Effects of Mushroom Stem Waste (Flammulina velutipes) on Laying Performance, Egg Quality and Serum Biochemical Indices
Effects of Mushroom Stem Waste (Flammulina velutipes) on Laying Performance, Egg Quality and Serum Biochemical Indices
Shad Mahfuz1,2, Shuyuan Wang1, Mo Chen1, Fei Zao1, Dong Zhen1, Zhongjun Liu4 and Hui Song1,3*
1School of Life Science, Jilin Agricultural University, Changchun, Jilin, P.R. China.
2Department of Animal Nutrition, Sylhet Agricultural University, Sylhet-3100, Bangladesh.
3Engineering Research Center of Chinese Ministry of Education for Edible and Medicinal Fungi, Changchun 130118, China.
4College of Chinese Medicine Materials, Jilin Agricultural University, Changchun, Jilin, P.R. China.
ABSTRACT
The extensive use of antibiotics in poultry industry with the purpose of increasing production performance has led to human health hazards. A driving force for the interest of using natural herbs is to eliminate the use of low-dose antibiotics in poultry production. Therefore, the aim of this study was to examine the effectiveness of Flammulina velutipes stem waste (FVW) inclusion in laying hens diet on production performance, egg quality and serum metabolic profile during the early phase of production. A total of 105 ISA Brown 18 wk old laying hens were grouped into 5 treatments with 7 replications of 3 hens each. Dietary treatments included a basal diet as control; antibiotic (0.05% flavomycin); 2% FVW; 4% FVW; and 6% FVW for 8 wk. Data were subjected to one-way analysis of variance using SPSS software followed by Duncan’s test probability of p<0.05. During the experimental period no significant differences were observed in egg production, egg mass, egg weight, feed intake and feed conversion ratio (FCR) among groups. Among the egg quality parameters haugh unit, egg shell color and egg yolk color were improved (p<0.05) in FVW fed groups than control and antibiotic groups. Serum immunoglobulin G (IgG) was higher (p<0.05) both in 4%FVW and 6%FVW fed groups than control and antibiotic fed groups. Serum immunoglobulin A (IgA) was found higher (p<0.05) in all mushroom inclusion groups than control and antibiotic fed groups. The concentrations of serum immunoglobulin M (IgM) were slightly higher in FVW fed groups compared with the other groups. Serum albumen concentration was increased (p<0.05) in 4%FVW fed group than control and antibiotics fed groups. Our study demonstrated that dietary inclusion of Flammulina velutipes mushroom stem waste could be used at 4%level as a unique feed supplement to improve egg quality, and health status of laying hens.
Article Information
Received 06 December 2017
Revised 12 May 2019
Accepted 20 June 2019
Available online 30 October 2019
Authors’ Contribution
SM conducted the experiments under the supervision of HS. SW, MC, FZ, DZ helped in mushroom preparation, experimental diets and participated in the animal experiment. SM, SW, MC, performed lab tests. SW, DZ helped in statistical analysis and formatting of manuscript. ZL, HS and SM managed the entire experiments and revised the manuscript.
Key words
Mushroom stem waste, Egg production, Egg shell color, Egg yolk color, Serum biochemistry
DOI: https://dx.doi.org/10.17582/journal.pjz/2020.52.1.255.262
* Corresponding author: [email protected]
0030-9923/2020/0001-0255 $ 9.00/0
Copyright 2020 Zoological Society of Pakistan
INTRODUCTION
Mushroom has long been reported as good dietary elements in human. The Flammulina fungus is also known as golden needle mushroom, or velvet stems or winter mushroom or lily mushroom with the four most worldwide distribution (Jing et al., 2014; Yang et al., 2016) and commonly found in China, being the largest producer, followed by Japan and South Korea (Mahfuz et al., 2017). It is the most popular edible mushrooms under the family Physalacriaceae, also has long been appreciated for its flavor, texture, medicinal and tonic attributes (Yang et al., 2011, 2016; Jing et al., 2014; Tang et al., 2016). F. velutipes is an excellent source of essential amino acids and vitamins, along with exhibits good antioxidant, anti-inflammatory, immunomodulatory, antitumor and cholesterol-lowering activities (Pang et al., 2007; Wu et al., 2010; Lee et al., 2013). The unique feature of this mushroom is that it contains rich in proteins, carbohydrates, and fiber (Ko et al., 2007).
