Efficacy of Chitosan on Performance of Tomato (Lycopersicon esculentum L.) Plant under Water Stress Condition
Efficacy of Chitosan on Performance of Tomato (Lycopersicon esculentum L.) Plant under Water Stress Condition
Hassnain1, Abdul Basit1*, Mehboob Alam1, Imran Ahmad1, Izhar Ullah1, Noor Alam2, Inayat Ullah3, Muhammad Areeb Khalid1, Muhammad Shair4 and Noor ul Ain1
1Department of Horticulture, The University of Agriculture, Peshawar, Khyber Pakhtunkhwa, Pakistan; 2Directorate of Floriculture, DHRD, National Agricultural Research Center, Islamabad, Pakistan; 3Department of Agricultural Mechanization, The University of Agriculture, Peshawar, Khyber Pakhtunkhwa, Pakistan; 4Department of Soil and Environmental Sciences, The University of Agriculture, Peshawar, Khyber Pakhtunkhwa, Pakistan.
Abstract | Drought stress is one of the main problems that cause a severe decrease in plant growth, development and yield. Application of chitosan helps to induce drought resistance and improved water use efficiency. Therefore, a research study was planned to investigate the effect of chitosan on growth and yield of tomato (Cv. Rio Grande) plant under water stress condition at Ornamental Nursery, Department of Horticulture, The University of Agriculture Peshawar, Pakistan, during 2018, using Completely Randomize Design (CRD) with two factors and repeated three times in a control (Glass house) environment. Different concentrations of chitosan (0, 50, 100,150 mg L-1) and various water stress intervals (3, 6, 9 and 12 days) were given to tomato plants from 15 days after transplantation. Results showed that various application of chitosan and water stress interval had a significant (P< 0.01) effect on almost all studied parameter. Tomato plants treated with 6 days water stress interval had maximum average plant height (82.69 cm), average number of leaves (104.02), leaf area (81.47 cm2), chlorophyll content (71.31 SPAD), relative water content (67.27%), excided leaf water retention (68.24%), number of fruit plant-1(24.66), average fruit weight (73.718 g) and yield (37.50-ton ha-1). Similarly, foliar application of chitosan at 100 mg L-1 resulted maximum average plant height (80.74 cm), average number of leaves (104.19), leaf area (81.05 cm2), chlorophyll content (72.29 SPAD), relative water content (66.82%), excised leaf water retention (66.58%), fruit plant-1 (24.33), average fruit weight (74.74 g) and yield (37.37 ton ha-1). It is concluded that 100 mg L-1chitosan application along with 6 days water interval had positive impact on growth and quality of tomato.
Received | March 16, 2019; Accepted | September 12, 2019; Published | January 15, 2020
*Correspondence | Abdul Basit, Department of Horticulture, The University of Agriculture, Peshawar, Khyber Pakhtunkhwa, Pakistan; Email: abdulbasithort97@gmail.com; mashalhort97@aup.edu.pk
Citation | Hassnain, M. Alam, I. Ahmad, A. Basit, I. Ullah, N. Alam, I. Ullah, M.A. Khalid, B. Muhammad and M. Shair. 2020. Efficacy of chitosan on performance of tomato (Lycopersicon esculentum L.) plant under water stress condition. Pakistan Journal of Agricultural Research, 33(1): 27-41.
DOI | http://dx.doi.org/10.17582/journal.pjar/2020/33.1.27.41
Keywords | Drought stress, Chitosan, Water use efficiency, Plant defense, Growth and yield, Tomato
Introduction
Tomato (Lycopersicum esculentum L.) is a famous member of family Solanaceae, one of the most important and widely used cultivated vegetable crop which is mostly grown in home gardens and by market gardeners worldwide. In Pakistan, tomato cultivated area during 2014-15 was 60700 ha with production of 570600 tons. In Khyber Pakhtunkhwa tomato was cultivated on an area of 13300 ha, while its production was 132000 tons (MNFSR, 2015). Tomato is an annual crop grown in warm season. Tomato start flowering 90-120 days after seed sowing and takes approximately 40-50 days to harvest after fruit setting depending on variety. Optimum temperature with a range of 20-27 0C is better for growth and yield, the temperature rises up 30 0C or fall below 10 0C affect tomato plant fruit setting (Iqbal et al., 2011).
Tomato is economically important due to its great yielding capacity for short duration. For increase production of tomato in order to meet the requirement of human population, it could be achieved through increasing the cultivated area or improving the land productivity especially under drought stress condition. Drought stress seems to be one of the main problems that cause a severe decrease in plant growth, development and yield (Thapa et al., 2011). Application of chitosan helps to induce drought resistance and improved water use efficiency in pepper and coffee (Dzung et al., 2011; Bittelli et al., 2001).
Water-stress conditions cause a marked suppression in plant photosynthetic efficiency, mainly due to the closing of stomata and inhibition of (Rubisco) enzyme (Lawlor and Cornic, 2002). The depressive effect of water stress on growth parameters may also be attributed to a drop-in leaf water content and a reduction in the assimilation of nitrogen compounds (Reddy et al., 2003), affecting the rate of cell division and enlargement. Drought stress also reduced the uptake of essential elements and photosynthetic capacity (Kandil et al., 2001) as well as the excessive accumulation of intermediate compounds such as reactive oxygen species (Yazdanpanah et al., 2011) which cause oxidative damage to DNA, lipid and proteins and consequently a decrease in plant growth. Finally, water stress leads to increases in abscisic acid which cause an inhibition of the growth (Abdalla, 2011). Chitosan is harmless to crops, animal and human. The molecule of chitosan triggers a defensive mechanism within the plant, which leads to the formation of physical and chemical barriers against invading different plant pathogens. Chitosan seems to be a natural biodegradable compound with low toxic in nature which is obtained from deacetylation of chitin and most application of the chitosan in agriculture is used for the stimulation of plant defense mechanisms (El-Hardrami et al., 2009).Chitosan seems to act as a stress tolerance inductor it enhanced a hyper sensible reaction and lignification, inducing lipid peroxidation, and production of defense against pathogens when directly applied to plant tissue (Ortiz et al., 2007). Seeds treated with chitosan reduced the mean germination time; increased germination index leads to improving seedling growth under low temperature stress and also reported that the application of chitosan reduced the vanadium toxicity when applied to wheat and barley in irradiated form (Tham et al., 2001). During drought stress foliar application of chitosan helps to reduce the loss of water from the leaves by including stomatal type closing compounds, which are able to decrease water loss from the leaf by improving plant biomass or yield of crop (Bittelli et al., 2001). Foliar application of chitosan helps to reduce the water stress effect on yield which may be due to increase in stomatal conductance under water stress and its role in reducing transpiration rate (Khan et al., 2002). Although not known the exact mechanisms by which chitosan stimulates growth and development of plants, it has been proposed that is involved in physiological processes, it prevents water loss via transpiration (Young et al., 2005). In this regard, the presence of stomatal closure has been demonstrated when sprinkled plants with chitosan, suggesting that the stimulatory effect of growth, after stomatal closure could be related to an antiperspirant effect on the ground (Bittelli et al., 2001), stating, moreover, foliar application of chitosan in potato reduced the effects of water stress (Jiao et al., 2012).
Keeping in view the importance of chitosan application, this study was designed to find out the effect of chitosan on plant growth and development attributes under water stress condition.
Materials and Methods
Experimental site
An experiment was conducted on “efficacy of chitosan on performance of tomato plant under water stress condition.” at Horticulture Nursery, Department of Horticulture, The University of Agriculture Peshawar, Pakistan in 2018.
