Effects of Aluminium Phosphide on the Behaviour, Haematology, Oxidative Stress Biomarkers and Biochemistry of African Catfish (Clarias gariepinus) Juvenile
Effects of Aluminium Phosphide on the Behaviour, Haematology, Oxidative Stress Biomarkers and Biochemistry of African Catfish (Clarias gariepinus) Juvenile
G.E. Odo1*, E.J. Agwu1, N.I. Ossai2, C.O. Ezea3, J. Madu1 and V. Eneje2
1Department of Zoology, University of Nigeria, Nsukka, Nigeria
2Enugu State University of Science and Technology, Enugu State
3Federal University of Technology, Owerri, Imo State
ABSTRACT
Aluminium phosphide is a cheap, effective and commonly used pesticide in agriculture for pest control in Nigeria. This study investigated the effects of aluminium phosphide on the behaviour, haematology, oxidative stress biomarkers and biochemistry of African catfish (C. gariepinus) juvenile on days 7, 14, 21 and 28. Fish were exposed to 0.175, 0.0875 and 0.035 mg/L corresponding to 1/10, 1/20 and 1/50 of the 96 h LC50 value (1.75) of the pesticide. Behavioral responses which include colouration of the fish skin and opercula colouration were observed. Aluminium phosphide elicited effects on the haematological parameters of the fish such as a decrease in Hb when compared to the control; reduction of MCV, MCH and MCHC to all exposed concentration of aluminium phosphide, significant increase in neutrophil, basophil, eosinophil and monocytes throughout the duration of exposure and decrease in the lymphocytes . The results on gill and liver tissues sampled showed concentration and time dependent significant increase (p < 0.005) in the values of malondialdehyde, catalase, superoxide dismutase, reduced glutathione, alkaline phosphatase (ALP), alanine aminotransferase (AST) and aspartate aminotransferase (AST). Similarly, aluminium phosphide enhanced levels of AST, ALT and ALP as both concentration and time dependent in elevation were recorded when compared to the control. The use of aluminium phosphide in agricultural fields near water bodies should be strongly monitored in view of the observed effects on this catfish fish physiology. The use of aluminium phosphide near water bodies should therefore strongly be monitored so that the health of aquatic organisms will not be impaired.
Article Information
Received 04 September 2015
Revised 13 January 2016
Accepted 02 May 2016
Available online 30 January 2017
Authors’ Contributions
GEO, EJA conceived, designed and supervised the project. NIO and COE performed experimental work and analysed the data. The manuscript was written by JM, VE and GEO.
Key words
Aluminium phosphide, Oxidative stress biomarkers, Clarias gariepinus
* Corresponding author: gregory.odo@unn.edu.ng
0030-9923/2016/0004-1161 $ 8.00/0
Copyright 2016 Zoological Society of Pakistan
DOI: http://dx.doi.org/10.17582/journal.pjz/2017.49.2.433.444
INTRODUCTION
The rising need to enhance agricultural practices and products has led to the global use of pesticides but these pesticides are hazardous substances which are eventually drained into the aquatic ecosystem where fishes live. Also, Agrochemicals are employed worldwide in agriculture to protect crop from pests, weeds, pathogens and parasites. One of these global pesticides is alunuium phosphide.
Aluminium phosphide is a cheap, effective and commonly used pesticide. It has also currently raised an interest in the past three decades, due to increased use in agricultural and non-agricultural purposes. Also, Glen (2014) reported that aluminium phosphide is used as both a fumigant for stored cereal and as a pesticide to kill small verminus mammals such as moles and rodents. Aluminium phosphide is an environmental toxicant and is among the other pesticides which cause environmentally-induced stresses to living organisms. These stresses frequently activate the endogenous production of reactive oxygen species (ROS), most of which are generated as side products of tissue/cellular respiration (Usman and Knowles, 2001). As a result, constant exposure to stressors may enhance ROS- mediated oxidative damage (Nishida, 2011). Oxidative stress is essentially an imbalance between the production of free radicals and the ability of the body to counteract or detoxify their harmful effects through neutralization (Nishida, 2011).
Oxidative stress is an unavoidable aspect of aerobic life. ROS are induced by substances such as transitional metal ions, pesticides, and petroleum pollutants (Slaninova et al., 2000; Lushchak, 2011). More so, mitochondrial respiration is the main endogenous source of ROS. Elevated production of ROS can cause oxidation of proteins and lipids, alterations in gene expression, and changes in cell radox status (Livingstone, 2003).
Mechanisms of antioxidant defenses in fish include the enzyme system and low molecular weight antioxidants (Givlio and Meyer, 2008). Superoxide dismutase (SOD) catalase (CAT), glutathione peroxides (GPX), and glutathione transferase (GST) are the main antioxidant enzymes and important indicators of oxidative stress (Nishida, 2011). Reduced glutathione (GSH) and oxidized glutathione disulphide (GSSG) play a key role in non-enzymatic antioxidant defense. Metal binding proteins such as territin ceruloplasmin and metallothioneins (MTs) have special functions in the detoxification of toxic metals and also play role in the metabolism and homeostasis of essential metals (Kelly et al., 1998). It has been reported that different fish species possess different isoforms of MTs (Smirnov et al., 2005).
Clarias gariepinus was selected for the present study because it is indigenous to Africa and can be found in other tropical countries of the world. It is of commercial importance and an aquaculture candidate that can narrow the gap between demand and supply of animal protein in developing countries. The species is also an attractive model of toxicological research because of its availability throughout the season, wide distribution in the environment and easy acclimatization to laboratory conditions.
There is a paucity of scientific documentation on aluminium phosphide effects on the behaviour, haematology, oxidative stress biomarkers and biochemistry in most indigenous fish species of Africa. Hence, this study investigated the effects of aluminium phosphide on the behaviour, haematology, oxidative stress biomarkers and biochemistry of African catfish (C. gariepinus) juvenile. The result of this study would provide information useful in management of aquatic systems and handling of chemical compound used in agriculture.
MATERIALS AND METHODS
The experimental fish
Freshwater African catfish C. gariepinus (Burchell 1822) (Familiy:Clariidae, Order: Siluriformes), mean standard length of 30.18±0.1cm and 159.8±5.4g weight were procured from Freedom Fisheries Ltd Nsukka, Enugu, Nigeria and transported to our wet laboratory where they were treated with 0.05% potassium permanganate ( KMnO4) for 2 min to avoid any dermal infections. The fish were acclimatized for three weeks in plastic tanks of 300 L capacity and fed ad libitum daily with commercial available food (Coppens International Helmond Netherlands) containing 35% crude protein. To maintain hygienic condition and prevent causes by food and feaces, feacal matter and other waste materials were siphoned off daily. Dead fish were removed with plastic forceps to avoid possible deterioration of water quality. During the period of acclimation, the water in the tanks was renewed daily with well aerated tap water. Feeding of the fish was terminated 24 h before the commencement of the acute toxicity test to determine the 96 h LC50 as suggested by Ward and Parrish (1982) and Reish and Oshida (1987). Ethical clearance from the University of Nigeria Committee on Experimental Animal Care (UNN-EGACC-006615) was obtained and strictly followed.
The mean water quality of the test water analyzed daily during the experimental period followed the standard methods (APHA, 2005). For the present study commercial formulation of aluminium phosphide manufactured by Orient Resources International Co., Ltd, China containing 58 g/L aluminium phosphide was purchased from the local market and used.