Several medicinal properties of mycelial and fruiting body samples of F. velutipes have been established in in-vitro experiment such as immunomodulatory effect via induction of cytokines and antifungal, antibacterial, antiviral, antioxidant, antiprotozoal, mitogenic activities (Badalyan and Hambardzumyan, 2001; Smiderle et al., 2006; Wang et al., 2012; Wu et al., 2014). F. velutipes stem waste (FVW) availability is abundant due to increased cultivation of this mushroom and stem is treated as an agricultural waste although having medicinal and nutritive values. F. velutipes are harvested, however, only the fruiting body is used as food stuff and the stem is discarded, leading to an environmental pollution (Mahfuz et al., 2017). Antibiotics have been extensively used in animal feed as feed additives and growth promoters (Kamran et al., 2016). But the extensive use of antibiotics has the potential to generate antibiotic-resistant bacteria in animal products, which subsequently leads to treatment failure for bacterial disease after transferring to humans through the food chain (Zhang et al., 2011; Sethiya, 2016).
Since last few years, animal researchers are searching for herbal feed resources which have no health hazard on improving poultry production (Dhama et al., 2015; Laudadio et al., 2015). At present, very limited studies have been carried out to examine the efficiency of FVW on laying hens. Taking consideration, the objective of this study was to estimate the possibility of disposed FVW as a feed supplement as well as a substitute for antibiotics on laying performance, egg quality, and serum biochemical indices in laying hens at the early phase of production.
MATERIALS AND METHODS
Birds and dietary treatment
A total of 105 ISA brown laying hens of 18 wk old were randomly assigned into 5 equal treatment groups, with 7 replications of 3 birds for each treatment. Feed and water were offered twice daily ad libitum. Hens were exposed to a 17 h light and 7 h dark period throughout the whole experiment (8 wk) from 18 wk to 26 wk. Hen’s house temperature was maintained at approximately 24°C. The experiment was carried out at the animal shed building under the college of Chinese Medicine Materials, Jilin Agricultural University from April 2017 to August 2017. The birds were handled according to by Chinese Guidelines for Animal Welfare and approved by the animal welfare committee of Jilin Agricultural University. Birds were given a standard basal diet considered as control group; antibiotic (0.05% flavomycin) group; 2%F. velutipes mushroom stem waste (FVW) fed group; 4% FVW fed group; and 6% FVW fed group respectively. The F. velutipes stem was collected from a domestic mushroom farm in Changchun. The collected stem was dried at 60°C temperature and transferred to feed mill for further uses. Mushroom and antibiotics were mixed with layer diet formulated per National Research Council (NRC, 1994) specification using a feed mixer in Feed Mill (Jilin Hanghong Animal Husbandry Co. Ltd, China). The FVW sample was prepared (0.01mm) for proximate component analysis following the method of AOAC (2004). The analyzed nutritional composition of the experimental diet was presented in Table I.
Laying hen performance
Egg production percentage was calculated by dividing the total number of eggs by hen-day and was expressed in percent. Egg weight was presented as the average egg weight per hen divided by the number of days during the experimental period, Egg mass was calculated as laying percentage multiplied by egg weight. Total feed intake was determined as the difference between feed offered and residual feed in trough feeders on weekly basis and FCR was then calculated as feed intake divided by egg mass respectively. Unmarketable eggs were considered based on curled eggs (broken eggs, abnormal shape etc).
Measurement of egg quality
For evaluation of egg quality, a total 105 eggs (21 eggs from each treatment group) were randomly selected on weekly basis (20 wk, 23 wk and 26 wk). Seven eggs per group were randomly selected from each evaluation period. Egg width and length were measured by digital caliper (0.01 mm, model 1116-150 Insize Co, Suzhou, China) and the Shape Index was measured by following the ratio of width and length of selected egg. Individual eggs were weighed and were broken to separate the yolk, albumen and egg shell. The yolks samples were then carefully raped with paper napkins to remove adhesive albumin and finally placed in previously weighed Petri dishes. The difference in the weight of each Petri dish after and before the introduction of the yolk was considered as the weight of the yolk. The eggshell (including shell membrane) was also socked by paper napkins and weighed by electronic weighing scale. The albumin weight was calculated from whole egg weight by subtracting the yolk and egg shell weight, methods described by Akbari et al. (2016). The yolk and albumen heights were determined using a tripod micrometer following the method described by Monira et al. (2003). Haugh unit was measured from egg weight and egg albumen height by following formula as described by Eisen et al. (1962).
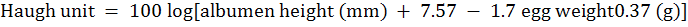
The relative weight of the egg shell, yolk and albumin were expressed in percentage of whole egg weight. Eggshell thickness was measured at three different locations (top, middle and bottom of the egg) using a micrometer (0.001 mm, Digital micrometer). Yolk index was calculated as a ratio of yolk width and yolk length.
