Effects of Dietary Fish Oil Replacement by Soybean Meal on Performance and Physiology of Rainbow Trout, Oncorhynchus mykiss
Effects of Dietary Fish Oil Replacement by Soybean Meal on Performance and Physiology of Rainbow Trout, Oncorhynchus mykiss
Muhammad Awais Nazir1, Khalid Mahmood Anjum1, Junaid Naseer1, Ahsan Anjum2, Aneela Zameer Durrani3, Saba Usman2, Muhammad Asif Munir1, Omer Naseer4 and Muhammad Usman3,*
1Department of Wildlife and Ecology, Faculty of Fisheries and Wildlife, University of Veterinary and Animal Sciences Ravi Campus, Pattoki
2Department of Pathology, Faculty of Veterinary Science, University of Veterinary and Animal Sciences, Lahore
3Department of Clinical Medicine and Surgery, University of Veterinary and Animal Sciences, Lahore
4Department of Clinical Medicine and Surgery, Cholistan University of Veterinary and Animal Sciences, Bahawalpur
ABSTRACT
This study was undertaken to investigate the impact of replacement of fishmeal (FM) with soybean meal (SBM) in diets of rainbow trout (Oncorhynchus mykiss). A total of 600 rainbow trout, with an initial mean body weight of 15.92 ± 0.34 g, were distributed randomly into twelve experimental tanks (50 fish per tank). Four isoenergetic and isonitrogenous feed diets were allocated to each treatment, with three replicates/treatment (12 fish/replicate). FM and fish oil were replaced gradually by SBM in the four diets. The inclusion of SBM in diets 1-4 was at the following respective levels of 0% (control), 29%, 35% and 51%. Growth performance of fish fed diet 4 was significantly lower than that of fish fed the other diets (P<0.05). The dietary inclusion of SBM up to level of 35% did not affect the blood biochemical parameters of rainbow trout, and was associated with acceptable growth performance. In conclusion, the SBM can be included in diet of Oncorhynchus mykiss at 35%, while keeping the level of FM and fish oil at 51%, and 1%, respectively.
Article Information
Received 30 June 2019
Revised 14 August 2019
Accepted 05 September 2019
Available online 30 November 2020
Authors’ Contribution
MAN carried out the research. KMA supervised the research. JN was member of supervisory committee. AA and SU helped in writing the manuscript. MAM and ON helped in laboratory work. AZD and MU proofread the manuscript.
Key words
Oncorhynchus mykiss, Fishmeal, Soybean meal, Growth, Hematology, Serum biochemistry.
DOI: https://dx.doi.org/10.17582/journal.pjz/20190630130612
* Corresponding author: [email protected]
0030-9923/2021/0001-0093 $ 9.00/0
Copyright 2021 Zoological Society of Pakistan
Introduction
In Pakistan, fishery plays a vital role in the income of people, especially those residing around the coastal areas. Oceanic and domestic types of fishery are activities occurring through the country (Hassan et al., 2007). Fishing contributes 2.12% of the agriculture sector, and 0.41% contribution to national gross domestic product (GDP) of Pakistan. During the period of July 2016 to March 2017 the total aquaculture production was 520,000 metric tons (Muhammad et al., 2017).
Trout has multiple species, inhabiting freshwater and saltwater, and belonging to the family Salmonidae (Şener, 2002). Rainbow trout is found in many countries of the world including USA, Brazil, Canada, Mexico, France, Germany, Nepal and India (Heidarieh et al., 2013). Three types of trout are commonly found in Pakistan namely, the brown trout (Salmo trutta), snow trout (Schizothorax plagiostomus) and rainbow trout (Oncorhynchus mykiss) (Korai et al., 2008). The recommended diet of the rainbow trout that meets the NITS nutritional requirement contains 2.5% crude protein, 15-20% fat, upto 20% carbohydrates and 1.5-3.0% crude fiber (Dogan and Bircan, 2015). There was an expansion in aquaculture during the last two decades, resulting in an increase in demand for their feed ingredients (Ávila et al., 2015). The ingredients level of the fish diet have to be included according to nutrient requirement of the breed (Alp et al., 2005). The fishmeal is globally included in these diets as the main source of protein. The unavailability and high cost of fishmeal (FM) created an impetus for nutritionist to seek for another sources of protein to include in the aquaculture meal that sustain an acceptable production, while lowering the cost of the formulated diet (Antolović et al., 2012).