Acute toxicity bioassay and behavioural responses
Acute toxicity bioassay to determine the 96 h LC50 values of aluminium phosphide was conducted as per standard methods (APHA, 2005). A set of ten fish were randomly exposed to aluminium phosphide concentrations of 1.5,1.75 mg/l, and 2 mg/l derived from a range finding test using plastic aquaria (60 x 30 x 30 cm size) containing 25 L aerated tap water. Another set of 10 fish was simultaneously maintained in equal amount of tap water but without the test insecticide and considered as the control. The experiments were set in triplicate to obtain the LC50 values of the test chemical. Fish were not fed throughout the experiment and lethality was the toxicity end point. Fish were visually examined daily and considered dead when no sudden swimming in response to gentle touch was observed. Dead fish were removed with plastic forceps and the mortality was recorded at intervals of 24, 48, 72 and 96 h. The LC50 value of the test insecticide for the fish at 24, 48, 72 and 96 h was determined by probit analysis (Finney, 1971). The behavioral responses of C. gariepinus at different concentrations of aluminium phosphide were observed from 24 to 96 h of the exposure.
Sublethal concentrations and tissue preparations
The 96 h LC50 value of aluminium phosphide in the present study was determined to be 1.75 mg/l. Based on this value, three sublethal concentrations of 0.035, 0.0875 and 0.175 mg/L corresponding to 1/50, 1/20 and 1/10 of aluminium phosphide respectively were prepared by serial dilution of the stock solution and used for the main experiment to determine the oxidative stress biomarkers, biochemical, behavioural responses and haematological parameters effects of Aluminium phosphide on the fish. A total of 30 acclimatized fish were exposed to each of the aforementioned sublethal test concentrations in triplicates of 10 fish per replicate. Control fish specimens were maintained in dechlorinated tap water without aluminium phosphide. The exposure lasted for 28 days after which the fish were withdrawn from the insecticides and kept for 7days in dechlorinated tap water for possible recovery. One fish from each replicate treatment group and control was sacrificed after anesthetizing with tricaine methanesulfonate (MS 222) to minimize stress. The liver and gill tissues were dissected out and weighed separately. The tissues were quickly rinsed in cold 0.9 NaCl solutions. The tissues from each triplicate experiment were pulled together and homogenized immediately in pre-chilled potassium phosphate buffer (1:10W/V.0.1M, pH 7.0). One part of the homogenate was used for the estimation of thiobarbituric acid reactive substances (TBARS) and the other part was further centrifuged for 20 min at 10,500 under 4°C to obtain the supernatant which was stored at 4°C for the estimation of other biochemical parameters. For each of the parameters, five determinations were made and the average recorded as mean ±SE.
Estimation of lipid peroxidation
Tissue lipid peroxidation (LPO) was determined by the estimation of TBARS as described by Sharma and Krishna-Murti (1968). The TBARS concentration was measured by the absorption at 535nm at molar extinction coefficient of 156 mM/cm. The specific activity was expressed in nanomoles of TBARS/mg protein
Assay of antioxidant enzymes and tissue biochemistry
The glutathione peroxide (GPx) activity was determined by monitoring the rate of NADPH oxidation at 340 nm by the coupled reaction with glutathione reductase. The specific activity was determined using the extinction coefficient 6.22 mMcm-1 (Lawrence and Burk, 1976). The values were expressed in unit /min/mg protein. Tissues CAT activities were determined spectrophotometrically by measuring the rate of H202 breakdown based decreasein absorbance at 240 nm (Aebi, 1984) and the values were expressed in U/mg protein. The SOD activity was assayed at 420 nm following the methods of Misra and Fridovich (1972). The assay is based on the oxidation of epinephrine-adrenochrome transition by the enzyme and expressed in U/mg protein. The tissue alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels were determined in the different homogenates following the methods of Reitman and Frankel (1957), while aluminium phosphide was measured using commercially available kit KEE GAD Biogen Pvt. Ltd India).
Estimation of haematological parameters
Blood samples were obtained through tail vein puncture using an 18 G, 1.5 syringe according to Schmidt et al. (1996) and 2 ml was decanted into plastic tubes containing the sodium salt of ethylene diamine tetra acetic acid (Na-EDTA) as anticoagulants. Whole blood (50 µl) was stained for enumeration of red blood cells (Rusia and Sood, 1992). The number of red blood cells per cubic millimeter of blood (RBC) and the total leukocytes count (WBC) were determined using a Neubauer-type hemocytometer with Toisson’s solution as the diluting fluid for RBC and Turk’s solution for WBC (Rusia and Sood, 1992). The haemoglobin level of blood was estimated following the cyanomethemoglobin method (Blaxhall and Daisley, 1973) with some modifications. Each blood sample (0.02 ml) was mixed with 4 ml Drabkin’s solution and allowed to stand for 10 min for proper colour development after which absorbance was read at 540 nm in Unican spectrophotometer against a blank. Haematocrit (PCV) was analyzed by centrifugation of the blood for 5 min at 14,000 × g in heparinized glass capillaries using a micro haematocrit centrifuge (Hawkesley and sons, Ltd., Lancing, UK) at room temperature (Nelson and Morris, 1989). The PCV was read after centrifugation using the microhematocrit reader and the result expressed as the percentage of the whole blood. Hematological indices such as MCHC, MCH and MCV were calculated according to formula proposed by Dacie and Lewis (2001):
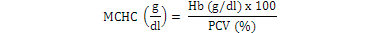
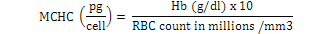

At the end of blood collection at each interval, the length and weight of fish specimens were measured and the liver and gills dissected out and weighed.
Statistical analysis
The data obtained, expressed as SE, were statically analyzed using the statistical package SPSS 17.0 computer program (SPSS Inc. Chicago, Illiois, USA). The data were subjected to three-way analysis of variance (ANOVA) to determine significant differences between tissues, concentration and sampling durations. A p-value less than 0.05 were considered statically significant.
RESULTS
The mean water qualities of the test water analyzed during the experimental period are presented in Table I.
Table I.- Physico-chemical parameters of the test water on C. gariepinus.
| Characteristics | Unit | Mean | Range |
| pH | - | 6.98 | 6.89-7.16 |
| Temperature |
0C |
26.40 | 25.10-27.0 |
| Conductivity |
µM cm-1 |
69.80 | 68.33-71.00 |
| Dissolved oxygen |
mg l-1 |
6.77 | 6.61-7.82 |
| Alkalinity |
mg l-1 |
24.16 | 25-27 |
| Total hardness |
mg l-1 |
6.04 |
5.99-6.28 |
Behavioural responses
The behavioural responses of the test fish were observed at 24 to 96 h of the exposure (Table II). In the control group, normal behavioural responses such as no hyperactivity, normal swimming patterns and fin movements were observed. Fish exposed to different concentrations of aluminium phosphide displayed behavioural abnormalities in response to the test chemical. At the initial exposure, fish were alert, stopped swimming and remained static in position in response to the sudden changes in the surrounding environment. After some few seconds, fish in the experimental group tried to avoid the test water by swimming rapidly and trying to jump out of the aquaria. Fish in the aquaria that had higher concentrations of the pesticides showed abnormal behavior and tried to avoid the test water by swimming very fast, jumping and displaying erratic with vigorous jerky movements, faster opercula movements and gulping of air indicate neurological impairment. Skin decoloration was mostly observed in fish that were exposed to the highest concentration of aluminium phosphide while it was least in the lowest concentration and this exhibited dermatological problems. This colour change in the fish was gray melanic colours through melanocyte-stimulating hormone–induced stimulation of melanin granule dispersed in the melanocytes. After sometime, the fish lost their balance and became exhausted owing to respiratory difficulty. They settled down weakly and tired at the bottom of the aquaria and this signified haematological difficulty. This clearly manifested a correlation between the skin decolouration and the hormone-enzyme activities.