Sensory evaluation of table eggs
A total of 35 eggs (7 eggs from each treatment) were selected at the end of the experiment to determine the
Table I. Experimental diet for layer (g/kg).
|
Ingredients |
Control |
Antibiotics |
2%FVW |
4%FVW |
6%FVW |
|
Maize corn |
557.0 |
556.5 |
544.0 |
525.0 |
510.0 |
|
Soyabean meal |
282.0 |
282.0 |
277.0 |
274.0 |
272.0 |
|
Soyabean Oil |
28.0 |
28.0 |
26.0 |
28.0 |
25.0 |
|
FVW a |
- |
- |
20.0 |
40.0 |
60.0 |
|
Lysine |
2.0 |
2.0 |
2.0 |
2.0 |
2.0 |
|
Methionine |
2.5 |
2.5 |
2.5 |
2.5 |
2.5 |
|
Dicalcium |
36.0 |
36.0 |
36.0 |
36.0 |
36.0 |
|
Limestone |
88.0 |
88.0 |
88.0 |
88.0 |
88.0 |
|
Common salt |
2.5 |
2.5 |
2.5 |
2.5 |
2.5 |
|
Vit- mineral premix b |
2.0 |
2.0 |
2.0 |
2.0 |
2.0 |
|
Antibiotics |
- |
0.5 |
- |
- |
- |
|
Total |
1000 |
1000 |
1000 |
1000 |
1000 |
|
Chemical analysisc |
|||||
|
DM (g/kg) |
932.2 |
932.2 |
933.3 |
933.55 |
935.50 |
|
CP (g/kg) |
170.0 |
170.0 |
169.80 |
169.90 |
170.03 |
|
Ca (g/kg) |
41.10 |
41.10 |
41.10 |
41.10 |
41.2 |
|
P (g/kg) |
7.20 |
7.20 |
7.20 |
7.20 |
7.20 |
|
EE (g/kg) |
52.3 |
52.3 |
50.40 |
52.20 |
49.30 |
|
CF (g/kg) |
25.6 |
25.5 |
29.60 |
33.7 |
37.90 |
|
Calculated analysis |
|||||
|
ME (MJ/kg) |
11.70 |
11.69 |
11.70 |
11.70 |
11.71 |
|
Lysine (g/kg ) |
10.5 |
10.5 |
10.40 |
10.4 |
10.4 |
|
Methionine (g/kg) |
5.0 |
5.0 |
5.0 |
4.9 |
4.9 |
|
Cystine (g/kg) |
2.8 |
2.8 |
2.8 |
2.7 |
2.7 |
aFVW: F. velutipes stem waste at 2%, 4% and 6%; FVW: Analyzed composition of F. velutipes mushroom stem waste (g/kg) DM 887.0 ±0.85; CP 12.15±0.49; CF 20.05±0.106; EE 2.7±0.014; Ash 10.5±0.085; Ca 4.0±0.1; P 6.2±0.28; Value are expressed as mean ± standard deviation (n=6); bProvided per kg of the complete diet: retinyl acetate, 4500 IU; cholecalciferol, 1200 IU; DL-α-tocopheryl acetate, 25000 IU; thiamin, 5000 mg; riboflavin, 20000 mg; phylloquinone,10000 mg; niacin, 45000 mg; pantothenic acid, 35000 mg; biotin, 1500 mg; folic acid, 3000 mg; cyanocobalamin, 40 mg; zinc, 45 mg; manganese 50 mg; iron, 30 mg; copper, 4 mg; cobalt, 120 μg; iodine, 1 mg; selenium, 120 μg; cDM= dry matter; CP= crude protein; Ca=calcium; P= phosphorus; EE=crude fat; CF=crude fiber; ME= metabolisable energy.
sensory test of table eggs. Selected eggs were evaluated by five departmental panelists. The panelists were requested to give score in terms of appearance and overall acceptability of eggs on 5-point scale (very poor to very good; 1 to 5). Egg shell color and yolk color were considered comparing with egg shell color fan (Robotmation Co. Ltd, Japan) and yolk color chart fan (Robotmation Co. Ltd, Japan) ranged 1-15 (very light -pale to very dark brown; 1 to 15).