Experimental design
Complete Randomized Design (CRD) with two factors was used during experimentation. Each treatment was repeated three times.
Nursery raising transplantation
The seeds were sown in nursery trays in the last week of January; a slight but frequent irrigation was applied to seedlings with the help of hand sprayer. The seedlings trays were placed in a greenhouse. The seedlings were transplanted when they reach to 5- 6 leaves stage and transplanted early in morning, and the seedlings were transplanted into plastic tubes and the tubes were transferred to a lathe house.
| Factor A: Water stress interval(days) |
Factor B:Chitosan level(mgL-1) |
|
S1 = 3 |
C1 = Control |
|
S2=6 |
C2 = 50 |
|
S3 =9 |
C3 = 100 |
|
S4 = 12 |
C4= 150 |
Field plastic tubes preparation
The Plastic tubes were prepared one week before transplantation of the crop. All the stone, stub, root or any other material which may result in barrier to the crop were removed. Plants were subjected to four water-stress treatments: WS0= 3 days (100% field capacity (FC), WS1= 6 Days water stress intervals (70% water capacity), WS2= 9 Days water stress intervals (50% WC) and WS3= 12 Days water stress intervals (30% WC).
Preparation of chitosan solution
Chitosan shows less solubility in water. Chitosan in required amount were dissolved in 100ml of double distill water with the addition of some drop of acid such as acetic acid to obtained four different concentrations of chitosan (0, 50, 100 and 150 mg. L-1), its volume was raised to 1000 ml. Different concentrations of chitosan were applied manually at different stages after water stress interval. The foliar spray was carried out with manual pump spray.
Parameters
Following attributes were analyzed during the experimental study.
Plant height (cm)
Plant height was calculated from bottom of plant to tip by using measuring tap when the plants reach its maximum growth stage and average was taken.
Number of leaves plant-1
The average numbers of leaves plant-1 were counted from five randomly selected plants of each treatment and their average was calculated.
Leaf area (cm2)
Five leaves were randomly selected per treatment and their area was calculated through leaf area meter and then the average was taken.
Chlorophyll content (SPAD)
It was measured through spade meter and five plants were randomly selected per treatment and then average was taken.
Relative water content (%)
Fresh leaves were gathered from every treatment and fresh weight (FW) of five leaves were taken and then set in refined water for 24 hours and then weighed again to get turgid weight (TW). From that point, the leaf section were dried for 48 hours at 72oC and then weighed again to get dried weight (DW). Relative water content (RWC) was determined utilizing accompanying equation (Egert and Tebini, 2002).
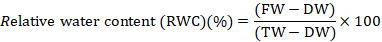
Excised leaf water retention (%)
The newly fresh leaves were collected for determining after harvest and left for 20 to 25oC at room temperature for 4hrs and reweighed for wilt weight after four hours (WW4hr). Excised leaf water retention (ELWR) was determined by using the following equation (Farshadfar et al., 2002).
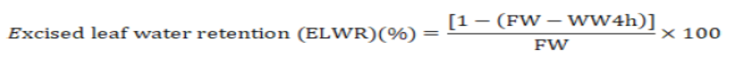
Number of fruits plant-1
The fruits produced were counted plant-1 from five randomly taken plants and their average was taken.
Fruit weight (gm)
For finding average fruit weight randomly selected fruit from each treatment was taken and then weighed by using digital balance and then their average was measured.
Yield (ton. ha-1)
For determining yield per hectare was recorded by using the following formula.
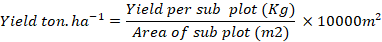
Statistical procedure
The collected data was subjected to analysis of variance (ANOVA) for various variables and analyzed by using Statistic 8.1 (Statistical software, Statistix, Analytical Software Inc, Tallahassee FL USA) used by Basit et al. (2018). LSD test at 1% level of significance was used for means comparison (Jan et al., 2009).
Results and Discussion
Plant height
It is obvious from Table 1 that different water stress intervals, foliar application of chitosan and their interaction had significantly affected plant height of tomato. Maximum plant height (82.69 cm) was noted in plants with 6 days water stress interval which was statistically similar with plant height (81.18 cm) of plants treated with 3 days water stress interval, while minimum plant height (65.93 cm) was recorded in plants treated with 12 days water stress interval. Regarding chitosan, maximum plant height (80.74 cm) was recorded in plants treated with 100 mg L-1 foliar application of chitosan, while minimum plant height (73.13 cm) was observed in control. The interaction of chitosan and water stress indicated that maximum plant height (87.60 cm) was observed in plants treated with 100 mg L-1 along with 6 days water stress interval while minimum plant height (61.30 cm) was noticed in plants treated with control treatment of chitosan foliar spray along with 12 days water stress interval (Figure 1).
Drought stress cause a major problem in reducing growth and quality attributes of soybean and white lupines (Abdalla, 2011; Hefny, 2011). Water stress reduce plant growth parameters which might be due to reduction in cell division, cell volume and cell elongation which have negative effect on plant growth (Banon et al., 2006). Among different water stress interval tomato have maximum plant height with 6 days interval. Our findings are in line with Shaimaa et al. (2018), they reported that drought stress has significantly decreased vegetative growth of sour orange specially plant height and stem diameter. It was found that foliar applications with chitosan resulted in higher vegetative growth and improvement in fruit quality of pepper, radish and cucumber (Farouk et al., 2008; Ghoname et al., 2010). Foliar application of chitosan at 100 mg L-1 had positive effect on growth attributes of tomato plant under normal stressed condition. The result of our experiment is in accordance with Guan et al. (2009) who investigated that chitosan had significant effect on plant growth which might be due increase in nutrients availability to the plants as well as uptake of essential nutrients by reducing harmful effect of free radicals (ORS) and by increasing antioxidants and enzyme activities. It is well known that water stress conditions cause a multitude of molecular, biochemical and physiological changes, thereby affecting plant growth and development (Boutraa, 2010). A decline in plant growth in response to water stress might be due either to decreases in cell elongation resulting from the inhibiting effect of water shortage on growth-promoting hormones which, in turn, lead to decreases in cell turgor, volume and eventually growth (Banon et al., 2006). The results of our experiments are also in agreement with Farouk and Ramadan (2012) who investigated that chitosan improved both growth and quality of cow pea such as, plant height, number of leaves and leaf area under water stress condition.
Table 1: Plant height (cm), No. of leaves plant-1, leaf area (cm2), Chlorophyll content (mgcm-2) and relative water content (%) of tomato plant as affected by chitosan concentration and Chlorophyll content water stress interval.
| Chitosan levels | Plant height (cm) |
No. of leaves plant-1 |
Leaf area (cm2) |
Chlorophyll content (SPAD) |
Relative water (mgL-1) content (%) |
| 0 | 71.13C | 88.00D | 68.64D | 54.77D | 49.26D |
| 50 | 75.40B | 92.91C | 72.08C | 58.51C | 55.90C |
| 100 | 80.74A | 104.19A | 81.05A | 72.29A | 66.82A |
| 150 | 76.24B | 100.03B | 77.27B | 65.10B | 59.47B |
| LSD≤0.01 | 1.62 | 3.00 | 1.99 | 3.52 | 3.26 |
| Water stress interval (days) | |||||
| 3 | 81.18A | 101.87A | 77.66B | 67.83 | 65.49A |
| 6 | 82.69A | 104.02A | 81.47A | 71.31A | 67.27A |
| 9 | 73.71B | 96.45B | 72.55C | 60.23B | 57.18B |
| 12 | 65.93C | 82.79C | 67.36D | 51.30C | 41.50C |
| LSD≤0.01 | 1.62 | 3.00 | 1.99 | 3.52 | 3.26 |
| Interaction | |||||
| Level of significance |
Fig. 1 |
NS | NS | NS |
Fig. 2 |
Means followed by dissimilar letters differs at 1% level of significance.
