Enhancing Resistance Level against Mungbean Yellow Mosaic Virus by Inducing Defense Related Enzymes in Mungbean
Enhancing Resistance Level against Mungbean Yellow Mosaic Virus by Inducing Defense Related Enzymes in Mungbean
Ummad-ud-Din Umar1, Syed Burhan-ud-Din2, Muhammad Fahad Khan1, Ateeq ur Rehman1, Syed Atif Hasan Naqvi1*, Muhammad Asif Zulfiqar3, Azhar Ali Khan3 and Naila Ilyas1
1Department of Plant Pathology, Faculty of Agricultural Sciences and Technology, Bahauddin Zakariya University, Multan, Pakistan; 2Agriculture Pest Warning and Quality Control of Pesticides, Rahim Yar Khan, Pakistan; 3Pakistan Agriculture Research Council, Research and Training Station, Bahauddin Zakariya University, Multan, Pakistan.
Abstract | Whitefly transmitted Begomoviruses diseases have appeared as irresistible problem in different crops of Pakistan. The research was carried out to estimate the probable role of induced systemic acquired resistance in mungbean against Mungbean yellow mosaic virus (MYMV) disease. Exogenous use of Salicylic acid (SA) and Benzothiadiazole (BTH) as elicitors enhanced the resistance in mungbean plants by triggering the SA pathway. Induced resistance was assessed by evaluating the appearance of symptoms and detection of virus titter through ELISA. Different concentrations of SA and BTH were exogenously applied to activate the inherent resistance of mungbean by the production of defense associated compounds. All treatments were supportive in suppressing plant infection as compared to infected control, but the most promising outcome of treatments was observed when SA and BTH were applied at the concentration of 5mM and 150mg/L respectively. Three weeks’ analysis of treated mungbean leaves with SA and BTH exhibited peak accumulation of phenols and defense related enzymatic antioxidants. Highest enzymatic activity was observed in treated plants followed by inoculation with MYMV. Increase in resistance by the application of SA and BTH, antioxidant enzymatic activities of SOD, POD, CAT were also increased for second week. This revealed that SA and BTH can be used as a potential source for the activation of resistance for better management of MYMV by improving protection level by the induction of systemic acquired resistance.
Received | November 06, 2018; Accepted | February 03, 2019; Published | February 28, 2019
*Correspondence | Syed Atif Hasan Naqvi, Department of Plant Pathology, Faculty of Agricultural Sciences and Technology, Bahauddin Zakariya University, Multan, Pakistan; Email: [email protected]
Citation | Umar, U.D., S.B. Din, M.F. Khan, A. Rehman, S.A.H. Naqvi, M.A. Zulfiqar, A.A. Khan and N. Ilyas. 2019. Enhancing resistance level against Mungbean Yellow Mosaic virus by inducing defense related enzymes in Mungbean. Pakistan Journal of Agricultural Research, 32(2): 241-251.
DOI | http://dx.doi.org/10.17582/journal.pjar/2019/32.2.241.251
Keywords | Induced resistance, Vigna radiata, Enzymatic antioxidants, MYMV
Introduction
The mungbean (Vigna radiata L) is also called mung/ green gram is dominant legume crop grown throughout the world for its edible seed and sprouts. Ninety percent of world mungbean is cultivated in Asia. In Pakistan mungbean is grown over an area of 133 thousand hectares with annual yield of 102 thousand tons. (Ministry of National Food Security and research GoP, 2015). Grain legumes are rich source of protein contents throughout south Asia, but they are affected by several pest and diseases and which resulted high yield losses and among them Mungbean Yellow Mosaic virus (MYMV) is very distressing disease during summer months in Pakistan and more than 50% yield losses were reported in mungbean. Whitefly transmitted MYMV belongs to family Geminiviridae and genus Begomovirus (Boss, 1999). According to an estimate more than US$ 300 million loss occurred due to this virus in different leguminous crops (Varma et al., 1992). Initial symptoms of disease arise on foliage in the form of small intermingle green and lemon color dots on young leaves that scattered on other leaves, puckering of leaves occurred and apex of growing areas are reduced in size and irregular light green and yellow specks appear on affected leaves. (Narini, 1960). If plants are attacked at seedling stage than 100% yield losses occurred (Usharani et al., 2004). These alterations emerging from disease causing organism may interrupt plant physiology and increase plant senescence and activate the defense mechanism. Systemic acquired resistance (SAR) is a natural phenomenon which activates in plants under stress conditions and signaling hormone like salicylic acid (SA) and Methyl Jasmontae (MeJ) a derivative of Jasmonic acid (JS) are produced