Serum biochemical indices
Blood samples were obtained from one bird of each replicate pen (7 birds per experimental group) via wing vein at the end of the experiment i.e. on d 182. Serum was obtained by centrifuged at 3000 × g for 20 min at 4°C (Legend Micro 17R centrifuge, Thermo Fisher, Germany) and were stored at -80°C until measuring serum biochemical indices and serum immunoglobulin concentration. The serum IgA, IgG, IgM, were measured using chicken specific IgA, IgG, IgM, ELISA Quantitation Kits (Shang Hai Lengton Biosicences Co. Ltd, China) according to the instructions of the manufacturer and absorbance was measured at 450 nm. Other biochemical parameters including total protein (g/L), albumen (g/L), urea-N (mmol/L), calcium (mmol/L) and phosphorus (mmol/L) were measured following the instruction of commercial diagnostic kits supplied by Nanjing Jiancheng Bioengineering Institute (Nanjing, China). The absorbance was measured at 562 nm for total protein; 630 nm for total
Table II. Effect of mushroom waste on laying performance1.
|
Parameters |
Control |
Antibiotics |
2%FVW |
4%FVW |
6%FVW |
SEM |
p value |
|
Hen day egg production (%) |
80.10 |
77.86 |
78.28 |
80.12 |
75.77 |
2.774 |
0.989 |
|
Egg weight (g/egg) |
56.34 |
55.37 |
55.74 |
55.81 |
56.56 |
0.491 |
0.951 |
|
Egg mass (g/d) |
45.43 |
43.41 |
44.12 |
45.03 |
43.29 |
1.834 |
0.995 |
|
Feed intake (g/d) |
107.46 |
106.00 |
105.18 |
104.51 |
104.70 |
1.519 |
0.977 |
|
FCR (g/g) |
2.52 |
2.70 |
2.57 |
2.49 |
2.73 |
0.121 |
0.965 |
|
Unmarketable eggs (%) |
2.46 |
1.54 |
1.64 |
0.95 |
1.15 |
0.263 |
0.433 |
1data represent the mean value of 7 replicates with 3 hens each treatment; Significantly different at p<0.05. SEM, pooled standard error of the means.
Table III. Effect of mushroom waste on egg quality1.
|
Parameters |
Control |
Antibiotics |
2%FVW |
4%FVW |
6%FVW |
SEM |
p value |
|
Shape index |
79.29 |
78.77 |
79.65 |
79.63 |
78.83 |
0.237 |
0.645 |
|
Shell weight (%) |
10.91 |
11.29 |
11.10 |
11.37 |
10.95 |
0.070 |
0.150 |
|
Shell thickness (mm) |
0.38 |
0.38 |
0.38 |
0.38 |
0.38 |
0.001 |
0.189 |
|
Yolk weight (%) |
21.85 |
20.86 |
21.18 |
21.09 |
20.77 |
0.126 |
0.054 |
|
Yolk index |
35.98 |
35.12 |
35.11 |
34.09 |
34.58 |
0.300 |
0.358 |
|
Albumen weight (%) |
67.23 |
67.85 |
67.73 |
67.54 |
68.28 |
0.149 |
0.246 |
|
Haugh unit |
80.29b |
79.99b |
81.55ab |
81.41ab |
82.86a |
0.294 |
0.014 |
1data represent the mean value of 7 replicates with 3 eggs each treatment (N=21); a, b, c-means in the same row with different letters are significantly different at p<0.05; SEM, pooled standard error of the means.
albumen; 640 nm for urea-N; 610 nm for calcium; and was 660 nm for phosphorus.
Statistical analysis
Data were subjected to one-way analysis of variance using SPSS (2006) software (version 20). Significant effects of dietary treatments on experimental groups were evaluated with Duncan’s test. Statements of statistical significance are based on a probability of p<0.05.
RESULTS AND DISCUSSION
The analyzed nutritional composition of F. velutipes mushroom stem waste (FVW) was presented in Table I. The chemical composition values of dry matter (DM), crude protein (CP), and crude fiber (CF) in FVW were mostly close to previously reported value (Ko et al., 2007; Lee et al. 2012) but the crude fat (EE), total mineral (ash), calcium (Ca), and phosphorus (P) were lower than the published value (Ko et al., 2007; Lee et al. 2012). This variation in chemical composition of FVW, might be associated with soil type, harvesting methods and environmental factors.
No significant effect was observed on hen day egg production, egg weight, egg mass, with dietary inclusion of F. velutipes stem waste (FVW). Compared with antibiotics fed group, egg weight was higher in FVW fed groups but the results were not significant. Feed conversion efficiency (FCR) and unmarketable eggs were improved at 4% FVW fed group but not significant. In addition, average daily feed intake (FI) was observed slightly lower with FVW fed groups although not significant (Table II). Similar finding was reported by Lee et al. (2014) who stated that fermented F. velutipes mycelium could improve egg size, egg mass and FCR but no significance effect in egg production in laying hens. Results associated with laying performance parameters in the current study differs from the past study must be correlated with mushroom types and different levels of inclusion in diet. Lee et al. (2012) reported that higher level up to 5% FV mycelium did not improve feed intake and other performance parameters in broiler which was also similar with this study for feed intake.