Number of leaves plant-1
The statistical analysis of data revealed that water stress interval and chitosan concentration show significant effect on number of leaves plant-1, while their interaction was found non-significant (Table 1). Maximum values for number of leaves plant-1 (104.02) was seen in plants under 6 days water stress interval statistically apart with number of leaves plant-1 (101.87) was recorded in plants have 3 days water stress interval, while minimum number of leaves plant-1 (82.79) was observed in plants under 12 days water stress interval. Regarding chitosan maximum values for number of leaves plant-1 (104.19) was observed in those plants which were treated with 100mg L-1 chitosan, while minimum values for number of leaves plant-1 (88.00) was found in control treatment.
Drought stress have negative effect in reducing growth attributes that might be attributed due to drop in leaf relative water content which inhibit leaf turgidity and osmosis as well as reduce the rate of cell division and cell elongation (Reddy et al., 2003). Among various water stresses, maximum number of leaves was found at 6 days water stress interval. Our results are in conformity with the findings of Shaimaa et al. (2018), they reported that among different irrigation interval, maximum number of leaves plant-1 was recorded under 7 days irrigation interval. Foliar application of chitosan at the rate of 100 mg L-1 improved early developmental stages by increasing number of leaves plant-1 in tomato plant. These finding are also in line with Jian et al. (2002) who investigated that application of chitosan increased plant height, number of branches, and also number of leaves plant-1 in rice.
Leaf area (cm2)
Different water stress intervals and foliar application of chitosan significantly affected leaf area (cm2), while their interaction was found non-significant on leaf area of tomato plant (Table 1). Maximum values for leaf area (81.47 cm2) were observed in plants treated with 6 days water stress interval while least leaf area (67.36 cm2) was observed in plants with 12 days water stress interval.Data regarding chitosan maximum leaf area (81.05 cm2) was recorded in plants with 100 mg L-1, whereas minimum leaf area (68.64 cm2) was observed in untreated plant.
Drought stress had significant effect on leaf area which resulted due to inhibition of cell division, cell elongation and also reduction of photosynthetic rate. The inhibiting effects of water stress on plant growth have previously been reported for soybean (Abdalla, 2011) and white lupins (Hefny, 2011). It is well known that water stress conditions cause a multitude of molecular, biochemical and physiological changes, thereby affecting plant growth and development (Boutraa, 2010). Banon et al. (2006) investigated that plant retarded its growth and vegetative parameter due to declining of cell elongation which in turn leads to decrease in turgidity of cell, volume and ultimately effect on plant photosynthesis as well blockage of xylem and phloem vessels, which inhibit translocation process. Drought stress cause significant decrease in plant photosynthetic productivity, which finally decrease CO2 diffusion into leaf and thus limits reduction leaf area (Lawlor and Cornic, 2002). During vegetative growth of tomato, there could be severe decrease in leaf area due to increase in water stress. Our finding are in agreement with Sarmadnia (1993) who noted that water stress reduced all vegetative growths specially plant height, total leaf area and had a negative effect on number of seeds per spike and spike length. According to our finding maximum leaf area of tomato crop were recorded when they are treated with chitosan concentration at 100 mg L-1. The findings of current experiment are in agreement with Sheikh and Al-Malik (2011) who investigated that chitosan at different concentration significantly enhanced bean plant height, leaf area and enhances other growth parameters. The results of our experiment are also in conformity with Mondal et al. (2013) on Mung bean; Chookhongkha et al. (2012) on chili; Mondal et al. (2012) on okra; Shehata et al. (2012) on cucumber; Abdel-Mawgoud et al. (2010) on strawberry and El-Tantawy (2009) on tomato.
Chlorophyll content (SPAD)
The statistical analysis of data revealed that chlorophyll content was significantly affected by different water stress intervals and chitosan concentration, while their interaction was non-significant. Maximum chlorophyll content (71.31 SPAD) was noticed in plants with 6 days water stress interval statistically similar with chlorophyll content (67.83 SPAD), while minimum chlorophyll content (51.30 SPAD) was recorded in plants treated with 12 days water stress interval.Regarding chitosan maximum chlorophyll content (72.29 SPAD) was noted at rate of 100 mg L-1, while minimum chlorophyll content (54.77 SPAD) was recorded in control.
The total chlorophyll content in tomato leaves significantly decrease under severs water stress condition. The decreasing rate of chlorophyll content under water stress might be due to reduced synthesis of main chlorophyll content pigments in tomato leaves. Nikolaeva et al. (2010); Kumar et al. (2011) reported that the reduction in chlorophyll content under drought condition is a common phenomenon. Results of the current research are in agreement with findings of Ahmad et al. (2016) on citrus; Karimi (2016) and Gupta et al. (2012) on wheat and Farouk and Ramadan (2012) on cowpea, who reported that chlorophyll content decreased due to higher water deficit condition. Foliar application of chitosan reduced the impact of water stress on photosynthetic rate which have the ability to enhance the endogenous cytokinins, which play a key role in improving chlorophyll content in leaves. Chitosan with different concentration increase chlorophyll content in tomato leaves. Our findings are conformity with Farouk et al. (2008) who reported that different concentration of chitosan enhanced chlorophyll content and also increased starch content in radish and cucumber plant. Our results are also in agreement with the findings of Chibu et al. (2002) observed that plants treated with chitosan had beneficial effect on chlorophyll content in the plants.
Relative water content (%)
It is obvious from Table 2 that various treatments of water stress, chitosan concentration and their interaction had significant effect on relative water content of tomato. Maximum relative water content (RWC) (67.27%) in tomato leaves was shown by plants with 6 days water stress interval statistically apart with RWC (65.49%) was given by plants with 3 days water stress interval, while minimum RWC (41.50%) was observed in tomato leaves plants treated with 12 days water stress interval. Regarding chitosan maximum relative water content RWC (66.82%) was observed in tomato leaves plants treated with 100 mg L-1 foliar application of chitosan, while the minimum RWC (49.26%) was noted in control. In case of interaction maximum relative water content RWC (73.97%) was noted in tomato leaves plants treated with 6 days water stress interval combined with 100 mg L-1 foliar application of chitosan while minimum RWC (29.28%) was observed in tomato leaves plants treated with 12 days water stress interval along with control treatment of chitosan (Figure 2).
Relative water content is an essential component of water status under drought condition (Carter and Paterson, 1985; Sinclair and Ludlow, 1985). Soil water potential and leaf water potential contributes basically to change in stem diameter, while the former is all around associated with changes under delayed drought stress (Katerji et al., 1994). RWC is directly connected with the cell volume, might be due to its relation with transpiration and water supply to the plants (Schonfeld et al., 1988). RWC has the ability to protect plant growth, and yield attributes from drought stress (Lilley and Ludlow, 1996; Liley and Fukai, 1994). According to our findings, tomato plant had more RWC at 6 days water stress interval. Similar results were reported by Schonfeld et al. (1988), they found that wheat have minimum RWC due to drought stress and maximum genotypic tolerance. Foliar application of chitosan might have the ability to expand cell layer and to improve antioxidant actives of tomato plant and keep up relative water content (RWC) in tomato leaves under drought condition. The results are similar with the findings of Bittelli et al. (2001) on pepper plants, who reported that plants treated with foliar application of chitosan enhanced the main transpiration rate by decreasing extra water usage up to 26- 43% and also keeping up growth and yield under water stress condition.