which provide pathway for the induction or activation of defense reaction. Depending on the developmental phase of plants, exogenous application of SA increases tolerance to stress. Against biotic and abiotic stresses, SA acts as an endogenous regulator and has definite role in defense mechanism. Benzothiadiazole (BTH) also involves in the activation of the SAR pathway downstream signals from the SA. Different type of proteins known as pathogenesis related proteins (PR), phenolic compounds, reactive oxygen species (ROS) and enzymatic and non-enzymatic antioxidants are produced by the activation of defense reaction which results in the detoxification of ROS produced by plant- pathogen interaction and protect plant from oxidative damage. Furthermore, these compounds could be acts as biochemical markers to evaluate the developing resistance. Therefore, an attempt was made for enhancing resistance against MBYMV by the exogenous application of SA and BTH. The resistance was characterized by evaluating symptom developed by viral infection, phenolic contents and enzymatic antioxidants like Superoxide dismutase (SOD), Peroxidase (POD), Catalase (CAT) and Phenylalanine ammonialyase (PAL). Application of plants with signaling molecules decrease enzyme activities and results in induction of resistance. Application of SA and BTH results in activation of plant defense mechanism and induces resistance in plant against pathogens. Salicylic acid is produced naturally in plants in stress or when they are attacked by pathogen which stimulates signal transduction pathway and induces local and systemic resistance (Datta et al., 2012; Maleck et al., 2000).
Materials and Methods
Evaluation of resistance in mungbean germplasm
Disease severity score was measured by using 0-5 disease scoring scale arrogated from Akhtar et al. (2009) after careful observation of initial symptoms of viral infection in the greensward (Table 1). Current method used for calibration of disease severity on visual basis contained percentage of disease plants were taken as varietal characteristics that either it is resistant variety or susceptible variety (Khattak et al., 2008). For assessment of MYMV severity, 10 plants from each row were selected randomly and tagged and disease severity was measured by using 0-5 disease scoring scale (Bashir and Zubair, 2009).
Table 1: Disease rating scale of Mungbean yellow mosaic virus (MYMV) from (0-5).
| Scale | % Infection | Visual Symptoms | Response |
| 0 | All plant free of virus symptoms | Complete absence of symptoms | Highly resistant |
| 1 | 1-10% | Small yellowish spots scattered on some leaves | Resistant |
| 2 | 11-20% | Yellowish bright spots common on leaves, easy to observe | Moderately resistant |
| 3 | 21-30% | Yellowish bright specks common on leaves, easy to observe with larger patches of symptoms | Moderately susceptible |
| 4 | 30-50% | Bright yellow specks or spots on all leaves, minor stunting of plants and less number of pods | Susceptible |
| 5 | > 50% | Yellowing or chlorosis of all leaves on whole plant, shortening of internode, severe stunting of plants with no yield or few flowers and deformed pods produced with small, immature and shriveled seeds | Highly susceptible |
Following formula was used for accurate calibration of disease severity index (Chaube, 1990),
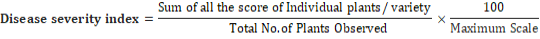
Application of resistance inducing compounds
Research trials were conducted under controlled conditions to study induction of resistance in mungbean against Mungbean yellow mosaic virus (MYMV). For this purpose, highly, susceptible variety of mungbean i.e. Kabuli mung was sown in earthen pots of 35cm filled with sand, peat and loam. Treatments were replicated thrice. After 20 days of sowing when leaves become mature, plants were treated exogenously with Salicylic acid (SA) and Benzohiadiazole (BTH) (Sigma, St. Louis, MO, USA) at different concentrations. After 24 hours of application mungbean plants were inoculated with viruliferous whiteflies (B. tabaci) reared on MYMV infected plants. The plants inoculated with virus and sprayed with distilled water without any application of elicitors were used as infected and healthy control respectively. The enzymatic activates i.e., PAL, POD, SOD, CAT and total Phenolic contents were determined after first, second and third week of post inoculation. The disease severity on each plant was calculated based on symptoms using disease rating scale. For the detection of virus titer all the treated plant was tested through DAS-ELISA (Clark and Adam, 1977) using Tomato Yellow Leaf Curl Bigemini virus polyclonal antibodies (AC, Diagnostics, USA) and has cross reactivity with other Gemini viruses.