Numerous studies investigated the replacement of FM protein and evaluating such replacement based on meeting the nutritional requirement, sustaining an acceptable production, and obtaining a cost-effective formulation (Alam et al., 1996; El-Sayed, 1999; Hidalgo et al., 1993). Among all the reported studies, the soybean meals (SBM) proved as a promising replacement to FM in feed of rainbow trout (Refstie et al., 2000). Apart of the 30% indigestible carbohydrates in SBM, it still can provide good balance of essential amino acids, high level of available protein, edibility to most fish species, associated with consistent supply in the world market at a reasonable price (Fafioye et al., 2005; Tacon et al., 1983).
Previous studies documented the success of replacing FM with SBM in aquafeeds (Abdel-Warith and Younis, 2013; Biswas et al., 2007; Hernández et al., 2007). There is a paucity of data availability regarding the replacement of FM by SBM in rainbow trout production. This study aimed at demonstration of the effect of inclusion of SBM in different levels of the rainbow trout on growth, hematology and serum biochemical values, hoping to determine the acceptable inclusion rate of SBM that can lead to normal growth without affecting the homeostasis of certain blood parameters.
Table I.- Percentage of the raw material in replacement of FM by SBM in the four experimental diets offered to rainbow trout.
|
Raw materials (g/100g) |
Percentage of raw material in the four diets |
|||
|
1 |
2 |
3 |
4 |
|
|
Fish meal |
82 |
57 |
51 |
35 |
|
Soybean meal |
0 |
29 |
35 |
51 |
|
Fish oil |
7 |
2 |
1 |
1 |
|
Carboxymethyl cellulose |
5 |
3 |
3 |
1 |
|
Starch |
3 |
6 |
7 |
9 |
|
Mineral premixa |
2 |
2 |
2 |
2 |
|
Vitamin premixa |
1 |
1 |
1 |
1 |
|
Total |
100 |
100 |
100 |
100 |
Premix detailed by Jalali et al. (2009).
Materials and methods
Replacement of FM by SBM in four diets
Four isocaloric and isonitrogenous diets were prepared with different inclusion rate of SBM. The increase in inclusion rate of the SBM was associated with a decrease in the inclusion rate of the FM. The protein content in all diets was adjusted to 43%. Table I presents the composition of all the four diets. Briefly, diet 1 is the control with 82% FM and 0.0% SBM. Diet 2, 3 and 4 were experimental diets with respective SBM levels of 29%, 35% and 51%, associated with the following respective inclusion rates of FM, equivalent to 57%, 51%, and 35%, respectively. Proximate analysis of all diets was performed and results are presented in Table II. The level of added soybean oil to mixed dried ingredients of the diets is shown in Table I. Diets were stored in plastic bags at -20°C before administration to the fish.
Table II.- Proximate analysis of diets.
|
Composition |
Diets |
|||
|
1 |
2 |
3 |
4 |
|
|
Dry matter |
94.43 |
94.41 |
94.40 |
94.42 |
|
Crude protein |
43.15 |
43.02 |
43.11 |
43.06 |
|
Crude fibre |
3.97 |
3.94 |
3.71 |
3.84 |
|
Lipid |
5.2 |
5.56 |
5.71 |
5.42 |
|
Ash |
10.28 |
9.92 |
10.11 |
9.57 |
|
Moisture |
4.32 |
4.31 |
4.29 |
4.67 |
|
Gross energy (kJ/kg) |
21.1 |
21.4 |
21.2 |
21.6 |
Fish culture and feeding regime
Six hundred rainbow trout fish, with mean body weight of 15.92±0.74 g, were obtained from a fish hatchery in the Department of Fisheries, University of Veterinary and Animal Sciences, Ravi campus Pattoki. The fish were randomly allocated four treatments, in which each treatment had three replicates (Tanks), each containing 50 fish in a volume of fresh water equivalent to 1200 L of fresh water. Levels of major characteristics of the fresh water in the tanks are presented in Table III. Each of the four different diets were offered to the fish present in three tanks (three replicates/treatment). Equal volume of distilled water was added to each diet before administering to fish, in order to make a wet paste that is easily digestible. Fish were fed twice a day, at 7:00 am and 16:00 pm., with a complete consumption by all differently treated fish, at each time of the day. The rearing of the fish was for a period of 12 weeks. It is worth noting that a photoperiod schedule of 12 h light and 12 h dark was set throughout the trial.