Table 2: Excised leaf water content (%), No. of fruits plant-1, fruit weight (g) and Yield (t.ha-1) of tomato plant as affected by chitosan concentration and water stress interval.
|
Chitosan levels (mgL-1) |
Excised leaf water content (%) |
No. of fruits plant-1 |
Fruit weight (g) |
Yield (t.ha-1) |
| 0 | 63.15B | 16.50D | 62.75C | 27.80D |
| 50 | 64.85AB | 19.66C | 67.99B | 30.89C |
| 100 | 66.58A | 24.33A | 74.74A | 37.37A |
| 150 | 63.92B | 22.16B | 68.93 | 34.24B |
| LSD≤0.01 | 1.88 | 1.47 | 2.40 | 1.09 |
| Water stress interval (days) | ||||
| 3 | 66.87A | 22.41B | 72.94A | 36.57A |
| 6 | 68.24A | 24.66A | 73.71A | 37.50A |
| 9 | 62.71B | 20.25C | 67.80B | 31.31B |
| 12 | 60.67 | 15.33D | 59.95C | 24.92C |
| LSD≤0.01 | 1.88 | 1.47 | 2.40 | 1.09 |
| Interaction | ||||
| Level of significance | NS | NS | NS | |
Means followed by dissimilar letters differs at 1% level of significance
Excised leaf water retention (%)
Mean (Table 2) reveled that different water stress interval and foliar concentration of chitosan had a significant effect on leaf water retention, while their interaction had no significant effect on excised water leaf retention (ELWR) of tomato plant. Maximum excised leaf water retention ELWR (68.24%) in tomato leaves was recorded by plants with 6 days water stress interval which was statistically similar with ELWR (66.87%), while minimum value of ELWR (60.67%) was observed in tomato leaves plants treated with 12 days water stress interval. Regarding chitosan maximum value of excised leaf water retention ELWR (66.58%) was observed in tomato leaves plants treated with 100 mg L-1 foliar application of chitosan, while the minimum value of ELWR (63.15%) was noted in control treatment.
Drought have main effect on excised leaf water retention (ELWR) and extract leaf water loss (ELWL) which are been accounted for as principle marker of water pressure in leaves (Merah, 2001). Water stress is characterized by a decline in cell water status, turgor and aggregate water capability of plant bringing about stomatal closure, wilting, and decrease in cell growth and development. Extreme water stress might bring about cessation of photosynthesis, aggravation of metabolism, loss of turgidity and lastly cell death (Bohnert and Jensen, 1996). It is well known, 80% of plant tissue component is water, and thus water loss may have evident adverse effects and can be considered as the most important limiting factors for growth and development of plants. Zabihi et al. (2014) mentioned that during drought stress water potential surrounding the plant is lower than natural status and plant will encounter difficultly in water absorption. They added that as a result of insufficient water supply, plant growth is inhibited by the decrease in leaf extension level, rates of cell division and enlargement. Also, the uptake of essential elements and photosynthetic capacity under water deficiency are reduced that reflected indirectly the formation of vegetative shoots, leaves and fresh or dry weights accumulation Farouk and Ramadan (2012), Nohong and Nompo (2015) and Ahmad et al. (2016). Moreover, water stress have a negative effects on leaf RWC, where leaf RWC reflecting the metabolic activity in tissues that declined markedly due to water deficiency. Leaf RWC reduction could have been due to unavailability of water in the soil, root system that are not able to compensate for water lost by transpiration through a reduction of absorbing surface Bolat et al. (2014). Roitsch and Ehneß (2000) demonstrated that cytokinins play a remarkable part in the regulation of source sink translocation. Under stressed conditions cytokinins progress cell division and elongation so that younger leaves could carry on as sink. Diminished cytokinins production by the roots is associated with drought stress, which can possibly hasten senescence in the older leaves by hindering its generation (Xu et al., 2016).
Drought stress significantly decrease ELWR capacity in tomato leaves. Our outcomes are correlated with Sinclair and Ludlow (1985) who reported that drought stress decrease ELWR and RWC in leaves. They further reported that different stress tolerant varieties might be able to give good results by avoiding drought through minimum water loss and retaining more water and, therefore, maintaining water status in leaves. The high RWC, an optimum ELWR and ELWL have been recommended as significant indicators of water status and also depends on crop variety to resist drought stress (Gunes et al., 2007; El-Tayeb, 2006). Foliar application of chitosan helps to retain more water in tomato crop, thus it helps to maintain water content in tomato leaves and reduce more loss of water from tomato leaves.
Number of Fruit plant-1
Mean (Table 2) revealed that that various water stress treatment and foliar application of chitosan was significantly affected fruit plant-1, while their interaction had nonsignificant effect on number of fruit plant-1. Maximum fruit plant-1 (24.66) was recorded in plants treated with 6 days water stress interval, while minimum fruit plant-1 (15.33) was observed in plants treated with 12 days water stress interval. Regarding chitosan, maximum fruit per plant (24.33) was noted in plants treated with 100 mg L-1 chitosan foliar spray, while minimum fruit plant-1 (16.50) was recorded in control treatment.
Water stress cause a major problem in vegetative growth under stress condition, particularly during shoot development, decrease cell division process, and reduce number of cell per unit (El-Adawy et al., 2003). According to findings of Ghoname et al. (2010) that chitosan have positive effect on growth and qualitative attributes of sweet pepper. Chitosan could enhance photosynthetic pigment and biochemical activities in plants that were directly related to fruits (sink) which helps to increase maximum number of fruits plant-1 (El-Tantawy, 2009). Among different concentration chitosan can enhance number of plant-1 at the rate of 100 mg L-1in tomato crop. The results of our research are in line with the findings of Mondal et al. (2013) who observed that number of fruit plant-1 in tomato improved with foliar application of chitosan at the rate of 75mg L-1. Our results are also similar with those of Chibu et al. (2002) who investigated that when rice and soybean treated with different concentration of chitosan during early growth stages significantly increased plant growth attributes and ultimately increase seed yield.
Fruit weight (g)
The analysis of variance in Table 2 for average fruit weight (g) showed that water stress treatment and foliar application of chitosan significantly affected average fruit weight, while their interaction was found non-significant. Maximum average fruit weight (73.71 g) was noticed in plants treated with 6 days water stress interval statistically followed by average fruit weight (73.71 g) was observed in plants have 3 days water stress interval, while minimum average fruit weight (59.95 g) was recorded in plants treated with 12 days water stress interval. The treatment with application of 100 mg L-1 chitosan registered more average fruit weight (74.74 g) as compared to average fruit weight (62.75 g) of untreated plants.
Water stress is mostly harmful to plants during growth and development by disturbing the electrolyte balance which definitely caused in reduction of some essential nutrients which are required during proper fruit development. Fruit weight significantly decreased due to increase in water stress interval. In some studies peach fruit size and weight were reduced by water stress (Crisosto et al., 1994; Li et al., 1989). Our result are in line with findings of Adballa (2011) they reported that water stress have negative effect on plant growth specially in soybean, groundnuts and white lupins. Foliar application of chitosan can increase growth parameter of tomato with optimum concentration at 100mg L-1. The results of our research is in accordance with Mukta et al. (2017), they reported that when strawberry plant treated with foliar spray of chitosan it resulted in increased fruit weight.