Egg quality characteristics of eggs consumed are important factors. Also, an increment in egg quality will enable egg producers to sell at higher price compared to low quality eggs (Metin et al., 2017). Haugh units were significantly (p<0.05) higher in 6%FVW fed group than the control and antibiotic groups. The other parameters of egg shape index, shell weight, shell thickness, yolk weight,
Table IV. Effect of mushroom waste on sensory evaluation of table eggs1.
|
Parameters |
Control |
Antibiotics |
2%FVW |
4%FVW |
6%FVW |
SEM |
p value |
|
Appearance |
3.09b |
3.06b |
3.31ab |
3.57a |
3.56a |
0.052 |
0.000 |
|
Shell color |
10.36bc |
10.30c |
10.66abc |
10.91ab |
11.17a |
0.097 |
0.012 |
|
Yolk color |
8.86b |
8.69b |
9.03ab |
9.13ab |
9.53a |
0.091 |
0.031 |
|
Aceeptability |
3.61b |
3.69b |
3.79ab |
4.13a |
4.09a |
0.064 |
0.019 |
1data represent the mean value of 7 eggs each treatment (N=7); a, b, c-means in the same row with different letters are significantly different at p<0.05; SEM, pooled standard error of the means.
Table V. Effect of mushroom waste on serum biochemical parameters in layer1.
|
Parameters |
Control |
Antibiotics |
2%FVW |
4%FVW |
6%FVW |
SEM |
p value |
|
Total Protein (g/L) |
44.48 |
43.69 |
45.20 |
46.37 |
48.05 |
0.993 |
0.692 |
|
Albumen (g/L) |
20.26b |
19.67b |
21.75ab |
23.24a |
21.80ab |
0.403 |
0.033 |
|
Urea -N (mmol/L) |
4.34 |
3.63 |
3.40 |
3.94 |
4.23 |
0.148 |
0.122 |
|
Calcium (mmol/L) |
3.22 |
3.48 |
3.75 |
3.70 |
3.79 |
0.073 |
0.064 |
|
Phosphorus (mmol/L) |
1.34 |
1.35 |
1.44 |
1.41 |
1.44 |
0.026 |
0.637 |
|
Serum IgA (mg/ml) |
5.18c |
4.70d |
6.18b |
6.28 ab |
6.47 a |
0.417 |
0.010 |
|
Serum IgG (mg/ml) |
5.21b |
4.61c |
5.03bc |
5.97a |
5.86a |
0.111 |
0.001 |
|
Serum IgM (mg/ml) |
2.62 |
2.19 |
5.40 |
3.79 |
4.12 |
0.424 |
0.094 |
1data represent the mean value of 7 hens per treatment. a, b, c, d-means in the same row with different letters are significantly different at p<0.05; SEM, pooled standard error of the means.
yolk index and albumen weight were not significantly different (p>0.05) among the experimental groups. However, shape index and albumen weight were slightly higher in FVW fed groups than antibiotic and control fed groups respectively, although the result were not significant (Table III). The improvement of egg quality especially, haugh unit were directly associated by dietary inclusion of FVW. In our observation FVW was a good source of crude protein (CP). Higher level of FVW supplement provided higher protein leads to increase albumin height as well as haugh unit. Dietary supplementation of herbal extract powder could improve the haugh unit of eggs in laying hens was reported by Metin et al. (2017). Lee et al. (2014) reported that dietary supplementation of 4%F. velutipes mycelium could improve egg shell weight, shell thickness, albumin height and haugh unit. The difference with other egg quality parameters with past study must be associated with mushroom types and inclusion level in this study. Similarly, Hwang et al. (2012) reported that dietary supplementation of oyster mushroom improved albumin height and haugh unit in laying hen.
The sensory evaluation results showed that the appearance, egg shell color, egg yolk color and the overall acceptability of table eggs were higher (p<0.05) in FVW fed group compared with antibiotic and control groups (Table IV). Higher acceptability of table eggs was reported from dietary supplementation of mushroom in laying hens on sensory evaluation (Hwang et al., 2012).