Table III.- Levels of major characteristics of fresh water in the tanks prepared for rearing the rainbow trout fish.
|
Major characteristics of water in tanks |
Levels |
|
Temperature |
13.4 ± 0.7 °C |
|
Dissolved oxygen concentration |
8.2 ± 0.3 mg/L |
|
pH level |
7.2 ± 0.4 |
|
Water salinity |
1-6% |
Growth performance
The growth performance was assessed by weighing all live fish at the end of 12 weeks of rearing, and recording the consumed feed. The percentage weight gain, specific growth rate (SGR), feed efficiency ratio (FER), and protein efficiency ratio (PER) were calculated according to documentation by Choi et al. (2016) and Yıldız et al. (2013). More specifically, the following formulae were followed in these calculations:

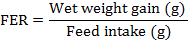
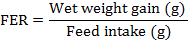
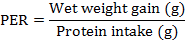
Hematological parameters
The hematological parameters were determined in non-coagulated blood that was collected at the end of the 12 weeks period, from the caudal tail blood vessel over heparin. The following hematological parameters were quantified namely, the red blood cell count, white blood cell count, hemoglobin concentration (Hgb), hematocrit percentage (Hct %), erythrocyte indices including the mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH) and mean corpuscular hemoglobin concentration (MCHC) (Hrubec and Smith, 2010).
Serum biochemical parameters
Plasma was separated from the non-coagulated blood by centrifugation at 3000 rpm for 10 min. The collected plasma was stored at -20°C for later analysis. The following parameters were quantified in the plasma namely the alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), lactate dehydrogenase (LDH), total protein (TP), serum albumin, cholesterol, triglycerides, and glucose level, by using commercial clinical chemistry kits (Biorex Diagnostics, Antrim, Northern Ireland) and an automated analyzer system reader (Roche, COBAS-MIRA Plus CC, Basel, Switzerland).
Statistical analysis
The means of measured parameters in the four treatments were compared statistically by one-way ANOVA at 5% confidence interval (P<0.05). The significant difference among the means was deduced from the analysis by post hoc Tukey’s test, using the SPSS version 23.
Results
Growth performance
At the end of day-84, data indicated that fish fed on 51% SBM in diet had least weight gain (P<0.05) as compared to other treatments. Similarly, SGR and FER were comparable decreased (P<0.05) in trout fed with diet 4 as compared to other three diets. However, no significant variation (P>0.05) was observed regarding PER value of trout fed on various types of diet as shown in Table IV.
Hematological and biochemical indices
Data demonstrated that trout fed with diet 4 had significantly (P<0.05) lowered WBC count, Hgb, Hct, MCV, MCH and MCHC when compared to other groups, but no significance difference (P>0.05) was observed on the basis of RBC count as shown in Table V.
Table V also revealed the impact of dietary inclusion of SBM on serum enzymatic activities such as ALT, AST, ALP and LDH as well as on TP, albumin, cholesterol, triglycerides and glucose level. No statistical difference (P>0.05) was noted in serum biochemical indices of trout fed on different concentrations of SBM.