Table II.- Effects of aluminium phosphide on behavioural responses of C. gariepinus juveniles.
| Duration | Conc. (mg/L) | Equilibrium status | Opercula movement | Fin movement | Opercula colouration | Skin discolouration |
| 24 h | Control | +++ | +++ | +++ | - | - |
| 1.5 | +++ | +++ | +++ | - | - | |
| 1.75 | +++ | ++ | ++ | ++ | + | |
| 2.0 | ++ | ++ | ++ | ++ | + | |
| 48 h | Control | +++ | +++ | +++ | - | - |
| 1.5 | +++ | +++ | +++ | - | - | |
| 1.75 | +++ | ++ | ++ | ++ | + | |
| 2.0 | ++ | ++ | ++ | ++ | + | |
| 72 h | Control | +++ | +++ | +++ | +++ | - |
| 1.5 | +++ | +++ | +++ | ++ | ++ | |
| 1.75 | ++ | ++ | ++ | ++ | +++ | |
| 2.0 | + | ++ | ++ | ++ | +++ | |
| 96 h | Control | +++ | +++ | +++ | - | - |
| 1.5 | ++ | +++ | +++ | - | ++ | |
| 1.75 | ++ | ++ | ++ | +++ | +++ | |
| 2.0 | ++ | ++ | ++ | +++ |
+++ |
- none, ++ mild, +++ strong.
Table III.- Effects of exposure to various sub lethal levels of AlP on RBC parameters in Clarias gariepinus.
| Parameters |
AlP (mgl-1) |
Exposure duration (days) |
|||
|
7 |
14 |
21 |
28 |
||
| PVC (%) | Control |
36.60 ± 2.15a1 |
36.06 ± 2.25a1 |
36.15 ± 1.18a1 |
36.25 ± 3.50a1 |
| 0.175 |
25.00 ± 2.17a2 |
26.50 ± 1.89a2 |
28.02 ± 0.95b2 |
29.03 ± 3.06b2 |
|
| 0.035 |
21.50 ± 1.18a3 |
23.00 ± 1.76a3 |
26.53 ± 1.01a2 |
27.52 ± 2.07c2 |
|
| 0.0875 |
25.00 ± 1.09a2 |
25.50 ± 2.86a2 |
27.03 ± 0.89a2 |
26.06 ± 2.08a23 |
|
| Hb(g/dL) | Control |
12.13 ± 1.63a1 |
12.03 ± 2.45a1 |
12.23 ± 2.45a1 |
12.06 ± 2.18a1 |
| 0.175 |
8.10 ± 1.14a2 |
8.10 ± 1.95a2 |
8.35 ± 1.16a2 |
8.95 ± 1.70a2 |
|
| 0.035 |
8.55 ± 1.06a2 |
7.60 ± 1.84a2 |
7.45 ± 1.18a2 |
8.50 ± 1.46a2 |
|
| 0.0875 |
8.50 ± 0.86a2 |
7.45 ± 1.05a2 |
7.55 ± 1.18a2 |
7.90 ± 1.02a2 |
|
|
WBC (×104/cells/mm3) |
Control |
10200 ± 10.11a1 |
10250 ± 9.06a1 |
10100 ± 9.80a1 |
10150 ± 10.40a1 |
| 0.175 |
8300 ± 10.06a2 |
9150 ± 9.85b2 |
8700 ± 9.40c2 |
8950 ± 11.40d2 |
|
| 0.035 |
8000 ± 10.42a3 |
9000 ± 9.45b2 |
7850 ± 6.40c3 |
8950 ± 10.01d2 |
|
| 0.0875 |
7900 ± 9.85a4 |
7150 ± 8.80b3 |
8200 ± 4.40c4 |
8500 ± 9.05d3 |
|
|
RBC(×106/cells/mm3) |
Control |
10.71 ± 1.19a1 |
10.60 ± 1.06a1 |
10.11 ± 1.14a1 |
10.60 ± 0.95a1 |
| 0.175 |
8.32 ± 1.06a1 |
9.30 ± 1.19a1 |
9.75 ± 0.90a1 |
10.08 ± 1.12a1 |
|
| 0.035 |
8.80 ± 1.10a1 |
9.55 ± 1.10a1 |
9.55 ± 1.14a1 |
10.79 ± 0.80a1 |
|
| 0.0875 |
9.25 ± 1.15a1 |
8.90 ± 0.90a1 |
10.50 ± 1.40a1 |
10.67 ± 0.80a1 |
|
| MCH (pg/cell) | Control |
11.32 ± 0.83a1 |
10.03 ± 0.72a1 |
9.89 ± 0.65a1 |
10.71 ± 0.49a1 |
| 0.175 |
9.74 ±0.81a1 |
8.70 ± 0.67a1 |
8.56 ± 0.24a1 |
8.88 ± 0.33a1 |
|
| 0.035 |
9.72 ± 0.67a1 |
7.95 ± 0.73a1 |
7.80 ± 0.55a1 |
7.88 ± 0.46a1 |
|
| 0.0875 |
9.91 ± 0.34a1 |
8.37 ± 0.53a1 |
7.18 ± 0.81a1 |
7.41 ± 0.38a1 |
|
| MCHC (g/dL) | Control |
33.14 ± 1.45a1 |
33.08 ± 1.50a1 |
32.60 ± 1.03a1 |
31.06 ± 2.02a1 |
| 0.175 |
32.41 ± 2.32a1 |
30.62 ± 1.96a1 |
29.81 ± 2.03a1 |
30.90 ± 1.82a1 |
|
| 0.035 |
39.88 ± 2.08a2 |
33.07 ± 1.18b1 |
28.11 ± 1.94c1 |
30.93 ± 1.16b1 |
|
| 0.0875 |
34.06 ± 2.11a1 |
29.34 ± 1.07b1 |
28.08 ± 1.06b1 |
30.41 ± 1.14b1 |
|
| MCV (fl/cell) | Control |
34.17 ± 2.06a1 |
33.20 ± 1.67a1 |
33.06 ± 1.01a1 |
34.03 ± 0.98a1 |
| 0.175 |
30.01 ± 1.13a1 |
28.50 ± 0.94a1 |
28.70 ± 1.09a1 |
28.19 ± 1.23a2 |
|
| 0.035 |
24.43 ± 1.06a2 |
24.15 ± 0.87a2 |
27.71 ± 0.97a2 |
25.53 ± 0.94a3 |
|
| 0.0875 |
27.03 ± 0.98a3 |
28.71 ± 0.77a1 |
25.77 ± 0.83ab3 |
24.44 ± 1.01b3 |
|
Values with different alphabetic (lowercase) superscripts differ significantly (p < 0.05) between different concentrations within the same exposure duration. Values with different numeric superscripts differ significantly (p < 0.05) between different exposure periods within the same concentration. Results are expressed as mean standard error of the mean. LlP, Aluminium phosphide.