Enzyme extraction
The activities of POD, SOD, CAT in mungbean leaves were assayed according to Aebi (1983). Three leaves samples of 0.2 g FW were collected from each treatment at 7, 14 and 21dpi and grind in liquid nitrogen with the help of pestle and mortar. 2ml of ice -cold 50mM phosphate buffer (pH 7.8) with 1mM ethylene diamine teraacetic acid (EDTA) was added and homogenized. The mixture was centrifuged at 1000 rpm for 15 min at 4oC. The supernatant was used as enzyme extract.
Superoxide dismutase (SOD) assay (EC 1.5.1.1)
Activity in leaves was determined based on its efficiency in restraining the photoreduction of Nitro blue tetrazolium (NBT) by the addition of Riboflavin. A volume of 3mL of reaction mixture containing (50 mM phosphate buffer (pH 7.8), 0.1mM EDTA, 130mM methionine,0.75 mM NBT, 0.02 mM riboflavin, and 0.1mL of enzyme extract) Fluorescent lamp of 40W was illuminated for 10 min. Reactions without enzyme extract exposed to light and in dark were used as calibration standards. Spectrophotometer (Biorad UV vis 3000 plus) was used for measuring absorbance of blank as control and reaction mixture at 560 nm. (Sun and Zigman, 1978).
Peroxidase (POD) assay ( EC 1.11.1.7)
POD activity was measured by the increase in absorbance at 470 nm due to formation of tetra guaiacol, an oxidation product of guaiacol (Chen et al., 2007). The reaction mixture contains 33.0 mM potassium phosphate buffer (pH 6.1), 16 mM guaiacol, 2 mM H2O2 and 200 µL of enzyme extract. The increase in absorbance at 470 nm was monitored for 3 min with and without addition of enzyme extract. POD activity (µmol H2O2 decomposed min-1 mg -1 protein) was estimated by using extinction coffection ( 26.6 mM-1 cm-1).
Catalase (CAT) assay ( EC 1.11.1.6)
The CAT activity was assayed by measuring the rate of decomposition of H2O2 using the method of Aebi (1984). The reaction mixture consist of 50mM phosphate buffer (pH 7.0), 12mM of H2O2 and 50 µL of enzyme extract . The rate of disapearence of H2O2 was followed by observing the rate of decrease in absorbance at 240 nm for 3 min. An extinction coefficient of 43.6 M–1 cm–1 was used for calculating the CAT activity (µmol min–1 mg–1 protein).
PAL(Phenylalanine ammonia Lyase) activity (EC4.3.1.24)
For the estimation of PAL activity leaf sample (1g ) was homogenized in 3mL of ice cold 0.1M sodium borate buffer, pH 7.0 containing 1.4mM of 2-mercatoethanol and insoluble PVP (Polyvenylpyrrolidone).The extract filter through cheese cloth and the filtrate centrifuge at 12000 rpm for 15 min and the supernantant was used as enzyme source. PAL activity was determined as the rate of conversion of L-phenylalanine to trans-cinnamic acid at 290 nm as described by Dickerson et al., (1984) . Sample containing enzyme extract was incubated with 0.1M borate buffer, pH 8.8 and 12mM L – phenylalanine in the same buffer for 30 min at 30oC.
Estimation of total phenolic contents ( EC 1.14.13.7)
For determination of total phenolic contents samples of mungbean leaves were collected from glass house trial, samples were kept in ice box during collection.For Phenolic content assay the modified Follin-Ciocalteu method was used (Ainsworth and Gillespie, 2007). For plotting Gallic acid standard curve 100 ppm stock solution of Gallic acid was prepared by dissolving 100 mg GA in 1 liter of water. The absorbance of the blue color developed was measured using Biorad UVvis 3000 plus spectrophotometer at 725 nm. The phenolic contents was expressed as µg gallic mg-1 protein. Total protein estimation was carried out by Bradford assay (Bradford, 1976).
Statistical analysis
Data was subjected to statistical to analysis of variance (ANOVA) using (Statistical software package 8.0. Institute Carry Inc; USA). Treatment means were compared using Fisher’s least significant differences (LSD) at (P = 0.05).
Results and Discussion
The results of the trial revealed that all the treatments have significantly help in reducing the disease severity as compared to the control. Disease severity of MYMV with respect to each treatment (Figure 1; Figure 2).
The maximum disease suppression was observed as compared to infected control when the treatments of SA@ 5 mM and BTH @150 mgL-1 were applied and the disease severity remained between 14% and 13.8% as compared to infected control where it was up to 45% (Figure 3).