Table IV.- Growth performance factors of the rainbow trout (Oncorhynchus mykiss) fed with different diets with increasing SBM and decreasing FM levels.
|
Parameters |
Diets |
|||
|
1 |
2 |
3 |
4 |
|
|
Initial weight (g) |
15.96 ± 0.85 |
15.92 ± 0.69 |
15.88 ± 0.87 |
15.88 ± 0.82 |
|
Final weight (g) |
126.56 ± 14.03a |
115.92 ± 10.92b |
110.78 ± 11.39b |
103.24 ± 12.14c |
|
Weight gain (%) |
695.11 ± 97.63a |
629.75 ± 77.70b |
599.36 ± 78.86b |
542.46 ± 86.14c |
|
SGR |
2.46 ± 0.14a |
2.35 ± 0.12b |
2.30 ± 0.13b |
2.20 ± 0.15c |
|
FER |
0.92 ± 0.11a |
0.90 ± 0.15b |
0.88 ± 0.17b |
0.85 ± 0.21c |
|
PER |
2.1 ± 0.2 |
2.0 ± 0.3 |
1.9 ± 0.1 |
1.8 ± 0.4 |
Diet 1 is control, with no inclusion of SBM. Diets 2, 3, and 4 has the following respective inclusion rate of SBM namely, 29%, 35%, and 51%, associated with the following respective inclusion rate of FM equivalent to 57%, 51%, and 35%. a-c, within a row, values (means ± SD) with different superscript letters were different (P<0.05) significantly. SGR, specific growth rate; FER, feed efficiency ratio; PER, protein efficiency ratio.
Table V.- Hematological parameters and serum biochemical indices in rainbow trout (Oncorhynchus mykiss) fed with different concentrations of soybean meal in diet.
|
Diets |
||||
|
1 |
2 |
3 |
4 |
|
|
Hematological parameters |
||||
|
RBC (×106µL) |
4.44 ± 0.05 |
4.45 ± 0.04 |
4.39 ± 0.05 |
4.35 ± 0.06 |
|
20.80 ± 1.25a |
20.27 ± 1.10ab |
20.21 ± 0.96ab |
20.13 ± 1.22b |
|
|
Hgb (g/dL) |
10.07 ± 0.13a |
10.06 ± 0.11a |
10.03 ± 0.11a |
09.94 ± 0.09b |
|
Hct (%) |
37.45 ± 0.39a |
37.28 ± 0.63ab |
37.24 ± 0.51ab |
37.05 ± 0.18b |
|
MCV (fL) |
86.07 ± 1.29a |
86.43 ± 1.37ab |
86.22 ± 1.31ab |
85.39 ± 1.19b |
|
MCH (pg/cel) |
23.09 ± 0.45a |
23.13 ± 0.56a |
22.96 ± 0.34ab |
22.78 ± 0.50b |
|
MCHC (%) |
27.24 ± 0.41a |
27.09 ± 0.35ab |
26.95 ± 0.33b |
26.83 ± 0.58b |
|
Biochemical indices |
||||
|
ALT (U/L) |
11.54 ± 1.22 |
11.98 ± 1.38 |
11.21 ± 1.15 |
11.01 ± 1.78 |
|
AST (U/L) |
307.82 ± 8.96 |
310.32 ± 7.98 |
311 ± 6.32 |
310 ± 9.51 |
|
ALP (U/L) |
1004.23 ± 124.74 |
1215.27 ± 35.45 |
1047.21 ± 112.32 |
1127.62 ± 93.24 |
|
LDH (U/L) |
1851.13 ± 105.65 |
1930.85 ± 67.85 |
1876.34 ± 113.42 |
1879.47 ± 98.50 |
|
TP (g/dL) |
5.22 ± 0.12 |
5.10 ± 0.50 |
5.09 ± 0.58 |
5.05 ± 0.33 |
|
Albumin (g/dL) |
0.73 ± 0.01 |
0.72 ± 0.03 |
0.70 ± 0.03 |
0.70 ± 0.02 |
|
Cholesterol (mg/dL) |
346.83 ± 2.73 |
346.18 ± 7.45 |
344.91 ± 8.32 |
343.21 ± 11.26 |
|
Triglycerides (mg/dL) |
133.25 ± 2.58 |
132.75 ± 5.66 |
132.98 ± 4.98 |
132.56 ± 3.71 |
|
Glucose (mg/dL) |
57.40 ± 2.88 |
57.07 ± 2.86 |
57.04 ± 4.20 |
56.62 ± 3.91 |
Diet 1 is control, with no inclusion of SBM. Diets 2, 3, and 4 has the following respective inclusion rate of SBM namely, 29%, 35%, and 51%, associated with the following respective inclusion rate of FM equivalent to 57%, 51%, and 35%. a-b, within a row, values (means ± SD) with different superscript letters were different (P<0.05) significantly. RBC, red blood cells; WBC, white blood cells; Hgb, hemoglobin; Hct, hematocrit; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALP, alkaline phosphatase; LDH, lactate dehydrogenase; TP, total protein.