Haematological parameters
The results of the haematological parameters of the control and experimental groups are presented in Table III. The red blood cells count (RBC) and haemoglobin in the experimental groups was significantly different from the control (p > 0.05) throughout the duration of the experiment and was significantly increased (p>0.05). There was significant difference in PCV values between the control and treated C. gariepinus on day 7 which subsequently increased significantly (p > 0.05) from day 14 of exposure. An aluminium phosiphide– induced dose and time dependent significant increase in the WBC count from day 14 onward (p >0.05) were observed while values of blood parameters (MCV, MCH and MCHC) in the experimental fish were not significantly different (p > 0.05) from the control group throughout the duration of the experiment. Changes in the mean values of the leukocyte differentials are presented in Table IV. There was dose and time dependent significant decrease (p < 0.05) in the levels of neutrophils when compared to the control throughout the experimental duration. The lymphocyte levels were significantly elevated (p > 0.05) from day 7 onward but the values of the monocytes, basophils and eosinophils were not significantly different (p>0.05) from the control.
Lipid peroxidation and antioxidant enzyme
The effects of different sub lethal concentrations of aluminium phosphide on lipid peroxidation in the form of TBARS formation and the responses of other antioxidant enzymes (GP, CAT and SOD) in the liver and gill tissues of C. gariepinus are presented in Table V. The LPO induction was both time and concentration dependent in both tissues with the lowest TBARS formation observed on day 7 of exposure. The values gradually increased as the experiment progressed with the highest formation recorded on day 28.
Administration of graded doses of aluminium phosphide did not cause significant changes in the liver MDA concentrations of juvenile catfish when compared to control values (p ≥ 0.05) (Table V). Time dependent declines were recorded in the liver MDA of fish treated with 0.175mg/L on days 14 – 28 when compared to day 7 (p ≤ 0.05).Similarly, significant decreases in the MDA concentrations was observed only on day 14 for the 0.175 and 0.0875 mg/L concentrations when compared to the control (p ≤ 0.05) in the gill. Time dependent elevations in the MDA concentrations were recorded for the 0.175 and 0.035 mg/L dosages on day 28 when compared to day 7. In the liver, significant changes occurred in the GSH concentrations of fish administered with 0.0875 mg/L and 0.035 mg/L on day 7 and day 14 respectively when compared to control values (p ≥ 0.05). In the gill of the fish, significant changes were recorded in the GSH values for the 0.0875 mg/L on day 14 when compared to the control values (p ≥ 0.05). Also, in the liver, significant time dependent declines were recorded in the SOD concentrations of fish administered 0.175 mg/L on day 14, and 0.035mg/L on day 21 when compared to day 7 values (p < 0.05).
| Parameters |
AlP (mgl-1) |
Exposure duration (days) |
|||
|
7 |
14 |
21 |
28 |
||
| Neutrophils | Control |
37.37 ± 4.54a1 |
37.07 ± 4.40a1 |
37.13 ± 3.70a1 |
37.11 ± 4.45a1 |
| 0.175 |
20.00 ± 3.45a2 |
22.50 ± 2.03a2 |
25.50 ± 2.40a2 |
31.10 ± 2.80a1 |
|
| 0.035 |
21.00 ± 2.16a2 |
18.04 ± 1.70a3 |
28.50 ± 2.45b23 |
30.05 ± 3.15b1 |
|
| 0.0875 |
19.50 ± 1.11a2 |
19.01 ± 1.16a3 |
29.05 ± 2.20b3 |
29.05 ± 2.45b1 |
|
| Basophils | Control |
0.00 ± 0.00a1 |
0.00 ± 0.00a1 |
0.00 ± 0.00a1 |
0.00 ± 0.00a1 |
| 0.175 |
1.00 ± 0.00a2 |
0.00 ± 0.00a1 |
0.00 ± 0.00a1 |
0.00 ± 0.00a1 |
|
| 0.035 |
0.00 ± 0.00a1 |
2.00 ± 0.01b2 |
1.50 ± 0.03b1 |
0.50 ± 0.02b2 |
|
| 0.0875 |
0.50 ± 0.01a2 |
1.00 ± 0.01b2 |
1.50 ± 0.22b1 |
1.00 ± 0.03b2 |
|
| Eosinophils | Control |
0.00 ± 0.00a1 |
0.00 ± 0.00a1 |
0.00 ± 0.00a1 |
0.00 ± 0.00a1 |
| 0.175 |
0.00 ± 0.00a1 |
0.50 ± 0.02b2 |
0.50 ± 0.03b2 |
0.50 ± 0.02b2 |
|
| 0.035 |
0.00 ± 0.00a1 |
0.50 ± 0.01b2 |
0.50 ± 0.02b2 |
0.50 ± 0.03b2 |
|
| 0.0875 |
0.50 ± 0.01b2 |
0.00 ± 0.00a1 |
1.50 ± 0.04b2 |
0.00 ± 0.00a1 |
|
| Monocytes | Control |
0.00 ± 0.00a1 |
0.00 ± 0.00a1 |
0.00 ± 0.00a1 |
0.00 ± 0.00a1 |
| 0.175 |
0.50 ± 0.01a2 |
1.00 ± 0.04a2 |
0.00 ± 0.00b1 |
0.00 ± 0.00b1 |
|
| 0.035 |
0.00 ± 0.00a1 |
0.50 ± 0.02b2 |
1.5 ± 0.05b2 |
0.50 ± 0.02b2 |
|
| 0.0875 |
1.00 ± 0.01a2 |
0.50 ± 0.02b2 |
0.5 ± 0.02b2 |
0.50 ± 0.02b2 |
|
| Lymphocytes | Control |
63.63 ± 3.64a1 |
63.24 ± 3.25a1 |
63.70 ± 3.01a1 |
63.50 ± 3.86a1 |
| 0.175 |
78.50 ± 2.85a2 |
76.02 ± 2.26a2 |
74.08 ± 3.11a2 |
68.50 ± 2.95b2 |
|
| 0.035 |
79.00 ± 2.51a2 |
79.03 ± 2.76a2 |
68.04 ± 4.02a2 |
68.52 ± 3.66b2 |
|
| 0.0875 |
78.50 ± 1.81a2 |
79.50 ± 2.45a2 |
68.05 ± 3.77b3 |
69.50 ± 3.76b2 |
|
Values with different alphabetic (lowercase) superscripts differ significantly (p < 0.05) between different concentrations within the same exposure duration. Values with different numeric superscripts differ significantly (p < 0.05) between different exposure periods within the same concentration. Results are expressed as mean standard error of the mean.
