22: 1-6.
Jiang, S.G., Huang, J.H., Zhou, F.L., Chen, X., Yang, Q.B., Wen, W.G. and Ma, Z.M., 2009. Observations of reproductive development and maturation of male Penaeus monodon reared in tidal and earthen ponds. Aquaculture, 292: 121-128. https://doi.org/10.1016/j.aquaculture.2009.03.054
Jiang, S., Zhou, F.L., Yang, Q.B., Huang, J.H., Yang, L.S. and Jiang, S.G., 2019. Impact of temperature stress on oxygen and energy metabolism in the hepatopancreas of the black tiger shrimp, Penaeus monodon (Crustacea: Decapoda: Penaeidae). Pakistan J. Zool., 51: 141-148. https:/ /doi.org/10.17582/journal.pjz/2019.51.1.141.148
Maheswarudu, G., Jose, J., Mohan, S. and Arputharaj, M.R., 2013. Brood stock dependent seed production and grow-out culture of green tiger shrimp Penaeus semisulcatus (De Haan, 1844) at Mandapam, South-east coast of India. Int. J. Agric., 124: 252-267.
Marsden, G., 2008. Factors affecting reproductive performance of the prawn, Penaeus monodon. Ph.D. thesis, Queensland University of Technology, pp. 45-55.
Menasveta, P., Sangpradub, S., Piyatiratitivorakul, S. and Fast, A.W., 1994. Effects of broodstock size and source on ovarian maturation and spawning of Penueus monodon Fabricius from the Gulf of Thailand. J. World Aquacult. Soc., 25: 41-49. https://doi.org/10.1111/j.1749-7345.1994.tb00802.x
Meeratana, P., Withyachamnarnkul, B., Damrongphol, P., Wongprasert, K., Suseangtham, A. and Sobhon, P., 2006. Serotonin induces ovarian maturation in giant freshwater prawn broodstock, Macrobrachium rosenbergii de Man. Aquaculture, 160: 315-325. https://doi.org/10.1016/j.aquaculture.2006.06.010
Nagur, B.K., Reddy, D.C. and Kalaram, V., 2014. Effect of eyestalk ablation on ovarian maturation in tiger shrimp Penaeus monodon (Fabricius) under different environmental conditions Middle-east. J. Sci. Res., 19: 1403-1405.
Palacios, E., Ilbarra, A.M., Ramirez, J.L., Portillo, G. and Racotta, I.S., 1998. Biochemical composition of eggs and nauplii in white Pacific shrimp, Penaeus vannamei (Boone), in relation to the physiological condition of spawners in a commercial hatchery. Aquacult. Res., 29: 183-189. https://doi.org/10.1111/j.1365-2109.1998.tb01123.x
Palacios, E., Racotta, I.S. and Apsa, A., 1999. Spawning frequency analysis of wild and pond-reared shrimp Penaeus vannamei broodstock under large-scale hatchery conditions. J. World Aquacult. Soc., 30: 180-191. https://doi.org/10.1111/j.1749-7345.1999.tb00865.x
Peixoto, S., Wasielesky, Jr. W., D’Incao, F. and Cavalli, R.O., 2003. Comparison of reproductive performance of similar-sized wild and captive Farfantepenaeus paulensis. J. World Aquacult. Soc., 34: 50-56. https://doi.org/10.1111/j.1749-7345.2003.tb00038.x
Peixoto, S., Coman, G.J., Arnold, S.J., Crocos, P.J. and Preston, N.P., 2005. Histological examination of final oocyte maturation and atresia in wild and domesticated Penaeus monodon (Fabricius) broodstock. Aquacult. Res., 36: 666-673. https://doi.org/10.1111/j.1365-2109.2005.01271.x
Pongtippatee, P., Vanichviriyakit, R., Chavadej, J., Plodpai, P., Pratoomchart, B., Sobhon, P. and Withyachumnarnkul, B., 2007. Acrosome reaction in the sperm of the black tiger shrimp Penaeus monodon (Decapoda, Penaidae). Aquacult. Res., 38: 1635-1644. https://doi.org/10.1111/j.1365-2109.2007.01824.x
Preston, N.P., Coman, G.J., Sellars, M.J., Cowley, J.A., Dixon, T.J., Li, Y. and Murphy, B.S., 2009. Advances in Penaeus monodon breeding and genetics. In: The rising tide: Proceedings of the Special Session on Sustainable Shrimp Farming, World Aquaculture 2009 (eds. C.L. Browdy and D.E. Jory). The World Aquaculture Society, Baton Rouge, Louisiana, USA.
Preston, N., Coman, G., Cowley, J., Moore, N. and Murphy, B., 2010. Black tiger breeding program yields record shrimp harvests in Australia. Global Aquaculture Advocate 95 (September/October).
Rao, K.J., 1991. Reproductive biology of the giant freshwater prawn Macrobrachium rosenbergii (De Man) from Lake Kolleru (Andhra Pradesh). Indian J. Anim. Sci., 61: 780-787.
Shailender, M., Amarnath, D., Kishor, B. and Suresh, B.C.H., 2013a. Effect of unilateral eyestalk ablation (UEA) on the reproductive success of giant fresh water prawn, Macrobrachium rosenbergii (De Man) in captivity. Int. J. Chem. Life Sci., 2: 1112-1120.
Shailender, M., Amarnath, D., Kishor, B. and Suresh, B.C.H., 2013b. Effect of unilateral eyestalk ablation in reproductive cycle of Penaeus monodon (Fabricius) after spawning under laboratory conditions. Int. J. Chem. Life Sci., 2: 1121-1125.
Vaca, A.A. and Jorge-Alfaro, J., 2000. Ovarian maturation and spawning in the white shrimp, Penaeus vannamei, by serotonin injection. Aquaculture, 18: 373-385. https://doi.org/10.1016/S0044-8486(99)00267-7
Wen, W., Yang, Q., Ma, Z., Jiang, S., Qiu, L., Huang, J., Zhou, F. and Qin, J.G., 2015. Comparison of ovarian maturation and spawning after unilateral eyestalk ablation of wild-caught and pond-reared Penaeus monodon. Spanish J. agric. Res., 13: e0402. https://doi.org/10.5424/sjar/2015133-7860
Wongprasert, K., Asuvapongpatana, S., Poltana, P., Tiensuwan, M. and Withyachumnarnkul, B., 2006. Serotonin stimulates ovarian maturation and spawning in the black tiger shrimp Penaeus monodon. Aquaculture, 261: 1447-1454. https://doi.org/10.1016/j.aquaculture.2006.08.044
Yang, L., Huang, J., Sun, M., Zhou, F., Yang, Q. and Wen, W., 2011. Investigation on status of carrying virus of wild Penaeus monodon from three resources. J. Shanghai Ocean Univ., 20: 546-552.
To share on other social networks, click on any share button. What are these?