Discussion
During the last few years, SBM is being used extensively as a replacement of FM in aquafeeds (Asche et al., 2013; García et al., 2015; Wu et al., 2016). FM is a most expensive protein source, let the nutritionist to find alternate economical plant protein source. SBM is a cost effective and easily available protein source of plant origin (Hien et al., 2015). Results of present research suggest that inclusion of SBM up to 35% in aquafeed was acceptable with no side effects on growth performance of rainbow trout. These results are according to previous documented literature. Inclusion of SBM up to 34% in diet of Juvenile Saddled Bream had no adverse effects on final weight gain, FCR and PER (Antolović et al., 2012). As a protein source, SBM could be included up to 30% in diet of yellowtail (Watanabe et al., 1992). For juvenile tin foil barb, diet containing up to 37% SBM could be used as an alternative to FM (Elangovan and Shim, 2000). Adding SBM up to 40% in diets of gilthead sea bream and of small size sharp snout was acceptable with no significant negative impact on their growth rate (Venou et al., 2006). Replacement of FP (75-90%) with SBM augmented with essential amino acids in aquafeed, could have beneficial effect on growth performance of hybrid striped bass and red drum (Gallagher, 1994).
FM in diet of blue catfish can totally be replaced by SBM if supplemented with methionine (Webster et al., 1992). Fish fed on heat treated soybean had the highest FCR, SGR, protein efficiency ratio and weight gain, thus confirming the possible use of soybean in aquafeed feed (Fafioye et al., 2005). Dietary inclusion of soybean meal supplemented with amino acids exhibit no significance impact on carcass composition of Nile Tilapia, Oreochromis niloticus (Abdel-Warith and Younis, 2013).
Our investigated hematological values in rainbow trout are almost similar to previously investigated values, which could be attributed to nearby dissolved oxygen concentration, freshwater temperature and pH level (Fazio et al., 2016). Hematological intervals have key role to monitor the health status of farmed channel fish (Tavares-Dias and Moraes, 2007). In current study, decreasing values of WBC count, Hct, MCV, MCH and MCHC were reported while increasing SBM level in diet of rainbow trout, suggesting that fish may have ability to consume fatty acids up to level of 35% in diet. Hgb level was not differ significantly in fish fed diet including SBM at level of 35%, indicating that oxygen carrying capacity of fish was not disturbed at this level of SBM. For diagnosis of anemia, MCH, MCH and MCHC are considered as major factors (Demir et al., 2014). Our findings suggest no anemic signs and symptoms in rainbow trout fed with SBM replacement. Multiple factors such as age, species, size, temperature as well as dietary composition are responsible for modifications in serum biochemical indices (Brunt and Austin, 2005; Li et al., 2015; Yeganeh et al., 2015). To our knowledge, very scarce literature has assessed the serum biochemical indices in response to partial replacement of dietary SBM in rainbow trout. Our results demonstrated that dietary inclusion of SBM at different concentration had no significant alterations in the levels of serum biochemical indices e.g. TP, albumin, cholesterol, triglycerides, glucose and in enzymatic activities including ALT, AST, ALP and LDH. Liver enzymatic parameters are often used as indicators of fish hepatotoxicity and tissue pathological changes (Nofouzi et al., 2017). Our results showed that inclusion of dietary SBM had no toxic effect on fish liver. Cholesterol lowering effects have been investigated in Tiger puffer (Takifugu rubripes) fed on SBM, which is in agreement to our results (Lim et al., 2011).
Conclusions
From this preliminary study, it can be concluded that partial replacement of SBM with FM up to concentration of 35% has beneficial effect on growth performance parameters. Moreover, dietary inclusion of SBM has no side effect on hematological and serum biochemical indices. Additional investigations are needed to be carried to evaluate the interaction between vegetable oils and various aquatic spices even on the molecular level.