Phenylalanine ammonia lyase (PAL) activity assay
The enzymatic activities after the induction of resistance through elicitors against MYMV were increased significantly as compared to both the virus infected and healthy controls. The PAL activity was increased in all the treatments significantly as compared to infected and healthy controls. Maximum PAL activity was observed in first week on the infected plants treated with 5mM S.A followed by the infected plants treated with 10mM. PAL activities tend to decrease after every week (Figure 4).
Peroxidase (POD) activity assay
The POD activity was increased in all treatments after application as compared to infected and healthy controls. During three weeks after application maximum POD activity was observed in second week on the infected plants treated with 10mM S.A (Figure 5).
Superoxide dismutase (SOD) assay
There was significant effect of S.A and BTH on SOD activity in second and third week after treatments as compared to the controls. In first week after application of treatments the SOD activity was very low but in second week, it increased and remained almost at higher levels. Where the SOD activity in infected plants treated with BTH was decreased in third week (Figure 6).
Catalase (CAT) activity assay
The CAT activity was greatly increased in second week after application. The most effective treatment which showed maximum CAT activity was 10mM SA followed by virus infection and BTH 150mg/ L applied after virus infection. The CAT activity was reduced greatly after second week (Figure 7).
Total phenolic contents
Total phenolic contents were increased significantly as compared to the healthy and inoculated controls. The effect of treatments on the production of total phenolic contents remained almost same during all three weeks after applications. Total phenolic contents were maximum in third week in all treated plants (Figure 8).
There was scarcity of resistant genetic source in mungbean, so innate defense of plant was enhanced by exogenous application of Salicylic acid and benzothiadiazole under controlled conditions on highly susceptible cultivar of mungbean.
Salicylic acid (SA) is plant hormone which is responsible in natural plant defense mechanism in response to pathogen attack and this signaling molecule is helpful in induction of resistance in plants. In case of lack of natural defense mechanism of plants, exogenous treatment of elicitor like SA (salicylic acid) and BTH (Benzothiadiazole) are helpful in the induction of resistance in plants. Enzymatic antioxidants are produced in plants and play an important role in induced systemic acquired resistance, furthermore act as a biochemical marker to evaluate the developing resistance as the result of elicitor application in plants. Mohammadi and Kazemi (2002) reported the increased activities of POD and CAT enzymes in Tomato yellow leaf curl virus (TYLCV) infected leaves and induced resistance in the host plant with increased ability of susceptible plants to withstand pathogens in a non-genetic way (Kagale, et al., 2004). As it is reported by Biswas et al. (2012) that the induced resistance prior to challenge infection raises the level of some signaling molecules which alerts the plants to produce rapidly some compounds after infection thus providing defense against the disease (Biswas et al., 2012). The phenomenon of induced resistance may involve the production of pathogenesis related proteins and antioxidative enzymes etc. Kagale et al. (2004) reported that elicitor applications involve induction
of systemic resistance by the activation of several defense related enzymes like SOD POD and PAL. The elevated level of these enzymes may be involved in the protection of the host plant against systemic infection which is according to previous findings that the increased level of phenolics could provide an adequate substrate to oxidative reactions catalyzed by POD make the medium unfavorable to the further development of pathogens (Lattanzio et al., 2006). Our study was like the findings of Radwan et al. (2006) who studied the application of SA on cucumber and pumpkin infected by Zucchini yellow mosaic virus (ZYMV) and the resultant enzymatic activity of PAL, POD, CAT and SOD activity was increased by three folds by application of SA. Our findings are also like findings of Loon (1989) who concluded that when plant is in stress by environmental factors or pathogen attack than a variety of pathogenesis
related proteins are accumulated in plants that provide resistance to plant. Antimicrobial quinones are produced by the oxidation of phenolic compounds by POD, which inhibit viruses by inactivating viral DNA and RNA (Dixon and Harrison, 1990). Elbadry et al. (2006) suggested that external application of SA was helpful in providing systemic acquired resistance against Bean yellow mosaic virus (BYMV) in faba beans by enhancing production of oxidative enzymes. Siddique et al. (2014) result revealed that SOD, CAT, PAL, POD, Phenolic contents and proteins played vital role in defense mechanism of cotton plant against CLCuBuV. This finding suggested that correlation exist between integral extent of enzymes activities and resistance of plant which acts as biochemical markers for determining compatibility of plant-virus relationship. External application of