Table V.- Activity of lipid peroxidation (TBARS, nmol TBARS mg protein) glutathione peroxidase (GPx, nmol min mg protein), catalase (CAT, umol min mg protein ) and superoxide dismutase (SOD, U mg protein ) in the liver and gill tissues of C. gariepinus exposed to sublethal concentration (0.24 and 0.47 mgL) of aluminium phosphide.
| Parameters | Tissue |
Concentration (mg/l) |
Exposure duration( days) |
|||
|
7 |
14 |
21 |
28 |
|||
| LPO | Gill | Control |
0.99 ± 0.04a1A |
0.98 ± 0.09a1A |
0.98 ± 0.13a1A |
0.97 ± 0.12a1A |
| 0.175 |
0.49 ± 0.06a2B |
0.72 ± 0.11b2B |
0.72 ± 0.06b2B |
0.91 ± 0.06c1B |
||
| 0.035 |
0.53 ± 0.06a2B |
0.59 ± 0.06a2B |
0.65 ± 0.01a2B |
0.69 ± 0.03a1B |
||
| 0.0875 |
0.34 ± 0.01a2A |
0.55 ± 0.03a2B |
0.67 ± 0.08b2B |
0.76 ± 0.04c1B |
||
| Liver | Control |
1.24 ± 0.14a1A |
1.23 ± 0.12a1A |
1.04 ± 0.10a1A |
1.11 ± 0.13a1A |
|
| 0.175 |
0.69 ± 0.07a2B |
0.80 ± 0.09a1B |
1.25 ± 0.16b1C |
1.41 ± 0.15b1B |
||
| 0.0355 |
0.50 ± 0.03a2B |
0.94 ± 0.03b1B |
1.58 ± 0.11c1C |
1.36 ± 0.10c1C |
||
| 0.0875 |
0.50 ± 0.02a2B |
0.64 ± 0.02a2B |
0.95 ± 0.09b1C |
1.34 ± 0.23c1C |
||
| CAT | Gill | Control |
0.74 ± 0.03a1A |
0.71 ± 0.08 a1A |
0.69 ± 0.06a1A |
0.72 ± 0.06a1A |
| 0.175 |
0.68 ± 0.06a1A |
0.60 ± 0.07a1A |
0.63 ± 0.05a1A |
0.58 ± 0.03a1A |
||
| 0.035 |
0.62 ± 0.04a1A |
0.63 ± 0.04a1A |
0.67 ± 0.07a1A |
0.54 ± 0.01a1A |
||
| 0.0875 |
0.70 ± 0.03a1A |
0.64 ± 0.04a1A |
0.67 ± 0.09a1A |
0.56 ± 0.04a1A |
||
| Liver | Control |
0.89 ± 0.14a1A |
0.86 ± 0.09a1A |
0.87 ± 0.10a1A |
0.85 ± 0.12a1A |
|
| 0.175 |
0.75 ± 0.07a1A |
0.62 ± 0.06a1A |
079 ± 0.06a1A |
0.59 ± 0.02a1A |
||
| 0.035 |
0.67 ± 0.07a1A |
0.61 ± 0.04a1A |
0.73 ± 0.08a1A |
0.58 ± 0.04a1A |
||
| 0.0875 |
0.63 ± 0.04a1A |
0.60 ± 0.02a1A |
0.63 ± 0.04a1A |
0.57 ± 0.08a1A |
||
| SOD | Gill | Control |
94.61 ± 4.56a1A |
94.30 ± 2.85a1A |
93.80 ± 3.64a1A |
94.04 ± 3.05a1A |
| 0.175 |
87.70 ± 3.87a2A |
82.22 ± 3.06b2A |
76.80 ± 2.86a2A |
76.21 ± 3.16a2A |
||
| 0.035 |
85.21 ± 3.56a2A |
77.90 ± 2.65b2A |
76.81 ± 2.56b2A |
73.20 ± 2.86a2A |
||
| 0.0875 |
85.31 ± 2.97a2A |
75.40 ± 2.67b3A |
75.80 ± 2.07a2A |
74.04 ± 2.96b2A |
||
| Liver | Control |
96.71 ± 6.45a1A |
96.43 ± 4.45a1A |
95.63 ± 4.6 a1A |
95.85 ± 5.95a1A |
|
| 0.175 |
88.41 ± 5.87a2A |
86.22 ± 3.24a2A |
81.07 ± 4.18b1A |
76.71 ± 4.16b2A |
||
| 0.035 |
84.81 ± 4.76a2A |
81.02 ± 2.45a3A |
78.72 ± 3.95b2A |
74.67 ± 3.24b2A |
||
| 0.0875 |
86.55 ± 3.86a2A |
80.91 ± 2.76a3A |
78.61 ± 2.65c2A |
75.11 ± 2.62c2A |
||
| GPx | Gill | Control |
86.61 ± 4.45a1A |
86.24 ± 3.08a1A |
85.48 ± 4.15a1A |
85.93 ± 3.30a1A |
| 0,175 |
30.32 ± 2.45a2C |
29.21 ± 2.45a2C |
26.71 ± 2.22b2C |
23.61 ± 2.45b2C |
||
| 0.035 |
31.21 ± 2.01a2C |
30.61 ± 3.45a2C |
27.11 ± 2.11a23C |
23.73 ± 2.65b2C |
||
| 0.0875 |
29.02 ± 1.19a2C |
28.30 ± 2.85a2C |
29.40 ± 1.95a3C |
25.43 ± 2.09b2C |
||
| Liver | Control |
40.44 ± 2.11a1B |
40.85 ± 3.30a1B |
41.12 ± 3.16a1B |
40.23 ± 3.45a1B |
|
| 0.175 |
30.11 ± 2.06a2C |
25.16 ± 2.65a2C |
26.11 ± 2.56a2C |
25.11 ± 3.09a2C |
||
| 0.035 |
30.42 ± 2.07a2C |
27.71 ± 2.56a2C |
25.83 ± 1.84a2C |
24.06 ± 2.08a2C |
||
| 0.0875 |
31.14 ± 1.85a2C |
27.70 ± 2.45a2C |
26.55 ± 1.63a2C |
24.65 ± 1.95a2C |
||
| GR | Gill | Control |
4.76 ± 1.18a1A |
4.23 ± 1.19a1A |
4.06 ± 1.45a1A |
4.12 ± 2.06a1A |
| 0.175 |
2.77 ± 1.06a1A |
2.67 ± 0.93a1A |
2.49 ± 1.13a1A |
2.23 ± 0.18a2A |
||
| 0.035 |
2.39 ± 0.93a1A |
2.41 ± 0.85a1A |
2.41 ± 0.56a1A |
2.41 ± 0.93a2A |
||
| 0.0875 |
2.58 ± 0.94a1A |
2.53 ± 0.74a1A |
2.36 ± 0.16a2A |
2.30 ± 0.84a2A |
||
| Liver | Control |
5.60 ± 0.95a1A |
5.36 ± 1.16a1A |
5.40 ± 1.14a1A |
5.03 ± 0.96a1A |
|
| 0.175l |
3.15 ± 0.75a1A |
3.33 ± 0.85a1A |
2.91 ± 0.86a1A |
2.57 ± 0.34a2A |
||
| 0.035 |
3.52 ± 0.65a1A |
3.17 ± 0.75a1A |
2.85 ± 0.56a1A |
2.50 ± 0.14a2A |
||
| 0.0875 |
3.49 ± 0.65a1A |
3.25 ± 0.36a1A |
3.25 ± 0.36a1A |
2.68 ± 0.13a2A |
||
| MDA | Gill | Control |
2.52±22a2 |
3.51±19a1 |
2.55±21a2 |
3.11±28a12 |
| 0.175 |
2.73±09a2 |
2.42±24b2 |
3.09±20a12 |
4.02±46a1 |
||
| 0.035 |
2.34±07a1 |
2.38±26b1 |
8.86±6.33a1 |
3.83±40a1 |
||
| 0.0875 |
2.42±11a2 |
3.02±18ab12 |
3.05±41a12 |
3.74±03a1 |
||
| Liver | Control |
2.96±40a1 |
3.21±46a1 |
2.46±09a1 |
3.09±44a1 |
|
| 0.175 |
2.84±06a1 |
3.16±11a2 |
2.80±08a2 |
4.06±19a2 |
||
| 0.035 |
2.57±11a1 |
2.58±15a1 |
3.35±63a1 |
3.35±50a1 |
||
| 0.0875 |
2.71±13a1 |
2.80±34a1 |
2.76±21a1 |
3.16±64a1 |
||
Values with different alphabetic (lower case) superscripts differ significantly (p<05) between exposure durations and tissue. Values with different numeric superscripts differ significantly (p<.05) between concentrations within exposure duration and tissue. Values with different alphabetic superscripts (upper case) differ significantly (p50.05) between tissues within exposure duration and concentration. Units of measurements are: lipid peroxidation (TBARS, nmol TBARS mg protein-1), glutathione peroxidase (GPx, nmol min-1mg protein-1), catalase (CAT, mmol min-1mg protein-1), glutathione reductase (GR, nmol min-1mg protein-1) and superoxide dismutase (SOD, U mg protein-1).