The effect of Flammunila velutipes stem waste (FVW) on serum biochemical parameters in laying hens were shown in Table V. Serum immunoglobulin G (IgG) was found higher (p<0.05) both in 4% FVW and 6%FVW fed groups compared with antibiotics and control fed group. Serum immunoglobulin A (IgA) was found higher (p<0.05) with FVW fed groups compared with antibiotics and control fed group. However, laying hens fed with FVW showed higher immunoglobulin M (IgM) in this study but the results were not significant. The polysaccharides in F. velutipes mushroom have strong immune modulatory activity that could enhance non-specific and specific immune responses in vitro (Wu et al., 2014; Tang et al., 2016; Mahfuz et al., 2017). Inclusion of oyster mushroom wastes at 1% level was able to improve some performance parameters and immunity in broiler chicks (Fard et al., 2014). Willis et al. (2013) also reported that certain mushrooms have immune enhancement activity. Higher concentration of IgG with FVW fed groups and lower of immunoglobulin M (IgM) was observed in laying hens fed with antibiotics in current study was supported by Huang et al. (2007). Serum albumen (g/L) concentration was increased (p<0.05) in 4% FVW fed group than control and antibiotics fed groups. No significant differences were observed for total protein (g/L), urea-N (mmol/L), calcium (mmol/L) and phosphorus (mmol/L) among experimental groups. However, total protein, calcium and phosphorus were found higher in FVW fed groups compared with control and antibiotics fed groups although the results were not significant. Blood biochemical parameters are usually related to the health status and are vital indicators of nutritional and physiological condition of birds and animals (Abd El-Hack et al., 2017). The content of total protein in poultry blood increases with age as a result of metabolic changes in animals and reflects various disorders of nutritional characters resulting from either insufficient or excess intake of proteins in feed mixtures (Pavlik et al., 2007). Serum total protein concentration, urea-N, calcium and phosphorus were not affected in this present study with dietary mushroom stem waste inclusion, as were noted in previous study by Li et al. (2016) who stated that there were no differences in serum total protein, serum glucose and aspartate amino transferase (AST) in hens fed with Chinese herb mixture. In contrast to Li et al. (2016) this study ensured the higher serum albumen concentration with FVW fed groups which might be correlated with different inclusion level and herbs. Higher serum albumen concentration with dietary supplementation of probiotics bacteria in laying hens was also reported by Abd El-Hack et al. (2017). Urea-N was lower in the current study with FVW fed groups than control. Ammonia-N is the microbial product that is known to have negative health effects on birds, animals and humans (Abd El-Hack et al., 2017). Serum calcium and phosphorus were slightly higher in FVW fed groups exhibiting that FV mushroom naturally contains higher amount of calcium, phosphorus and other nutrients.
CONCLUSION
The present study found that FVW could be a good source of natural feed additives that can improve serum immunoglobulin concentration, serum albumen deposition, egg quality including haugh unit, egg shell color, egg yolk color, egg weight, in laying hen without hampering normal egg production rate. Flammunila velutipes mushroom stem waste can be used as a natural supplement as well as substitute for antibiotic in laying hens.
ACKNOWLEDGEMENTS
This work was supported by “Innovation Platform for Economic Fungi in Jilin Province” [Grant No. 2014-2016] Changchun, P.R. China.
Statement of conflicts of interest
There is no conflict of interest relevant to this publication.
REFERENCES
Abd El-Hack, M.E.A., Mahgoub, S.A., Alagawany, M. and Ashour, E.A., 2017. Improving productive performance and mitigating harmful emissions from laying hen excreta via feeding on graded levels of corn DDGS with or without Bacillus subtilis probiotic. J. Anim. Physiol. Anim. Nutr., 101: 904-913. https://doi.org/10.1111/jpn.12522
Akbari, M.K.R., Golian, A. and Zarghi, H., 2016. Effect of digestible methionine plus cystine concentration on performance, egg quality and blood metabolites in laying hens. Br. Poult. Sci., 57: 403-414. https://doi.org/10.1080/00071668.2016.1173199
AOAC., 2004. Official methods for analysis, 13th ed. Association of Official Analytical Chemists, Washington, DC.