SA in appropriate concentration significantly reduce systemic infection of MYMV before inoculation and no prominent symptom of MYMV was observed at100µM concentration of SA (Vanacker et al., 2000). Primary and secondary metabolism is involved in the induction of resistance by SA against MYMV which depend on enhanced protein synthesis during metabolic reprogramming. Recognition of proteins depicting huge profusion, associated with process of photosynthesis is essential analysis which recovers virus induced destruction of photosynthesis process and gave increased metabolites that are needed for re distribution of available defense resources. Our findings agree with Mandal (2010). Elicitors are capable and imitate in recognition of pathogen by plants, hence activate the delicate and easy defense capability in plants. Activity of PAL by application of SA and BTH showed an increase in POD activity was increased. The effect or activity of these compounds on other antioxidants like CAT, SOD was also higher by these elicitors. Phenolic compounds accumulation and activity of several defense related enzymes were increased as they were triggered by elicitor. SA application is helpful in activation of defense proteins in plants (Clarke et al., 1998). The results of this investigation suggested that when SA was applied to plants infested with viruses than Pathogenesis related proteins (PR) inhibit production of viral replication and movement protein Ashfaq et al. (2010) also observed the increase in total proteins of virus infected Urdbean (Vigna mungo L.) plant due to viral and PR proteins. It has been known for some time that SA modulates the induction of SAR following pathogen attack (Ryals et al., 1996) Loon et al. (1994) concluded that PR proteins has strong defense ability against viral infection. In our whole study on induction of resistance in highly susceptible variety Kabuli mung under controlled conditions we observed that by application of elicitor compounds BTH and SA activity of defense related antioxidants like SOD, POD, PAL, CAT and total proteins and phenolic contents were higher in mungbean leaves that were treated with elicitor compounds and in healthy control as compared to infected control our study coincides with investigation of many scientists their results also showed that the external application of elicitor molecules enhances plant defense system by producing higher level of SOD, POD, CAT, phenols and proteins. Nandi et al. (2005) that the most appropriate defense mechanism of plants was linked with metabolic variation that are integrated in plants are interconnected with our results in their results leaf curl infection in tomato plant was reduced by application of SA and activities of SOD, POD, CAT was increased in treated plants as compared to infected control. Our results are linked with the study that SA and BTH application significantly increases oxidative enzymes accumulation in Tobbacco plant infected with Tobacco mosaic virus (TMV) (Zechmann et al., 2003). The generation of reactive oxygen species (ROS) is one of the initial responses of plants to pathogens (Mehdy, 1994; Vanacker et al., 2000). As a major scavenger in antioxidant enzyme systems that protect cellular membranes and organelles from AOS in plants, SOD converts superoxide anion radicals to hydrogen peroxide and oxygen by disproportion (Fridovich, 1986). PAL, a key enzyme in phenylpropanoid metabolism, plays a significant role in the synthesis of various secondary metabolites (e.g., phenols, phenylpropanoids, and lignin and salicylic acid monomers) that are involved in plant immunity and induce resistance by PGPR (Hamid et al., 2004). These secondary metabolites are in the restriction and invasion of virus (Luo et al., 2011). BTH-treated Arabidopsis thaliana plants were showed resistance to infection by turnip crinkle virus, Pseudomonas syringae and Peronospora parasitica (Lawton et al., 1996). Mungbean plants treated with BTH and SA reduced the percentage of infected plants and displayed less severe symptoms. The reduction in disease severity was directly correlated with the concentration of BTH and SA and the time between application and virus inoculation.
Conclusions and Recommendations
It is concluded that an exogenous application of SA and BTH could offer a good source for the management of MYMV by inducing resistance in highly species of mungbean.
Acknowledgments
This manuscript is a part of thesis of Ms. Naila Ilyas, submitted to Higher Education Commission of Pakistan via Department of Plant Pathology, Bahauddin Zakariay University, Multan on 14-12-2016.
Author’s Contribution
Ummad ud Din Umar and Ateeq ur Rehman conceived the idea and wrote the Introduction and discussion. Syed Burhan-ud-Din wrote the abstract while Muhammad Fahad Khan and Naila Ilyas performed the biochemical analysis. Syed Atif Hasan Naqvi did the SPSS analysis. Methodology was designed by Azhar Ali Khan and reference section was managed by Muhammad Asif Zulfiqar.
References
Aebi HE. 1983. Catalase, In: Methods of enzymatic analysis. Bergmeyer, H.U. (Ed.). Verlag Chemie Weinhem. 273-286.
Aebi, H. (1984) Catalase in Vitro. Methods Enzymology, 105, 121-126.
Ainsworth EA, Gillespie KM. 2007. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin-Ciocalteu reagent. Nat Protoc 2(4): 875-877.