Acknowledgements
Authors are thankful to chairman, Department of Pathology, University of Veterinary and Animal Sciences main Lahore, Pakistan, for providing laboratory and other necessities towards completion of research work.
Statement of conflict of interest
The authors declare that there are no conflicts of interests regarding the publication of this article.
References
Abdel-Warith, A.W.M. and Younis, E.M., 2013. Influence of dietary inclusion of full-fat soybean meal and amino acids supplementation on growth and digestive enzymes activity of Nile tilapia, Oreochromis niloticus. Turk. J. Fish. aquat. Sci., 13: 69-77.
Alam, M., Maughan, O. and Matter, W., 1996. Growth response of indigenous and exotic carp species to different protein sources in pelleted feeds. Aquacul. Res., 27: 673-679. https://doi.org/10.1111/j.1365-2109.1996.tb01302.x
Alp, A., Kara, C. and Büyükcapar, H.M., 2005. Age, growth and diet composition of the resident brown trout, Salmo trutta Macrostigma Dumeril 1858, in fırnız stream of the River Ceyhan, Turkey. Turk. J. Vet. Anim. Sci., 29: 285-295.
Antolović, N., Kožu, V., Antolović, M. and Bolotin, J., 2012. Effects of partial replacement of fishmeal by soybean meal on growth of juvenile saddled bream (Sparidae). Turk. J. Fish. aquat. Sci., 12: 247-252.
Asche, F., Oglend, A. and Tveteras, S., 2013. Regime shifts in the fishmeal/soybean meal price ratio. J. agric. Econ., 64: 97-111. https://doi.org/10.1111/j.1477-9552.2012.00357.x
Ávila, D.S., Sánchez, E.A., Hernández, L.H.H., Araiza, M.F. and López, O.A., 2015. Addition of yeast and/or phytase to diets with soybean meal as main protein source: Effects on growth, excretion and lysozyme activity in juvenile rainbow trout (Oncorhynchus mykiss Walbaum). Turk. J. Fish aquat. Sci., 15: 215-222.
Biswas, A.K., Kaku, H., Ji, S.C., Seoka, M. and Takii, K., 2007. Use of soybean meal and phytase for partial replacement of fishmeal in the diet of red sea bream, Pagrus major. Aquaculture, 267: 284-291. https://doi.org/10.1016/j.aquaculture.2007.01.014
Brunt, J. and Austin, B., 2005. Use of a probiotic to control lactococcosis and streptococcosis in rainbow trout, Oncorhynchus mykiss (Walbaum). J. Fish Dis., 28: 693-701. https://doi.org/10.1111/j.1365-2761.2005.00672.x
Choi, J., Rahman, M.M., Lee, S.Y., Chang, K.H. and Lee, S.M., 2016. Effects of dietary inclusion of fermented soybean meal with Phaffia rhodozyma on growth, muscle pigmentation, and antioxidant activity of juvenile rainbow trout (Oncorhynchus mykiss). Turk. J. Fish. aquat. Sci., 16: 91-101.
Demir, O., Türker, A., Acar, Ü. and Kesbiç, O.S., 2014. Effects of dietary fish oil replacement by unrefined peanut oil on the growth, serum biochemical and hematological parameters of Mozambique Tilapia juveniles (Oreochromis mossambicus). Turk. J. Fish. aquat. Sci., 14: 887-892. https://doi.org/10.4194/1303-2712-v14_4_06
Dogan, G. and Bircan, R., 2015. The Effects of diets containing hazelnut meal supplemented with synthetic lysine and methionine on development of rainbow trout, Oncorhynchus mykiss. Turk. J. Fish. aquat. Sci., 15: 119-126.
El-Sayed, A.F.M., 1999. Alternative dietary protein sources for farmed tilapia, Oreochromis spp. Aquaculture, 179: 149-168. https://doi.org/10.1016/S0044-8486(99)00159-3
Elangovan, A. and Shim, K., 2000. The influence of replacing fishmeal partially in the diet with soybean meal on growth and body composition of juvenile tin foil barb (Barbodes altus). Aquaculture, 189: 133-144. https://doi.org/10.1016/S0044-8486(00)00365-3
Fafioye, O., Fagade, S., Aa Adebisi, J.O. and Omoyinmi, G., 2005. Effects of dietary soybeans (Glycine max (L.) Merr.) on growth and body composition of African catfish (Clarias gariepinus, Burchell) fingerlings. Turk. J. Fish. aquat. Sci., 5: 11-15.