Badalyan, S.M. and Hambardzumyan, L.A., 2001. Investigation of immunomodulating activity of the medicinal mushroom Flammulina velutipes (Curt.: Fr.) P. Karst. in vitro. cytokine induction by fruiting body extract. Int. J. Med. Mushr., 3: 110-111. https://doi.org/10.1615/IntJMedMushr.v3.i2-3.290
Dhama, K., Latheef, S.K., Mani, S., Samad, H.A., Karthik, K., Tiwari, R., Khan, R.U., Alagawany, M., Farag, M.R. and Alam, G.M., 2015. Multiple beneficial applications and modes of action of herbs in poultry health and production-A review. Int. J. Pharmacol., 11: 152-176. https://doi.org/10.3923/ijp.2015.152.176
Eisen, E., Bohren, B. and McKean, H., 1962. The Haugh unit as a measure of egg albumen quality. Poult. Sci., 41: 1461-1468. https://doi.org/10.3382/ps.0411461
Fard, S.H., Toghyani, M. and Tabeidian, S.A., 2014. Effect of oyster mushroom wastes on performance, immune responses and intestinal morphology of broiler chickens. Int. J. Recycl. Org. Waste Agric., 3: 141-146. https://doi.org/10.1007/s40093-014-0076-9
Huang, R., Deng, Z., Yang, C., Yin, Y., Xie, M.Y., Wu, G., Li, T., Li, L., Tang, Z., Kang, P., Hou, Z., Deng, D., Xiang, H., Kong, X.F. and Guo, Y., 2007. Dietary oligochitosan supplementation enhances immune status of broilers. J. Sci. Fd. Agric., 87: 153-159. https://doi.org/10.1002/jsfa.2694
Hwang, J.A., Hossain, M.E., Yun, D.H., Moon, S.T., Kim, G.M. and Yang, C.J., 2012. Effect of shiitake [Lentinula edodes (Berk.) Pegler] mushroom on laying performance, egg quality, fatty acid composition and cholesterol concentration of eggs in layer chickens. J. med. Pl. Res., 6: 146-153. https://doi.org/10.5897/JMPR11.1351
Jing, P., Zhao, S.J., Lu, M.M., Cai, Z., Pang, J. and Song, L.H., 2014. Multiple-fingerprint analysis for investigating quality control of Flammulina velutipes fruiting body polysaccharides. J. Agric. Fd. Chem., 62: 12128-12133. https://doi.org/10.1021/jf504349r
Kamran, S., Khushi, M. and Rubina, N., 2016. Effect of herbal extracts on serum minerals, lipid profile and anti-NDV-HI antibody levels of vaccinated broiler chicks. Pakistan J. Zool., 48: 1715-1719.
Ko, W.C., Liu, W.C., Tsang, Y.T. and Hsieh, C.W., 2007. Kinetics of winter mushrooms (Flammulina velutipes) microstructure and quality changes during thermal processing. J. Fd. Engin., 81: 587-598. https://doi.org/10.1016/j.jfoodeng.2006.12.009
Laudadio, V., Lorusso, V., Lastella, N., Dharna, K., Karthik, K., Tiwari, R., Alam, G.M. and Tufarelli, V., 2015. Enhancement of nutraceutical value of table eggs through poultry feeding strategies. Int. J. Pharmacol., 11: 201-212. https://doi.org/10.3923/ijp.2015.201.212
Lee, H.G., Kim, M.J. and Lee, J.S., 2014. Effects of dietary fermented Flammulina velutipes mycelium on performance and egg quality in laying hens. Int. J. Poult. Sci., 13: 637-644. https://doi.org/10.3923/ijps.2014.637.644
Lee, S.B., Choi, Y.H., Cho, S.K., Shin, T.S., Cho, B.W., Kang, H.S., Kim, K.K., Kim, S.K. and Lee, H.G., 2012. Effects of dietary Flammulina velutipes mycelium on broiler chick performance, pathogenic bacterial counts in caecal contents and amount of NH3 in excreta. J. Anim. Sci. Technol. (Korea)., 54: 341-347. https://doi.org/10.5187/JAST.2012.54.5.341
Lee, Y.T., Lee, S.S., Sun, H.L., Lu, K.H., Ku, M.S., Sheu, J.N., Ko, J.L. and Lue, K.H., 2013. Effect of the fungal immunomodulatory protein FIP-fve on airway inflammation and cytokine production in mouse asthma model. Cytokine, 61: 237-244. https://doi.org/10.1016/j.cyto.2012.09.024
Li, X., He, W., Wang, Z. and Xu, T., 2016. Effects of Chinese herbal mixture on performance, egg quality and blood biochemical parameters of laying hens. J. Anim. Physiol. Anim. Nutr., 100: 1041-1049. https://doi.org/10.1111/jpn.12473
Mahfuz, S., Hui, S. and Zongjun, L., 2017. Improved production performance and health status with winter mushroom stem (Flammulina velutipes) in laying chicken: review. Int. J. Poult. Sci., 16: 112-117. https://doi.org/10.3923/ijps.2017.112.117
Metin, D., Asuman, A.D., Köksal, K., Ecevit, E., Harun, C. and Mohammad, M.T., 2017. Effect of carrot (Daucus carota) leaf powder on external and internal egg characteristics of Hy-Line white laying hens. Pakistan J. Zool., 49: 129-137.