Yield (tons ha-1)
It is quite clear from the Table 2 that water stress interval, foliar spray of chitosan and their interaction significantly affected yield (ton ha-1) of tomato plant. Maximum tomato yield (37.50 ton ha-1) was given by plants with 6 days water stress interval which was statistically similar with yield (36.57 ton hac-1) recorded in plants with 3 days water stress interval, while minimum yield (24.92 ton ha-1) was noted in plants treated with 12 days water stress interval. Regarding chitosan, maximum value of tomato yield (37.37 ton ha-1) was noticed in plants sprayed with 100 mg L-1 chitosan followed by yield (34.24 ton ha-1) observed in plant treated with 150 mg L-1 chitosan, while minimum tomato yield (27.80 ton ha-1) was perceived in control. In case of interaction maximum tomato yield (43.51 tons ha-1) was shown by 6 days water stress interval along with 100 mg L-1 chitosan foliar spray, while lowest yield (20.06 ton ha-1) was given by 12 days water stress interval with control treatment of chitosan (Figure 3).
Water is a main limiting factor which reduces the production of horticulture crops and most harvest plants are damaged due to severe water stress. The regular and control water supply throughout the growing season resulted in high yield and good quality of fruit. During growing stages increasing the rate of water supply resulted in higher yield (Sander et al., 1989; Vittum et al., 1962). Our results are in accordance with Costa et al. (2008) who reported that yield and yield components of crops were badly affected by water stress. According to findings of Liu et al. (2003) who suggested that water stress resulted in reduction of yield because of increase in rate of flowers drop, which ultimately resulted in reduction of yield. Our outcomes are correlated with Bittelli et al. (2001) who studied that pepper plants when treated with foliar application of chitosan decreased the gaseous exchange and transpiration from the leaves, decreased the more demand of water use and providing water to biomass and yield during low water availability to the plant. The reduced yield may be due to the negative effect of water stress on the number of branches and leaves per plant as well as leaf area (Table 1), resulting in a reduction in the supply of carbon assimilate and photosynthetic rate by plants and consequently less biomass produced as well as decreased translocation of assimilates towards the developing fruits (Kumar et al., 1994). In addition, yield may be reduced under drought conditions due to increasing the rate of flower abscission and pod abortion (Liu et al., 2003). A decreased rate of carbohydrate flux from leaves to reproductive structures has been reported to control pod set in well-watered plants (Kokubun et al., 2001; Setter et al., 2001). On the other side, the increase in Phaseolus yield due to chitosan application may be due to its effects in stimulating physiological processes, improving vegetative growth, followed by active translocation of photo assimilates from source to sink tissues. The increases in plant biomass may be due to improving photosynthetic machinery (Khan et al., 2002). The role of chitosan in alleviating the harmful effect of water stress on yield may be due to an increase in stomatal conductance and net photosynthetic CO2-fixation activity under water stress (Khan et al., 2002), and to its role in reducing transpiration to save water. Current findings are in accordance with the results of Mondal et al. (2013) who reported that pod number and yield in mung bean increased by the application of chitosan. Current results are also supported by the results of Ghoname et al. (2010), Chibu and Shibayam (1999) they investigated that dry weights of land rice and sweet pepper increased by the application of 0.1 and 0.5% foliar application of chitosan.
Conclusions and Recommendations
It is observed from findings of research study that tomato plants treated with foliar application of chitosan at the rate of 100 mg L-1 resulted in maximum average plant height (cm), average number of leaves, leaf area (cm2), chlorophyll content (SPAD), relative water content (%), excised leaf water retention (%), average fruit weight (g), number of fruit plant-1, yield (tons. ha-1). Water stress treatment with 6 days interval combined with foliar spray of chitosan at the rate of 100 mg L-1 are recommended for acquiring maximum growth and yield of tomato under water stress condition.
Author’s Contribution
Hassnain, I. Ahmad: Concieved and designed the study
I. Ahmad, Hassnain, A. Basit: Performed the experiment.
Hassnain, A. Basit, I. Ullah: Analyzed the data
A. Basit, Hassnain, I. Ullah, M.A. Khalid, M.. Shair, B.M.: Contributed the chemical/ materials/ analysis tools.
A. Basit, I. Ullah, N. Alam: Wrote the paper.
References
Abdalla, M.M. 2011. Beneficial effect of diatomite on growth, the biochemical contents and polymorphic DNA in lupines albus plants grown under water stress condition, Agric. Bio. J. North Am. 2: 207-220. https://doi.org/10.5251/abjna.2011.2.2.207.220
Abd-El-HG and A.M. Bankok. 2015. Response of tomato plants to salicylic acid and chitosan under infection with tomato mosaic virus. American Eurasian J. Agric. Engviron. Sci. 15(8):1520-1529.
Abdel-Mawgoud, A.M.R., A.S. Tantawy and M.A. El-Nemr. 2010. Growth and yield responses of strawberry plants to chitosan application. Eur. J. Sci. Res. 39(1): 170-177.
Agriculture, O.A.C. and K. Helrich. 1990. Official methods of analysis of AOAC: Food.
Ahmad, A. O, M. Dutt, F. G. Gmitter and J. W. Grosser. 2016. Somatic Embryogenesis: Still a Relevant Technique in Citrus Improvement. In: Germana M., Lambardi M. (eds) In Vitro Embryogenesis in Higher Plants. Methods in Molecular Biology, vol 1359. Humana Press, New York, NY
Ali, A., M.T.M. Muhammad, K. Sijam and Y. Sidiqui. 2011. Effect of chitosan coating on the physio-chemical characteristics of Eksotica II papaya (Carica Papaya L.) fruit during cold storage. Food Chem. 124(2): 620-626. https://doi.org/10.1016/j.foodchem.2010.06.085
Allan, C.R. and L.A. Hadwiger. 1979. The fungicidal effect of chitosan on fungi of varying cell wall composition. Exp. Mycol. 3: 285-287. https://doi.org/10.1016/S0147-5975(79)80054-7
Banon, S.J., J. Ochoa, J.A. Franco, J.J. Alarcon and M.J. Sanchez-Blanco. 2006. Hardening of colander seedlings by deficit irrigation and low air humidity. Environ. Exp. Bot. 56: 36-43. https://doi.org/10.1016/j.envexpbot.2004.12.004
Basit, A., K. Shah, M.U. Rahman, L. Xing, X. Zuo, M. Han, N. Alam, F. Khan, I. Ahmed and M.A. Khalid. 2018. Salicylic acid an emerging growth and flower inducing hormone in marigold (Tagetes sp. L.). Pure Appl. Biol. 7(4): 1301-1308. https://doi.org/10.19045/bspab.2018.700151
Bittelli, M., M. Flury, G.S. Campbell and E.J. Nichols. 2001. Reduction of transpiration through foliar application of chitosan. Agric. For. Meteorol. 107(3):167-175. https://doi.org/10.1016/S0168-1923(00)00242-2
Bittelli, M., M. Flury, G.S. Campbell and E.J. Nichols. 2001. Reduction of transpiration through foliar application of chitosan. Agric. For. Meteorol. 107(32): 167-175. https://doi.org/10.1016/S0168-1923(00)00242-2
Bohnert, H.J. and R.G. Jensen. 1996. Strategies for engineering water stress tolerance in plants. Trends Biotechnol. 14(3): 89–97. https://doi.org/10.1016/0167-7799(96)80929-2
Bolat, I., M. Dikilitas, S. Ercisli, A. Ikinci and T. Tonkaz. 2014. The effect of water stress on some morphological, physiological, and biochemical characteristics and bud success on apple and quince rootstocks. Sci. World J. 8. https://doi.org/10.1155/2014/769732
Boutraa, T. 2010. Improvement of water use efficiency in irrigated agriculture: a review. J. Agron. 9: 1–8. https://doi.org/10.3923/ja.2010.1.8
Carter, J. E. Jr. and R. P. Paterson. 1985. Use of Relative Water Content as a Selection Tool for Drought Tolerance in Soybeans. Argon J. Abstr ASA Madison. 21:77.