Table VI.- Effects of aluminium phosphide on biochemical parameters on C. gariepinus.
| Parameters | Tissue |
Concen tration (mg/l) |
Exposure duration (days) |
|||
|
7 |
14 |
21 |
28 |
|||
| ALT | Liver | Control |
39.67±4.91ab1 |
0.67±88a2 |
13.00±2.08ab2 |
14.33±1.45ab2 |
| 0.175 |
31.67±4.18b1 |
14.00±5.29a2 |
10.00±58b2 |
11.00±1.00b2 |
||
| 0.035 |
51.33±2.60a1 |
9.33±1.20a2 |
15.00±2.08ab2 |
15.67±1.45a2 |
||
| 0.0875 |
46.00±3.21a1 |
17.67±3.48a2 |
17.33±65a2 |
16.33±88a2 |
||
| Gill | Control |
32.00±6.11ab1 |
13.00±1.00a2 |
14.67±2.67ab1 |
17.33±1.76a2 |
|
| 0.175 |
23.00±58b1 |
11.00±1.53ab2 |
13.00±00a2 |
11.33±88b2 |
||
| 0.035 |
40.00±4.04a1 |
8.00±1.55b3 |
19.33±2.40a2 |
17.67±1.45a2 |
||
| 0.0875 |
37.33±4.06a1 |
13.67±1.20a2 |
14.33±1.20a2 |
16.33±1.88ab2 |
||
| AST | Liver | Control |
13.00±3.21a2 |
51.0015.04a1 |
60.00±10.00a1 |
47.00±8.74a1 |
| 0.175 |
10.33±88a2 |
45.00±6.11a1 |
40.335.46b1 |
33.67±2.60a1 |
||
| 0.035 |
14.00±2.08a2 |
76.00±17.58a1 |
70.33±1.86a1 |
53.00±7.00a1 |
||
| 0.0875 |
17.00±1.53a2 |
73.00±2.52a1 |
66.00±1.73a1 |
49.67±2.73a2 |
||
| Gill | Control |
10.00±2.03b2 |
77.67±21.18a1 |
57.33±10.71ab1 |
34.00±11.72a12 |
|
| 0.175 |
9.00±1.53b2 |
38.00±6.11a1 |
35.00±1.00b1 |
39.33±1.53a1 |
||
| 0.035 |
11.00±1.00b2 |
69.67±20.85a1 |
63.33±6.17a1 |
48.33±4.98a1 |
||
| 0.0875 |
17.33±1.86a3 |
68.33±4.63a1 |
70.33±5.78a1 |
52.33±2.85a2 |
||
| ALP | Liver | Control |
159.87±39.43a1 |
127.26±8.92b1 |
132.49±6.06a1 |
118.02±4.91a1 |
| 0.175 |
166.52±27.11a1 |
121.59±5.35b12 |
112.83±8.66b3 |
122.69±3.33a12 |
||
| 0.035 |
181.99±8.88a1 |
150.45±7.31a2 |
131.42±51a23 |
121.17±8.63a3 |
||
| 0.0875 |
156.06±14.23a1 |
136.26±2.83ab12 |
134.01±2.81a12 |
123.34±2.11a2 |
||
| Gill | Control |
147.01±10.27a1 |
132.44±3.26a12 |
134.03±7.62a12 |
120.09±2.02a2 |
|
| 0.175 |
157.82±14.2a1 |
125.37±01a2 |
130.53±3.672 |
117.54±5.98a2 |
||
| 0.0175 |
142.87±5.24a1 |
139.06±7.79a12 |
13.84±4.73a12 |
119.25±5.44a2 |
||
| 0.0875 |
167.27±2.13a1 |
139.06±5.16a2 |
132.64±2.15a23 |
123.86±3.38a3 |
||
Mean values with different alphabets as superscripts in a column are significant (p < 0.05). ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase.
In the gill, the graded doses of aluminum phosphide did not elicit any significant changes in the SOD concentrations when compared with control values (p ≥ 0.05). Significant time – dependent reduction in the SOD concentrations were recorded on day 14 for the 0.175 and 0.0875 mg/L concentrations when compared to day 7 values (p ≤ 0.05). Significant change (decline) in the liver catalase concentration occurred only on day 21 for the 0.175 mg/L treatment (p ≤ 0.05), while other changes were not significant (p ≥ 0.05). Significant time dependent changes in the gill catalase concentrations of juvenile catfish were only recorded on days 14 and 21 when compared to the day 7 value (p ≤ 0.05) while other variations were similar (p ≥ 0.05).
Effect on biochemical parameters
The changes in tissue ALP, AST and ALT are presented in Table V1. Results showed time and concentration dependant significant decrease in ALP in both tissues throughout the experimental duration. There were fluctuations values of AST and ALT in the liver when compared to the control. This mixed trend was observed in the values in the gill tissue. While ALT values were comparable to the control throughout the experimental duration, AST values significantly increased from day 5 of exposure.
DISCUSSION
The C. gariepinus were exposed to varying concentrations of aluminium phosphide and this manifested various effects on the behaviour of the fish which included graying of the epidermal layer of the skin, yellowish colouration of the operculum, fin and opercula movement and eventually death. This was similar to the behavioural response observed by Edeh (2012) who reported that C. gariepinus fingerlings exhibited such changes in opercula movement and respiratory distress when exposed to varying concentrations of cassava effluents which contains the toxicant cyanide. These observations also corresponded with those of Oti (2002) who reported dark coloration, respiratory distress and increased opercula movements were observed when fishes were exposed to various concentrations of toxicants. This colour change in the fish was gray melanic colours through melanocyte-stimulating hormone –induced stimulation of melanin granule dispersed in the melanocytes. This behavior of pigment-containing cells is controlled by both the nervous and endocrine systems with more rapid changes typically reflecting neural control (Fujii, 2000).
The percentage mortality of C. gariepinus increased with increasing concentrations of aluminium phosphide. Moreover, in this study, the toxicity level of aluminium phosphide on C. gariepinus was found to be 1.75 mg/L. This was higher than the 1.05mg/L and lower than 13.6mg/L reported by Usman and Knowles (2001) and Das and Murkherjee (2003) when organophosphate derivatives-based pesticide were exposed to Labeo rohita fingerlings and larvae and adults of Helic oerpaze and Agrotis ipsolonm respectively. However, the 96 h LC50 value 1.75 mg/l is higher than 0.750 mg/l reported by Yaji et al. (2011) when Oreochromis niloticus were exposed to organophosphate commercial formulation pesticide.