Monira, K.N., Salahuddin, M. and Miah, G., 2003. Effect of breed and holding period on egg quality characteristics of chicken. Int. J. Poult. Sci., 2: 261-263. https://doi.org/10.3923/ijps.2003.261.263
NRC, 1994. Nutrient requirements of poultry, 9th rev. ed. Natl. Acad. Press, Washington, DC, US.
Pang, X., Yao, W., Yang, X., Xie, C., Liu, D., Zhang, J. and Gao, X., 2007. Purification, characterization and biological activity on hepatocytes of a polysaccharide from Flammulina velutipes mycelium. Carbohydr. Polym., 70: 291-297. https://doi.org/10.1016/j.carbpol.2007.04.010
Pavlik, A., Pokludová, M., Zapletal, D. and Jelínek, P., 2007. Effects of housing systems on biochemical indicators of blood plasma in laying hens. Acta Vet. Brno, 339-347. https://doi.org/10.2754/avb200776030339
Sethiya, N., 2016. Review on natural growth promoters available for improving gut health of poultry: an alternative to antibiotic growth promoters. Asian J. Poult. Sci., 10: 01-29. https://doi.org/10.3923/ajpsaj.2016.1.29
Smiderle, F.R., Carbonero, E.R., Mellinger, C.G., Sassaki, G.L., Gorin, P.A.J. and Iacomini, M., 2006. Structural characterization of a polysaccharide and a beta-glucan isolated from the edible mushroom Flammulina velutipes. Phytochemistry, 67: 2189-2196. https://doi.org/10.1016/j.phytochem.2006.06.022
SPSS, 2006. Statistical software package for the social sciences. SPSS, Int., USA. 15.0.
Tang, C., Hoo, P.C.X., Tan, L.T.H., Pusparajah, P., Khan, T.M., Lee, L.H., Goh, B.H. and Chan, K.G., 2016. Golden needle mushroom: A culinary medicine with evidenced-based biological activities and health promoting properties. Front. Pharmacol., 7: 474. https://doi.org/10.3389/fphar.2016.00474
Wang, Y., Bao, L., Yang, X., Li, L., Li, S., Gao, H., Yao, X.S., Wen, H. and Liu, H.W., 2012. Bioactive sesquiterpenoids from the solid culture of the edible mushroom Flammulina velutipes growing on cooked rice. Fd. Chem., 132: 1346-1353. https://doi.org/10.1016/j.foodchem.2011.11.117
Willis, W., Wall, D., Isikhuemhen, O., Jackson, J., Ibrahim, S., Hurley, S. and Anike, F., 2013. Effect of level and type of mushroom on performance, blood parameters and natural coccidiosis infection in floor-reared broilers. Open Mycol. J., l7: 1-6. https://doi.org/10.2174/1874437001307010001
Wu, D.M., Duan, W.Q., Liu, Y. and Cen, Y., 2010. Anti-inflammatory effect of the polysaccharides of Golden needle mushroom in burned rats. Int. J. Biol. Macromol., 46: 100-103. https://doi.org/10.1016/j.ijbiomac.2009.10.013
Wu, M., Luo, X., Xu, X., Wei, W., Yu, M., Jiang, N., Ye, L., Yang, Z. and Fei, X., 2014. Antioxidant and immunomodulatory activities of a polysaccharide from Flammulina velutipes. J. Trad. Chin. Med., 34: 733-740. https://doi.org/10.1016/S0254-6272(15)30089-3
Yang, W., Fang, Y., Liang, J. and Hu, Q., 2011. Optimization of ultrasonic extraction of Flammulina velutipes polysaccharides and evaluation of its acetylcholinesterase inhibitory activity. Fd. Res. Int., 44: 1269-1275. https://doi.org/10.1016/j.foodres.2010.11.027
Yang, W., Yu, J., Pei, F., Mariga, A.M., Ma, N., Fang, Y. and Hu, Q., 2016. Effect of hot air drying on volatile compounds of Flammulina velutipes detected by HS-SPME–GC–MS and electronic nose. Fd. Chem., 196: 860-866. https://doi.org/10.1016/j.foodchem.2015.09.097
Zhang, D., Hu, H., Rao, Q. and Zhao, Z., 2011. Synergistic effects and physiological responses of selected bacterial isolates from animal feed to four natural antimicrobials and two antibiotics. Foodborne Pathog. Dis., 8: 1055-1062. https://doi.org/10.1089/fpd.2010.0817
To share on other social networks, click on any share button. What are these?