Akhtar, K.P., Kitsanachandee, R., Srinives, P., Abbas, G., Asghar, M.J., Shah, T.M. Atta, B.M., Chatchawankanphanich, O., Sarwar, G., Ahmad, M., and N. Sarwar.2009. Field evaluation of mungbean recombinant inbred lines against mungbean yellow mosaic disease using new disease scale in Thailand. Plant Pathol. J., 25:422-428.
Ashfaq, M., M.A. Khan, N. Javed, S.M. Mughal, M. Shahid and S.T. Sahi. 2010. Effect of Urdbean leaf crinkle virus infection on total soluble protein and antioxidant enzymes in black gram plants. Pak. J. Bot. 42: 447-454.
Bashir, M., and M. Zubair. 2002. Identification of resistance in urdbean (Vinga mungo) against two different viral diseases. Pak. J. Bot., 34(1): 49-51.
Biswas, K. K., V. G. Malathi, and A. Varma. 2012. Resistance in mungbean against five variants of Mungbean Yellow Mosaic Virus. Ind. J. Virol., 16: 27-31.
Boss, L. 1999. Plant Viruses: Unique and Intriguing Pathogens: A Text Book of Plant Virology, Backhuys Publishers, the Netherlands. 305-306 pp.
Bradford M M 1976 A rapid and sensitive method for quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254.
Chaube. 1990. Plant Disease Management Principles and Practices. CRS Press, Boca Raton: 319.
Chen, H. M., A. L. Chien, C. G. Kuo, C. M. Chien, H. C. Sun, C. C. Huang, Y. C. Lin, and H. M. Ku. 2007. Development of a molecular marker for a bruchids (Callosobruchus chinensis L.) resistance gene in mungbean. Euphytica, 157, 113- 122.
Clark, M. E., and A. N. Adams 1977. Characteristics of the microplate method of enzyme-linked immunosorbent assay for the detection of plant viruses. J. Gen. Virol. 34:475-483.
Clarke, J.D., Liu, Y., Klessig, D.F., and Dong, X. 1998. Uncoupling PR gene expression from NPR1 and bacterial resistance: Characterization of the dominant Arabidopsis cpr6–1 mutant. Plant Cell 10, 557–569.
Datta, S., S. Gangwar, S. Kumar, S. Gupta, R. Rai, M. Kaashyap, P. Singh, S. K.Chaturvedi, B. B. Singh, N. Nadarajan. 2012. Genetic diversity in selected Indian mungbean [Vigna radiata (L.) wilczek] cultivars using RAPD markers. American Journal of Plant Sciences, 3: 1085-1091.
Dickerson D P., Pascholati S F, Hagerman A E, Butler L G and Nicholson R L 1984 Phenylalanine ammonia-lyase and hydroxyl cinnamate: CoA ligase in maize mesocotyls inoculated with Helminthosporium maydis or Helminthosporium carbonum. Physiol. Plant Pathol. 25, 111–123.
Dixon RA, Harrison MJ. 1990. Activation, structure and organization of genes involved in microbial defense in plants. Adv. Genet. 28:165–234.
Elbadry, M., R. M. Taha, K. A. Eldougdoug and H. G. Eldin. 2006. Induction of systemic resistance in faba bean (Vicia faba L.) to Bean Yellow Mosaic potyvirus (BYMV) via seed bacterization with plant growth promoting rhizobacteria. J. Pl. Dis. Prot. 113: 51–247.
Francki, R.I.B., D.W. Mossop and T.Hatta. 1979. Cucumber mosaic virus. CMI/AAB Descriptions of Plant Viruses, No. 213.
Fridovich. 1986. Superoxide dismutases: Advances in Enzymology and Related Areas of Molecular Biology. 58: 61–97.
Government of Pakistan. 2015. Economic survey of Pakistan, finance and economic affairs division, Islamabad: 22 pp.
Hamid, S. and D.J. Robinson. 2004. Begomoviruses from mungbeans in Pakistan: epitope profiles. DNA A sequences and phytogenic relationships. Arch virol, 149:809-819.
Kagale S., Marimuthu T., Thayumanavan B., Nandakumar R., Samiyappan R. 2004. Antimicrobial activity activity and induction of systemic resistance in rice by leaf extract of Datura metel against Rhizoctonia solani and Xanthomonas oryzae pv. oryzae. Physiological and Molecular Plant Pathology, 65:91–100.
Khattak, G.S.S., I. Saeed and S.A. Shah. 2008. Breeding high yielding and disease resistant mungbean (Vigna radiata (L.) Wilczek) genotypes. Pak J Bot. 40:1411- 1417.
Lattanzio, V., V.M.T. Lattanzio and A. Cardinali. 2006. Role of phenolics in the resistance mechanisms of plants against fungal pathogens and insects. Phytochem. Adv. Res. 23-67.