Exposure of C.gariepinus to sublethal concentrations of aluminium phosphide elicited effects on the haematological parameters of the fish. There was a decrease in Hb when compared to the control; the reduction in this parameter may be attributed to haemolysis caused by the drug action on the fish. This is also an indication that the Hb biosynthetic was adversely affected. This could limit the oxygen-carrying capacity of the fish blood. The decrease may also be attributed to the limit in erythrocyte synthesis due to impaired osmoregulation across the gill epithelium (Saravanan et al., 2011; Pereira et al., 2013). During the treatment, aluminium phosphide can be absorbed easily through vascularized surfaces like the gastro intestinal tracks (GIT) and the gills. This absorption was made possible due to the lipophilic nature of aluminium phosphide that enable it pass easily through the membranes hence the observed decrease of Hb in the hematological parameters in our present study. Further, blood indices are often subjected to variations depending upon stress and environmental factors ( Hlavova, 1993). Exposure of aluminium phosphide stimulate the T-lymphocyte cells in the lymphomyeliod tissue as defense mechanism against the stressor hence the observed proliferation of WBC in the peripheral blood (Campbell, 1996). WBCs are involved in the regulation of immunological function in many organisms and the observed increase in many organisms in the WBC count in aluminium phosphide-treated fish after some days of exposure indicated immune and protective response. Similarly, to these present finding, Saravanan et al. (2012) reported a significant increase in WBCs in C. carprio exposed to pharmaceutical drugs clofibric acid and diclofenac. Significant increase in WBCs has also been reported in Cirrhinus mirigala (Saravanan et al., 2012) after exposure to different concentrations of pharmaceutical drug ibuprofen. The data on MCV, MCH and MCHC showed a significant decrease to all exposed concentration of aluminium phosphide. The decreased MCV shows that erythrocytes shrunk as a result of water imbalance, stress or the production of immature erythrocytes. The significant reduction in MCV in aluminium phosphide-treated fish, when compared to the control, is an evidence of the production and subsequent release of immature erythrocytes into the general circulation to compensate for the adverse effects of on the haemopoietic tissues of the fish. The significant reduction in MCH and MCHC is a good indication of defective Hb biosynthesis in the fish. Similar decreases in MCH, MCV, and MCHC have been reported in fish exposed to different concentrations of pharmaceutical drugs (Kasagala and Pathiratne, 2008; Velisek et al., 2009; Li et al., 2011). Changes in leucocyte differentials are recognized as a sensitive indicator of environmental stress and provide an overview of the integrity of the immune system (Cole et al., 2001). Neutrophils and lymphocytes make up the majority of WBC and proliferate in circulation in response to stress (Jain, 1993; Thrall, 2004). The observed significant increase in neutrophil, basophil, eosinophil and monocytes throughout the duration of exposure may have been provoked by the stress imposed by aluminium phosphide on the fish. The lymphocytes however, decreased as duration lasted from 7-28 days.
Exposure of fish to different contaminants have been known to present substantial variability effects in most physiological and biochemical variables (Sadauskas-Henrique et al., 2011). Antioxidant levels, enzymological activity and haematological profiles are widely used as indicators to assess toxic stress, functional status and homeostasis in animals (Saravanan et al., 2012; Gecit et al., 2014). In the present study, our data demonstrated that exposure to sublethal concentration of aluminium phosphide increased in a time and concentration-dependent manner, for example in the levels of MDA in both tissues (gill and liver). Time dependent declines were recorded in the liver MDA values on the gill for 0.175 mg/l and 0.0875 mg/l on day 7 but on day 28, there was time dependent elevation thus reflecting increased oxidative stress and lipoperoxidation. Cellular oxidative stress results when the balance between pro-oxidants and antioxidants are disrupted leading to excessive generation of reactive oxygen species (Dabas et al., 2011). ROS generated can react with biological molecules and cause increase in lipid peroxidation, DNA damage and protein oxidation resulting in disturbance in cell physiological processes (Tejada et al., 2007). MDA is one of the end products in the lipid peroxidation process and thus the elevated values of MDA values obtained in the present study are in agreement with previous reports in fish exposed to different herbicides (Modesto et al., 2011; Guilherme et al., 2012; Blahova et al., 2013) and other toxicants (Li et al., 2011; Zhang et al., 2013; Adeyemi, 2013). CAT on the other hand degrades hydrogen peroxide which results from the degradation of the anion superoxide by the enzyme SOD. Under exposure to aluminium phosphide, in C. gariepinus catalase in the fish liver showed significant elevation on day 14 when compared to the control values and no significant change was observed in the gills. In addition, no significant change was recorded in SOD of the fish tissues. SOD plays an important role and helps to convert superoxide radical to hydrogen peroxide for possible conversion to water and molecular water by CAT (Shao and Dong, 2012). This minor elevation of CAT probably was not sufficient to remove the ROS and neutralize oxidative stress as significant increase in MDA observed in the tissues throughout the duration of the experiment. This suggested that oxidative stress can be imposed by the presence of the pesticide aluminium phosphide and the ROS produced may subsequently react with biomolecules resulting in oxidative stress to cellular components. Similar results have been reported in C. gariepinus exposed to butachlor by Farombi et al. (2008). The effects of aluminium phosphide on biochemical parameters such as AST, ALT and ALP have been used widely in ecotoxicological studies to assess stress induced by various contaminants in the environment (El-Sayeed, 2007). Furthermore, enzymes such as AST and ALT have been used to determine pollution exposure and serve as good bioindicators (Larvanya et al., 2011). In the present study, exposure of C. gariepinus to aluminium phosphide enhanced levels of AST, ALT and ALP as we recorded both concentration dependent and time dependent in elevation which however varied and were comparable to the control throughout the exposure. This is in agreement with the work of Nwani et al. (2014) whose results showed concentration and time dependent increase in biochemical parameters of tissues of C. gariepinus exposed to the herbicide, Primextra. The increase in these parameters indicated enhanced transamination which is a sensitive indicator of stress imposed by the pesticide. Increased transaminations during pesticide challenge have attributed to the need to meet higher energy demand by fish (Saravanan et al., 2012) Increased activities of these parameters were also observed in common carp, Cyprinus carpio exposed to the herbicide, Gordoprin Plus, with triazine and S-metalochlor as active ingredients (Dobsikova et al., 2011) and in other fish exposed to different environmental toxicants (Prusty et al., 2011; Saravanan et al., 2012).
CONCLUSION
This study showed various effects of A. phosphide on the behaviour of the fish which included graying of the epidermal layer of the skin, yellowish colouration of the operculum, fin and ope+rcula movement and eventually death. Also, sublethal concentrations of aluminium phosphide elicited effects on the haematological parameters of the fish such as a decrease in Hb when compared to the control; reduction of MCV, MCH and MCHC to all exposed concentration of aluminium phosphide, significant increase in neutrophil, basophil, eosinophil and monocytes throughout the duration of exposure and decrease in the lymphocytes. Similarly, aluminium phosphide enhanced levels of AST, ALT and ALP as both concentration dependent and time dependent in elevation were recorded when compared to the control. The use of aluminium phosphide in agricultural fields near water bodies should be strongly monitored in view of the observed effects on this catfish fish physiology. The environmental authorities especially in Nigeria needs to set up new environmental laws as well as environmental assessment criteria to regulate the usage of aluminium phosphide and other pesticides. This will help reduce the influx of these toxicants entering into the aquatic environment.
Statement of conflict of interest
Authors have declared no conflict of interest.