Castel, J.R. and A. Buj. 1990. Response of salustiana oranges to high frequency deficit irrigation. Irrig. Sci. Agric. Sci. 11: 121-127. https://doi.org/10.1007/BF00188448
Chang, Z., G. Qin, X. Xu, B. Li and S. Tian. 2007. Proteome approach characterized protein induced by antagonistic yeast and salicylic acid in peach fruit. J. Proteome Res. 6: 1677-1688. https://doi.org/10.1021/pr060483r
Chibu, H. and H. Shibayama. 1999. Effects of chitosan applications on the early growth of several crops. Rep. Japan Kyushu Branch Crop Sci. Soc. 65: 83-87.
Chibu, H., H. Shibayama and S. Arima. 2002. Effects of chitosan application on the shoot growth of rice and soybean. Jape. J. Crop Sci.71: 206-211. https://doi.org/10.1626/jcs.71.206
Chirkov, S.N, N. Surguchova and J.G. Atabekov. 1994. Chitosan inhibits systemic infections caused by DNA containing plant viruses. Arch. Phytopathol. Plant Prot. 29: 21-24. https://doi.org/10.1080/03235409409383089
Choi, Y.S., Y.M. Kim, O.J. Hwang, Y.J. Han, S.Y. Kim and J.I. Kim. 2013. Overexpression of Arabidopsis (ABF3) gene confers enhanced tolerance to drought and heat stress in creeping bentgrass. Plant Biotechnol. 7: 165-173. https://doi.org/10.1007/s11816-012-0245-0
Colla, G., Y. Rouphael, M. Cardarelli, D. Massa, A. Salerno and E. Rea. 2006. Yield, fruit quality and mineral composition of grafted melon plants grown under saline conditions. J. Hort. Sci. Biotechnol. 81(1): 146-152. https://doi.org/10.1080/14620316.2006.11512041
Composition; Additives; natural contaminants, vol. II. AOAC, Arlington.
Costa, C.L., A.K.S. Lobato, N. Oliveir, C.F. Maia and G. Alves. 2008. Biochemical and physiological responses in two Vigna unguiculata (L.) Walp. Cultivars under water stress. J. Agron. 7: 98-101. https://doi.org/10.3923/ja.2008.98.101
Crisosto, C.H., R.S. Johnson, J.G. Luza and G.M. Crisosto. 1994. Irrigation regimes affect fruit soluble solids concentration and rate of water loss of ‘O’Henry’ peaches. Hort. Sci. 29: 1169-1171. https://doi.org/10.21273/HORTSCI.29.10.1169
Dang, H.M., M. Serrano and D. Valero. 2012. Alginate coating preserve fruit quality and bioactive compounds during storage of sweet cherry fruit. Food Bioprocess Tech. 5: 2990-2997. https://doi.org/10.1007/s11947-011-0599-2
Dong, H., L. Cheng, J. Tan, K. Zhen and Y. Jiang. 2004. Effect of chitosan coating on quality and shelf life of peeled litchi fruit. J. Food Eng. 64: 355-358. https://doi.org/10.1016/j.jfoodeng.2003.11.003
Drevinskas, T., G. Naujokaityte, A. Maruška, M. Kaya, I. Sargin, R.C. Daubaras and L. Esoniene. 2017. Effect of molecular weight of chitosan on the shelf life and other quality parameters of three different cultivars of kiwi fruit (Actinidia kolomikta L.). Carbohydr. Polym. 173: 269-275. https://doi.org/10.1016/j.carbpol.2017.06.002
Dzung, N.A., V.T.P. Khanh and T.T. Dzung. 2011. Research on impact of chitosan oligomers on biophysical characteristics, growth, development and drought resistance of coffee. Carbo. Poly. 84(2): 751-755. https://doi.org/10.1016/j.carbpol.2010.07.066
Egert, M. and T. Tevini. 2002. Influence of drought on some physiological parameters symptomatic for oxidative stress in leaves of chives (Allium schoenoprasum L.). 48: 43-49. https://doi.org/10.1016/S0098-8472(02)00008-4
El-Adawy, T. A., E. H. Rahma, A. A. El-Bedawey and A. E. El-Beltagy. 2003. Nutritional potential and functional properties of germinated mung bean, pea and lentil seeds. Plant Foods Hum Nutr. 58(3): 1-13. https://doi.org/10.1023/B:QUAL.0000040339.48521.75
El-Belgqaty, A.S., M.T. EL-Said, M.H. Sawsan, M.G. Hasniya and A.M. Abo-El. 1984. Effect of different water regimes on tomato. An. Agric. Sci. Ain. Sh. Univ. 29: 1937-1956.
El-Miniawy, S.M. Ragab, S. Youssef and A. Metwally. 2013. Response of strawberry plants to foliar spraying of chitosan. Res. J. Agric. Biol. Sci. 9(6): 366-372.
El-Tanahy, R.M., Asmaa, M. Mona, M. Abde and H.A. Aisha. 2012. Effect of Chitosan Doses and Nitrogen Sources on the growth, yield and seed quality of cowpea. Aus. J. Basic. Appl. Sci. 6(4): 115-121.
El-Tantawy, E.M. 2009. Behavior of tomato plants as affected by spraying with chitosan and amino-fort as natural stimulator substances under application of soil organic amendments. Pak. J. Biol. sci. 12(17): 1164-1173. https://doi.org/10.3923/pjbs.2009.1164.1173
El-Tayeb, M.A. 2006. Differential response of two Vicia faba L. cultivars to drought: Growth, pigments, lipid, peroxidation, organic solutes, catalase, and peroxidase activity. Acta Agron. Hungaricavol. 54: 25–37. https://doi.org/10.1556/AAgr.54.2006.1.3
Faoro, F. 2018. Induced systemic resistance against systemic viruses (accessed on 8 April 2018).
Farouk, S. and A.R. Amany. 2012. Improving growth and yield of cowpea by foliar application of chitosan under water stress. Egypt. J. Biol. 14: 14-26. https://doi.org/10.4314/ejb.v14i1.2
Farouk, S., K.M. Ghoneem and A.A. Abeer. 2008. Induction and expression of systematic resistance to downy mildew disease in cucumber plant by elicitors. Egypt. J. Phyto-patholo. 1-2: 95-111.
Farshadfar, E., A. Afarinesh and J. Sutka. 2002. Inheritance of drought tolerance in maize crop. CRC.30: 3-4.
Gayed, A.A.N.A., S.A.M.A. Shaarawi, M.A. Elkhishen and N.R.A. Elsherbini. 2017. Pre-harvest application of calcium chloride and chitosan on fruit quality and storability of ‘Early Swelling’ peach during cold storage. Ciência Agrotecnol. 41: 220–231. https://doi.org/10.1590/1413-70542017412005917
Ghoname, A.A., M.A. EL-Nemr, A.M.R. Abdel-Mawgoud and W.A. El-Tohamy. 2010. Enhancement of sweet pepper crop growth and production by application of biological, organic and nutritional solutions. Res. J. Agric. Biol. Sci.. 6(7): 349-355.
Goell, A. and Y. Levy. 1970. The effect of irrigation stress on the fruit quality. Proc. XVIII. Intr. Hort. Cong. 1: 221-222.