Lawton, K., Friedrich, L., Hunt, M., Weymann, K., Staub, T., Kessmann, H., and Ryals, J. 1996. Benzothiadiazole induces disease resistance in Arabidopsis by activation of the systemic acquired resistance signal transduction pathway. Plant J. 10, 71-82.
Ling, K.S. and A. Levi. 2007. Sources of resistance to Zucchini yellow mosaic virus in Lagenaria siceraria germplasm. Hort. Sci. 42: 1124–1126.
Loon, V.L.C. 1989. Sterss proteins in infected plants. In: T. Kosuge and E.W. Nester (eds.), Plant-Microbe Interact. Mol. Genet. Perspect. pp.198–237. MacGraw-Hill Publ. Comp. New York, USA.
Loon, V.L.C., W.S. Pierpoint, T. Boller and V. Conejero. 1994. Recommendations for naming plant pathogenesis-related proteins. Pl. Mol. Biol. Rep. 12: 245–264. https://doi.org/10.1007/BF02668748
Luo, Y., J. Shang, P. Zhao, D. Xi, S. Yuan and H. Lin. 2011. Application of jasmonic acid followed by salicylic acid inhibits Cucumber mosaic virus replication. Plant Pathol. J. 27: 53-58. https://doi.org/10.5423/PPJ.2011.27.1.053
Maleck, K., A. Levine, T. Eulgem, A. Morgan, J. Schmid and K. Lawton. 2000. The transcriptome of Arabidopsis thaliana during systemic acquired resistance. Nat. Genet. 26: 403–409. https://doi.org/10.1038/82521
Mandal, S., 2010. Induction of phenolics, lignina and key defense enzymes in eggplant (Solanum melongena L.) roots in response to elicitors. Afr. J. Biotechnol. 9 (47), 8038–8047.
Mehdy MC., 1994 Active oxygen species in plant defense against pathogens. Plant Physiol 105: 467-472.
Mohammadi, M. and H. Kazemi, 2002. Changes in peroxidase and polyphenol oxidase activities in susceptible and resistant wheat heads inoculated with Fusarium graminearum and induced resistance. Plant Sci., 162: 491-498.
Nandi A, Moeder W, Kachroo P, Klessig DF, Shah J. 2005. Arabidopsis ssi-2 conferred susceptibility to Botrytis cinerea is dependent on EDS5 and PAD4 . Mol Plant-Microbe Interact 18:363–370.
Naqvi, S.M., M.A. Rustamani, T. Hussain and M.A. Talpur. 1995. Relative resistance of mungbean varieties to whitefly and yellow mosaic. Proc. Pak. Zool. Conf. 15: 247-251.
Nariani, T.K. 1960. Yellow mosaic of mung (Phaseolus aureus). India. Phytopathol. 13: 24-29.
Nath, P.D. 1994. Effect of sowing time on the incidence of yellow mosaic virus disease and whitefly population on greengram. Ann. Agric. Res. 15(2): 174-177.
Radwan, D. E. M., Lu, G., Fayez, K. A. and Mahmoud, S. Y., 2006. Protective action of salicylic acid against bean yellow mosaic virus infection in Vicia faba leaves. J. Pl. Physio. 165: 845-857.
Radwan, D.E.M., K.A. Fayez, S.Y. Mahmoud, A. Hamad and G. Lu. 2007. Salicylic acid alleviates growth inhibition and oxidative stress caused by Zucchini yellow mosaic virus infection in Cucurbita pepo leaves. Physiol. Mol. Plant Pathol. 69: 172-181. https://doi.org/10.1016/j.pmpp.2007.04.004
Ryals J, Neuenschwander U, Willits M, Molina A, Steiner HY, Hunt M. 1996. Systemic acquired resistance. Plant Cell, 8: 1809-1819.
Saavedra, T.D.L., M.A. García-Neria and R.F. Rivera-Bustamante. 2013. Benzothiadiazole (BTH) induces resistance to Pepper golden mosaic virus (PepGMV) in pepper (Capsicum annuum L.). Biol. Res. 46: 333-340. https://doi.org/10.4067/S0716-97602013000400004
Sun, M., and S. Zigman. 1978. Assay for superoxide dismutase based on epinephrine autoxidation. Anal. Biochem. 90: 81-89.
Nair, N.G. and Y.L. Nene. 1973. Studies on the yellow mosaic of urdbean (Phaseolus mungo L.) caused by mungbean yellow mosaic virus. II. virus-vector relationships. India. J. Farm Sci. 1: 62-70.