Fazio, F., Saoca, C., Piccione, G., Kesbiç, O.S. and Acar, Ü., 2016. Comparative study of some hematological and biochemical parameters of Italian and Turkish farmed rainbow trout Oncorhynchus mykiss (Walbaum, 1792). Turk. J. Fish. aquat. Sci., 16: 715-721. https://doi.org/10.4194/1303-2712-v16_3_25
Gallagher, M.L., 1994. The use of soybean meal as a replacement for fishmeal in diets for hybrid striped bass (Morone saxatilis × M. chrysops). Aquaculture, 126: 119-127. https://doi.org/10.1016/0044-8486(94)90253-4
García, V., Celada, J.D., González, R., Carral, J.M., Sáez-Royuela, M. and González, Á., 2015. Response of juvenile tench (Tinca tinca L.) fed practical diets with different protein contents and substitution levels of fishmeal by soybean meal. Aquacul. Res., 46: 28-38. https://doi.org/10.1111/are.12154
Hassan, A., Ishaq, M., Farooq, A. and Sadozai, S.H., 2007. Economics of trout fish farming in the northern areas of Pakistan. Sarhad J. Agric., 23: 407-410.
Heidarieh, M., Mirvaghefi, A.R., Sepahi, A., Sheikhzadeh, N., Alishahbazfar, A. and Akbari, M., 2013. Effects of dietary aloe vera on growth performance, skin and gastrointestine morphology in rainbow trout (Oncorhynchus mykiss). Turk. J. Fish. aquat. Sci., 13: 367-373. https://doi.org/10.4194/1303-2712-v13_2_20
Hernández, M., Martínez, F., Jover, M. and García, B.G., 2007. Effects of partial replacement of fishmeal by soybean meal in sharpsnout seabream (Diplodus puntazzo) diet. Aquaculture, 263: 159-167. https://doi.org/10.1016/j.aquaculture.2006.07.040
Hidalgo, M., Sanz, A., Gallego, M.G., Suarez, M. and De La Higuera, M., 1993. Feeding of the European eel Anguilla anguilla L. influence of dietary carbohydrate level. Comp. Biochem. Physiol. A Physiol., 105: 165-169. https://doi.org/10.1016/0300-9629(93)90190-F
Hien, T.T.T., Be, T.T., Lee, C.M. and Bengtson, D.A., 2015. Development of formulated diets for snakehead (Channa striata and Channa micropeltes): Can phytase and taurine supplementation increase use of soybean meal to replace fishmeal? Aquaculture, 448: 334-340. https://doi.org/10.1016/j.aquaculture.2015.06.020
Hrubec, T.C. and Smith, S.A., 2010: Hematology of fishes. In: Schalm’s veterinary hematology (eds. D.J. Weiss and K.J. Wardrop), 6th edn. Blackwell Publishing Ltd., Singapore, pp. 994-1003.
Korai, A., Sahato, G., Lashari, K. and Arbani, S., 2008. Biodiversity in relation to physicochemical properties of Keenjhar Lake, Thatta district, Sindh, Pakistan. Turk. J. Fish. aquat. Sci., 8: 259-268.