Gol, N.B. and T.V.R. Rao. 2011. Effect of chitosan coating on physio- chemical propeeties and shelf life extension of tomato. Int. J. Fruit Sci. 11(2): 119-135. https://doi.org/10.1080/15538362.2011.578512
Goy, R.C., D.D. Britto and O.B. Assis. 2009. A review of the antimicrobial activity of chitosan. Polimers. 19. https://doi.org/10.1590/S0104-14282009000300013
Grill, E. and H. Ziegler. 1998. A plant’s dilemma. Sci. 282(5387): 252- 253. https://doi.org/10.1126/science.282.5387.252
Guan, Y., H. Jin, W. Xian-Ju and S. Chen-xia. 2009. Seed priming with chitosan improves maize germination and seedling growth in relation to physiological changes under low temperature stress. J. Zhejiang Univ. Sci. 10(6): 427-433. https://doi.org/10.1631/jzus.B0820373
Gupta, O. P., V. Permar, V. Koundal, U. D. Singh and S. Praveen. 2012. MicroRNA regulated defense responses in Triticum aestivum L. during Puccinia graminis f.sp. tritici infection. Mol Biol Rep. 39:817 -824.
Han, C., Y. Zhao, S.W. Leonard and L.G.M. Gorris. 1996. Prolongation of the shelf life of perishable food products using biodegradable films and coatings. Lebensm. Wiss. Technol. 29: 10-17. https://doi.org/10.1006/fstl.1996.0002
Hefny, M.M. 2011. Agronomical and biochemical responses of white lupin (Lupinus albus L.) genotypes to contrasting water regimes and inoculation treatments. J. Am. Sci. 7(3): 187-198.
Ibrahim, E.A. and W.A. Ramadan. 2015. Effect of zinc foliar spray alone and combined with humic acid or/and chitosan on growth, nutrient elements content and yield of dry bean (Phaseolus vulgaris L.) plants sown at different dates. Sci. Hort. 184: 101-105. https://doi.org/10.1016/j.scienta.2014.11.010
Jan, M.T., P. Shah, P.A. Hollington, M.J. Khan and Q. Sohail. 2009. Agriculture research: Design and analysis, a monograph. NWFP Agric. Univ. Pesh. Pak.
Jian, L., Z.M. Cheng, H.B. Gen, Z.M. Jian, Z.W. Chang and S.W. Guo Wei and H.Y. Ting. 2002. The biological effect of Chitosan on rice growth. Acta Agric. Shanghai 18: 31-34.
Jiao, Z., Y. Li, J. Li, X. Xu, H. Li, D. Lu and J. Wang. 2012. Effects of Exogenous Chitosan on Physiological Characteristics of Potato Seedlings under Drought Stress and Rehydration. Potato Res. 55(3-4): 293-301. https://doi.org/10.1007/s11540-012-9223-8
Karimi, J. and S. Mohsenzadeh. 2016. Effects of Silicon Oxide Nanoparticles on Growth and Physiology of Wheat Seedlings. 63(1): 119-123. https://doi.org/10.1134/S1021443716010106
Katerji N., F. Tardieu, O. Bethenod and P. Quetin. 1994. Behavior of maize stem diameter during drying cycles: comparison of two methods for detecting water stress. Crop Sci. 34 (1): 165–169. https://doi.org/10.2135/cropsci1994.0011183X003400010029x
Khan, M.H., K.L.B. Singha and S.K. Panda. 2002. Changes in antioxidant levels in Oryza sativa L. roots subjected to NaCl salinity stress. Acta Physiol. Plant. 24: 145–148. https://doi.org/10.1007/s11738-002-0004-x
Khan, W.M., B. Prithiviraj and D. Smiyh. 2002. Effect of foliar application of chitin oligosaccharides on photosynthesis of maize and soybean. Photosynthetica. 40(4): 621-626. https://doi.org/10.1023/A:1024320606812
Kokubun, M., S. Shimada and M. Takahashi. 2001. Flower abortion caused by pre anthesis water deficit is not attributed to impairment of pollen in soybean. Crop Sci. 41: 1517-1521. https://doi.org/10.2135/cropsci2001.4151517x
Kumar, A., D.P. Singh and P. Singh 1994. Influence of water stress on photosynthesis, transpiration, water use efficiency and yield of Brassica juncea L. Field Crops Res. 37: 95-101. https://doi.org/10.1016/0378-4290(94)90037-X
Lawlor, D.W. and G. Cornic. 2002. Photosynthetic carbon assimilation and associated metabolism in relation to water deficits in higher plants. Plant Cell Environ. 25: 275– 294. https://doi.org/10.1046/j.0016-8025.2001.00814.x
Leung, J. and J. Giraudat. 1998. Abscisic acid and signal transduction. Plant Mol. Biol. 49: 199-222. https://doi.org/10.1146/annurev.arplant.49.1.199
Li, S.H., J.G. Huguet, P.G. Schoch and P. Orlando. 1989. Response of peach tree growth and cropping to soil water deficit at various phonological stages of fruit development. J. Hort. Sci. 64: 541-552. https://doi.org/10.1080/14620316.1989.11515989
Liang, C., F. Yuan, F. Liu, Y. Wang and Y. Gao. 2014. Structure and antimicrobial mechanism of –polylysine chitosan conjugates through Maillard reaction. Int. J. Biol. Macromol. 70: 427-434. https://doi.org/10.1016/j.ijbiomac.2014.07.012
Liu, F., M.N. Andersen and C.R. Jensen. 2003. Loss of pod set caused by drought stress is associated with water status and ABA content of reproductive structures in soybean. Funct. Plant Biol. 30: 271–280. https://doi.org/10.1071/FP02185
Lobato, A.K.S., N.C.F. Oliveira, R.C.L. Costa, F.B.G. Santos, F.J.R. Cruz and H.D. Laughing. 2008. Biochemical and physiological behavior of Walnut (Vigna unguiculataL) under water stress during the vegetative phase. Asian J. Plant Sci. 7(1): 44-49. https://doi.org/10.3923/ajps.2008.44.49
Malekpoor, F., Pirbalouti, A.G. and A. Salimi. 2016. Effect of foliar application of chitosan on morphological and physiological characteristics of basil under reduced irrigation. Res. Crops. 17(2): 354-359. https://doi.org/10.5958/2348-7542.2016.00060.7
Merah, O. 2001. Potential Importance Of Water Status Traits For Durum Wheat Improvement Under Mediterranean Conditions. Journal Of Agricultural Science, Cambridge. 137: 139-145.
Mishra, S., K.S. Jagadeesh, P.U. Krishnaraj and S. Prem. 2014. Biocontrol of tomato leaf curl virus (ToLCV) in tomato with chitosan supplemented formulations of pseudomonas sp. under field conditions. Aust. J. Crop Sci. 8: 347-355.
MNFSR. 2015. Fruit, vegetables and condiments statistics of Pakistan 2014-2015. GoP, Minist. Nat. Food Sec. Res., Islamabad Pakistan.
Mondal, M., M. Malek, A. Putech, M. Ismail, M. Ashrafuzaman and L. Naher. 2012. Effect of foliar application of chitosan on growth and yield in okra. Aust. J. Crop Sci. 6(5): 918.
Mondal, M.M.A., M.A. Malek, A.B., Puteh and M.R. Ismail. 2013. Foliar application of chitosan on growth and yield attributes in mung bean. Bangladesh J. Bot. 41: 179-183. https://doi.org/10.3329/bjb.v42i1.15910
Mukta, J. A., M. Rahman, A. As Sabir, D. R. Gupta, M. Z. Surovy, M. Rahman, and M. T. Islam. 2017. Chitosan and plant probiotics application enhance growth and yield of strawberry. Biocatalysis Agric. Biotech. 11:9-18. https://doi.org/10.1016/j.bcab.2017.05.005
Nikolaeva, M. K., S. N. Maevskaya, A. G. Shugaev and N. G. Bukhov. 2010. Effect of drought on chlorophyll content and antioxidant enzyme activities in leaves of three wheat cultivars varying in productivity. Russian J. Plant Physiol. 57: 87 -95.