Siddiquea, Z., K.P. Akhtar, A. Hameed, N. Sarwar, I.U. Haq and S.A. Khan. 2014. Biochemical alterations in leaves of resistant and susceptible cotton genotypes infected systemically by cotton leaf curl Burewala virus. J. Plant Interact. 9 (1): 702–711. https://doi.org/10.1080/17429145.2014.905800
Singh, S.K., L.P. Awasthi, S. Singh and N.K. Sharma. 2011. Protection of mungbean and urdbean crops against vector borne mungbean yellow mosaic virus through botanicals. Curr. Bot. 2(2): 08-11.
Singh, D.P. 1981. Breeding for resistance to diseases in greengram and blackgram. Theor. Appl. Genet. 59: 1-10. https://doi.org/10.1007/BF00275766
Socheath, O. and S.C. Filomena. 2016. Effect of exogenous application of salicylic acid on the severity of tomato leaf curl disease. JISAAS 22 (1): 137-145.
Srivastava, A.K. and R.K. Prajapati. 2012. Influence of weather parameters on outbreak of mungbean yellow mosaic virus in black gram (vigna mungo L.) of bundelkh and zone of central India. J. Agric. Phys. 12: (2)143-151.
Subrata. K.,2011. Salicylic acid ameliorates susceptible vigna mungo cultivar to mungbean yellow mosaic India virus infection. Sci. Cult. 78: 5–6.
Sudha, M., A. Karthikeyan, P. Nagarajan, M. Raveendran, N. Senthil, M. Pandiyan, K. Angappan, J. Ramalingam, M. Bharathi, R. Rabindran, K. Veluthambi and P. Balasubramanian. 2013. Screening of mungbean (Vigna radiata) germplasm for resistance to Mungbean yellow mosaic virus using agroinoculation. Can. J. Plant Pathol. 35: (3) 424–430. https://doi.org/10.1080/07060661.2013.827134
Thakur, M. and B.S. Sohal. 2013. Role of elicitors in inducing resistance in plants against pathogen infection: A review. ISRN Biochem. 2013: 1-1 0.
Usharani, K.S., B. Surendranath, Q.M.R. Haq and V.G. Malathi. 2004. Yellow mosaic virus infecting soybean in northern India is distinct from the species-infecting soybean in southern and western India. Curr. Sci. 86: 6. 845-850.
Vanacker van Acker, S.A.B.E., D.J. van den Berg, M.N.J.L. Tromp, D.H. Griffioen, W.P. van Bennekom and W.J.F. Vijgh. 1996. Structural aspects of antioxidant activity of flavonoids. Free Radic. Biol. Med. 20: 331–342. https://doi.org/10.1016/0891-5849(95)02047-0
Varma, A., A.K. Dhar and B. Mandal. 1992. MYMV transmission and control in India. In: Mungbean yellow mosaic disease (S.K. Green, D. Kim, ed.). Asian Vegetable Res. Dev. Centre, Taipei, Taiwan. Pp. 8–27.
Varma, A., B. Mandal and V.G. Malathi. 1998. Putative location of common region and coat protein gene of black gram isolate of mungbean yellow mosaic Gemini virus. J. Plant Biochem. Biotechnol. 7: 7–12. https://doi.org/10.1007/BF03263026
Venkata, S. and S. Kotakadi. 2012. Biochemical studies of sunflower necrosis tospovirus infecting sunflower (Helianthus annuss) an edible oil seed crop of India. J. Microbiol. Biotech. Res. 2 (3): 458-463.
Verma, H.N. and L.P. Awasthi. 1979. Isolation of the virus inhibitor from the root extracts of Boerhaavia diffusa inducing systemic resistance by plants. Can. J. Bot. 57: 1214–1217. https://doi.org/10.1139/b79-146
Vimala, R. and M. Suriachandraselvan. 2009. Induced resistance in bhedi against powdery mildew by foliar application of salicylic acid. J. Biopest. 2: 111–114.
White, R.F. 1979. Acetylsalicylic acid (aspirin) induces resistance to tobacco mosaic virus in tobacco. Virol. 99: 410-412. https://doi.org/10.1016/0042-6822(79)90019-9
Zechmam, B., M. Muller and G. Zelling. 2003. Cytological modification in Zucchini yellow mosaic virus (ZYMV) infected styrian pumpkin plants. Arch. Virol. 148: 1119-1133. https://doi.org/10.1007/s00705-003-0005-0
To share on other social networks, click on any share button. What are these?