Li, C., Liu, P., Ji, H., Huang, J. and Zhang, W., 2015. Dietary n3 highly unsaturated fatty acids affect the biological and serum biochemical parameters, tissue fatty acid profile, antioxidation status and expression of lipid-metabolism-related genes in grass carp, Ctenopharyngodon idellus. Aquacul. Nutr., 21: 373-383. https://doi.org/10.1111/anu.12169
Lim, S.J., Kim, S.S., Ko, G.Y., Song, J.W., Oh, D.H., Kim, J.D., Kim, J.U. and Lee, K.J., 2011. Fishmeal replacement by soybean meal in diets for Tiger puffer, Takifugu rubripes. Aquaculture, 313: 165-170. https://doi.org/10.1016/j.aquaculture.2011.01.007
Muhammad, A.A., Farooq, S., Rabbaniha, M. and Hameed, A., 2017. Occurrence of Gardiner’s butterfly fish, Chaetodon gardineri (Norman, 1939),(Chaetodontidae) in coastal waters of Pakistan. J. Basic appl. Sci., 13: 182-184. https://doi.org/10.6000/1927-5129.2017.13.31
Nofouzi, K., Aghapour, M., Ezazi, A., Sheikhzadeh, N., Tukmechi, A., Khordadmehr, M., Akbari, M., Tahapour, K. and Mousavi, M., 2017. Effects of Verbascum speciosum on growth performance, intestinal histology, immune system and biochemical parameters in rainbow trout (Oncorhynchus mykiss). Turk. J. Fish. aquat. Sci., 17: 145-152.
Refstie, S., Korsøen, Ø.J., Storebakken, T., Baeverfjord, G., Lein, I. and Roem, A.J., 2000. Differing nutritional responses to dietary soybean meal in rainbow trout (Oncorhynchus mykiss) and Atlantic salmon (Salmo salar). Aquaculture, 190: 49-63. https://doi.org/10.1016/S0044-8486(00)00382-3
Şener, E., 2002. Farming of the rainbow trout, Oncorhynchus mykiss, in the black sea Region of Turkey. Turk. J. Fish. aquat. Sci., 2: 93-96.
Tacon, A., Haaster, J., Featherstone, P., Kerr, K. and Jackson, A., 1983. Studies on the utilization of full-fat soybean and solvent extracted soybean meal in a complete diet for rainbow trout. Nippon Suisan Gakk., 49: 1437-1443. https://doi.org/10.2331/suisan.49.1437
Tavares-Dias, M. and Moraes, F., 2007. Haematological and biochemical reference intervals for farmed channel catfish. J. Fish Biol., 71: 383-388. https://doi.org/10.1111/j.1095-8649.2007.01494.x
Venou, B., Alexis, M., Fountoulaki, E. and Haralabous, J., 2006. Effects of extrusion and inclusion level of soybean meal on diet digestibility, performance and nutrient utilization of gilthead sea bream (Sparus aurata). Aquaculture, 261: 343-356. https://doi.org/10.1016/j.aquaculture.2006.07.030
Watanabe, T., Viyakarn, V., Kimura, H., Ogawa, K. and Okamoto, N., 1992. Utilization of soybean meal as a protein source in a newly developed soft-dry pellet for yellowtail. Nippon Suisan Gakk., 58: 1761-1773. https://doi.org/10.2331/suisan.58.1761
Webster, C.D., Yancey, D.H. and Tidwell, J.H., 1992. Effect of partially or totally replacing fishmeal with soybean meal on growth of blue catfish (Ictalurus furcatus). Aquaculture, 103: 141-152. https://doi.org/10.1016/0044-8486(92)90408-D
Wu, Y., Wang, Y., Ren, G., Qin, J. and Kim, S., 2016. Improvement of fishmeal replacements by soybean meal and soy protein concentrate in golden pompano diet through γ-ray irradiation. Aquacul. Nutr., 22: 873-880. https://doi.org/10.1111/anu.12303
Yeganeh, S., Teimouri, M. and Amirkolaie, A.K., 2015. Dietary effects of Spirulina platensis on hematological and serum biochemical parameters of rainbow trout (Oncorhynchus mykiss). Res. Vet. Sci., 101: 84-88. https://doi.org/10.1016/j.rvsc.2015.06.002
Yıldız, M., Eroldoğan, O.T., Engin, K., Gülçubuk, A. and Baltacı, M.A., 2013. Effects of dietary cottonseed and/or canola oil inclusion on the growth performance, FA composition and organ histology of the juvenile rainbow trout, Oncorhynchus mykiss. Turk. J. Fish. aquat. Sci., 13: 453-464. https://doi.org/10.4194/1303-2712-v13_3_08
To share on other social networks, click on any share button. What are these?