Nohong, B. and S. Nompo. 2015. Effect of water stress on growth, yield, proline and soluble sugars contents of Signal grass and Napier grass species. Am-Euras J. Sustainable Agric. 9(5): 14-21.
Ortiz, O.H., A.M. Benavides, R.M. Villarreal, H.R. Rodríguez and K.A. Romenus. 2007. Enzymatic activity in tomato fruits as a response to chemical elicitors. J. Mexi. Chemi. Socie. 51(3): 141-144.
Petriccione, M., F. Mastrobuoni, M.S. Pasquariello, L. Zampella, E. Nobis, G. Capriolo and M. Scortichini. 2015. Effect of chitosan coating on the post-harvest quality and antioxidant enzyme system response of strawberry fruit during cold storage. Foods. 4: 501-523. https://doi.org/10.3390/foods4040501
Ray, S.R., M.J.H. Bhuiyan, M.A. Hossain, A.K. Hasan and S. Sharmin. 2016. Chitosan ameliorates growth and biochemical attributes in mung bean varieties under saline condition. Res. Agric. Livest. Fish. 3(1): 45-51. https://doi.org/10.3329/ralf.v3i1.27857
Reddy, T.Y., V.R. Reddy and N. Anbumozhi. 2003. Physiological response of groundnut to drought stress and its amelioration. Plant Growth Regul. 41(3): 75-78. https://doi.org/10.1023/A:1027353430164
Roitsch, T and R. Ehneß. 2000. Regulation of source/sink relations by cytokinins. Plant Growth Regul. 32(2): 359–367. https://doi.org/10.1023/A:1010781500705
Sander, D.C., T.A. Hovell, M.S.S. Hile, L. Hodges, D. Meek and C.J. Peen. 1889. Yield and quality of processing tomato in response to irrigation rate and schedule. J. Am. Sco. Hort. Sci. 114(4): 904-908.
Sarmadnia, G.H. 1993. The importance of environmental stresses in agronomy. Articles of the first congress agronomy and plant breeding of Iran. Coll. Agric. Karaj.
Sathiyabama, M., G. Akila and C.R. Einstein. 2014. Chitosan-induced defense responses in tomato plants against early blight disease caused by Alternaria solani (Ellis and Martin) Sorauer. Arch. Phytopathol. Plant Prot. 47: 1777-1787. https://doi.org/10.1080/03235408.2013.858423
Schonfeld, M.A., R.C. Johnson, B.F. Carver and D.F. Mornhinweg. 1988. Water relations in winter wheat as drought resistance indicators. Crop Sci. 28: 526-531. https://doi.org/10.2135/cropsci1988.0011183X002800030021x
Setter, T.L., B.A. Flannigan and J. Melkonian. 2001. Loss of kernel set due to water deficit and shade in maize: carbohydrate supplies, abscisic acid, and cytokinins. Crop Sci. 41: 1530–1540. https://doi.org/10.2135/cropsci2001.4151530x
Shehata, S., Z. Fawzy and H. El-Ramady. 2012. Response of cucumber plants to foliar application of chitosan and yeast under greenhouse conditions. Aust. J. Basic Appl. Sci. 6: 63-71.
Sheikha, S.A. and F.M. Al-Malki. 2011. Growth and chlorophyll responses of bean plants to chitosan applications. Euro. J. Sci. Res. 50(1): 124-134.
Sinclair, T. R. and M. M. Ludlow. 1985. Who taught plants thermodynamics? The unfilled potential of plant water potential. Aust. J. Plant Physiol. 12:213-217.
Smirnoff, N. 1996. The function and metabolism of ascorbic acid content in plants Ann. Bot. 78: 661-669. https://doi.org/10.1006/anbo.1996.0175
Tham, L.X., N.S. Nagasawa, N.S. Matsuhashi, I.T.O. Ishioka and T. Kume. 2001. Effect of radiation-degraded chitosan on plants stressed with vanadium. Radia. Phys. Chem. 61(2): 171-175. https://doi.org/10.1016/S0969-806X(00)00388-1
Thapa, G.D., M. Dey, L. Sahoo and S.K. Panda. 2011. An insight into the drought stress induced alterations in plants. Biol. Plant. 55(4): 603. https://doi.org/10.1007/s10535-011-0158-8
Tuzel, Y., M.A. UI and I.H. Tuzel. 1994. Effect of different irrigation intevals and rate on spring season glasshouse tomato production. Active Hort. 366: 389-396. https://doi.org/10.17660/ActaHortic.1994.366.48
Ulla, V.K., K. Angelika and K. Harald. 1999. Effect of different water supply on the plant growth and fruit quality of tomato (lycopersicum esculentum L.). J. plant. Nutr. Soil Sci. 162: 583-588. https://doi.org/10.1002/(SICI)1522-2624(199912)162:6<583::AID-JPLN583>3.0.CO;2-P
Verrreyne, J.S., E. Rabe and K.I. Theron. 2001. The effect of combined deficit irrigation and summer trunk girdling on the internal fruit quality of ‘Marisol’ clementines. Sci. Hort. 91: 25-37. https://doi.org/10.1016/S0304-4238(01)00233-3
Willmer, C.M. and M. Pricker. 1996. Stomata. (2nd edn), Champand Hall, London. https://doi.org/10.1007/978-94-011-0579-8
Xu, Y., P. Burgess, X. Zhang and B. Huang. 2016. Enhancing cytokinin synthesis by overexpressing ipt alleviated drought inhibition of root growth through activating ROS-scavenging systems in Agrostis stolonifera. J. Exp. Bot. 67(6): 1979–1992. https://doi.org/10.1093/jxb/erw019
Ya-jing, G., J. Hu, X. Wang and C. Shao. 2009. Seed priming with chitosan improves maize germination and seedling growth in relation to physiological changes under low temperature stress. J. Zhejiang Univ. Sci. 10(6): 427-433. https://doi.org/10.1631/jzus.B0820373
Yang, F., J. Hu, J. Li, X. Wu and Y. Qian. 2009. Chitosan enhances leaf membrane stability and antioxidant enzyme activities in apple seedlings under drought stress. Plant Gro. Reg. 58: 131–136. https://doi.org/10.1007/s10725-009-9361-4
Yazdanpanah, S., A. Baghizadeh and F. Abbassi. 2011. The interaction between drought stress and salicylic and ascorbic acids on some biochemical characteristics of Satureja hortensis. African J. Agric. Res. 6(4): 798-807.
Young, S.L., H.K. Yong and B.K. Sung. 2005. Changes in the respiration, growth, and vitamin C content of soybean sprouts in response to chitosan of different molecular weights. Hort. Sci. 40(5-8): 1333-1335. https://doi.org/10.21273/HORTSCI.40.5.1333
Zagzog, O.A., M.M. Gad and N.K. Hafez. 2017. Effect of nano-chitosan on vegetative growth, fruiting and resistance of malformation of mango. Trends Horti. 6: 673-681.
Zhili, J., L. Yong, L. Juanjuan, X. Xiaoyan, L. Hui, L. Dianqiu and W. Jingying. 2012. Effects of exogenous chitosan on physiological characteristics of potato seedlings under drought stress and rehydration. EAPR. Potato Res. 55: 293–301. https://doi.org/10.1007/s11540-012-9223-8
To share on other social networks, click on any share button. What are these?