REFERENCES
Aebi, I.H.L., 1984. Catalase in vitro. Meth. Enzymol., 105: 121-126, Academic Press, Orland. https://doi.org/10.1016/S0076-6879(84)05016-3
Amiard, T.C., Rainbow, P.S., Amiard, J.C., Barka, S. and Pellenin, J., 2006. Methallothioneins in aquatic invertebrates: Their role in metal detoxification and their use as biomarkers. Aquat. Toxicol., 76: 160-202. https://doi.org/10.1016/j.aquatox.2005.08.015
Blahov, A.J., Pihalova L, and Hostovsky, M., 2013. Oxidative stress response in Zebra fish Danio reri after subchronic exposure to antrazine. Fd. Chemist. Toxicol., 61: 82-85. https://doi.org/10.1016/j.fct.2013.02.041
Blaxhall, P.C. and Daisley, K.W., 1973. Routine haematological methods for use with fish blood. J. Fish Biol., 5: 771–781. https://doi.org/10.1111/j.1095-8649.1973.tb04510.x
Cole, M.B., Arnold, D.E., Watten, B.J. and Krise, W.F., 2001. Haematological and physiological responses of brookcharr to untreated and limestone neutralised acid. J. Fish Biol., 59: 79–91. https://doi.org/10.1111/j.1095-8649.2001.tb02339.x
Dabas, A., Nagpure, N.S. and Kumar, R., 2011. Assessment of tissue specific effect of cadmium on antioxidant defense system and lipid peroxidation in freshwater murrl, Channa punctatus. Fish Physiol. Biochem., 38: 469-482. https://doi.org/10.1007/s10695-011-9527-7
Dacie, J.V. and Lewis, S.M., 1984. Practical hematology. 6th ed. Churchill, New York, London.
Das, A.C. and Mukherjee, D., 2003. Effect of the herbicides oxadiazon and oxyfluoren on phosphates solubility microorganisms and their persistence in rice fields. Chemosphere, 53: 217-221. https://doi.org/10.1016/S0045-6535(03)00440-5
Dobiskova, R., Blahova, J. and Modra, H., 2011. The effects of acute exposure to herbicide Gardoprim plus Gold 500 SC on hematological and biochemical indicators and hisopathological changes in common carp (Cyprinus carpio). Acta Vet. Brno, 80: 359-363. https://doi.org/10.2754/avb201180040359
El-Ssyed, Y.S., Saad, T.T. and El-Bahr, S.M., 2007. Acute intoxication of deltamethrin in monsex Nile tilapia, Oreochromis niloticus with special reference to the clinical biochemical and hematological effects. Environ. Toxicol. Pharmacol., 24: 212-217. https://doi.org/10.1016/j.etap.2007.05.006
Finney, D.J., 1971. Probit analysis, 3rd edition Cambridge University Press, Cambridge, pp. 20.
Fujii, R., 2000. Pig cell research ,The regulation of motile activity in fish chromatophores. Pigment Cell Res., 13: 300-319. https://doi.org/10.1034/j.1600-0749.2000.130502.x
Givllo, R.T. and Meyer, J.N., 2008. Reactive oxygen species and oxidative stress. In: The toxicology of fishes (eds R.T. Di-Givlio and D.E. Hinton). CRC Press, Taylor and Francis Group, pp. 273-324.
Glen, V.D.K., 2014. Effects of atrazine in fish, amphibians, and reptiles: an analysis based on quantitative weight of evidence. Rev. Toxicol., 44: 1-66.
Guilherme, S., Gaivao, I., Santos, M.A. and Pacheco, M., 2012. DNA damage in fish (Anguilla anguilla) exposed to a glyphosate based herbicide elucidation of organ-specificity and the role of oxidative stress. Mutat. Res. Gen. Toxicol. Environ. Res., 743: 1-9. https://doi.org/10.1016/j.mrgentox.2011.10.017
Hlavova, V., 1993. Reference values of the haematological indices in grayling Thymallus thymallus Linnaeus). Comp. Biochem. Physiol., 105A: 525–532. https://doi.org/10.1016/0300-9629(93)90429-8
Jain, N.C., 1993. Essentials of veterinary haematology. Blackwell, Philadelphia.
Kasagala, K.H.D.T. and Pathrinem, A., 2008. Effects of waterborne chloramphenicol and oxytetracycline exposure on haematological parameters and phagocytic activity in the blood of Koi carp, Cyprinus carpio. In: Diseases in an Asian acqaculture VI (eds. M.G., Bondad-Reantaso, C.V., Mohan, M., Crumlish and R.P. Subasinghe). Asian Fisheries Society, Fish Health Section. Malina, Philippine, pp. 283-296.
Kelly, S.A., Aavailla, C.H.M., Brady, T.C., Abramo, K. H. and Levin, E.D., 1998. Oxidadtive stress in toxicology: established mammalian and emerging piscine model systems. Environ. Hlth. Persp., 106: 375-384. https://doi.org/10.1289/ehp.98106375
Lavanya, S., Ramesh, M. and Kavitha, C., 2011. Hematological, and biochemical responses of of Indian major carp Catla catla during chronic sublethal exposure to inorganic arsenic. Chemosphere, 82: 265-275. https://doi.org/10.1016/j.chemosphere.2010.10.071
Lawrence, R.A. and Burk, R.F., 1976. Glutathione peroxidase activity in selenium deficient rat liver. Biochem. biophys. Res. Commun., 71: 952-958. https://doi.org/10.1016/0006-291X(76)90747-6
Li, Z.H., Velisek. J., Zlabek, V. and Grabic, R., 2011. Chronic toxicity of verapamil on juvenile rainbow trout (Oncorhynchus mykiss): Effect on morphological indices, hematological parameters and antioxidant responses. J. Haz. Mat., 185: 870-880. https://doi.org/10.1016/j.jhazmat.2010.09.102
Lushchak, V., 2011. Environmentally induced oxidative stress in aquatic animals. Aquat Toxicol., 1: 13-30. https://doi.org/10.1016/j.aquatox.2010.10.006
Modesto, K. A. and Martinez, C.B.R., 2010. Roundup cause oxidative stress in liver and inhibits acetylcholinesterase in muscles and brain of catfish Prochilodus lineatus. Chemosphere, 78: 294-299. https://doi.org/10.1016/j.chemosphere.2009.10.047
Nelson, D.A. and Morris, M.W., 1989. Basic methodology, hematology and coagulation, part IV. In: Clinical diagnosis and management by laboratory methods (eds. D.A. Nelson and J.B. Henry JB). 17th ed. Philadelphia (PA): W.B. Saunders, pp. 578-625.
Nishida, B., 2011. The chemical process of oxidative stress by copper (II) and iron (III) ions in several nervous degenerative disorders. Monatsh. Furchem., 142: 375-384.
Nwani, C.D., Ifo, T.C., Nwamba, O.H., Ejere, V.C., Onyishi, C.G., Oluah, N.S., Ikwuagwu, E.O. and Odo, G.E., 2015. Oxidative stress and biochemical responses in the tissues of African catfish Clarias gariepinus juvenile following exposure to primextra herbicide. Drug Chem. Toxicol., 38: 275-285. https://doi.org/10.3109/01480545.2014.947503
Ohkawa, H., Yagi, K. and Osishi, N., 1979. Assay for lipid peroxide in animal tissue by thiobarbituric acid reaction. Anals. Biochem., 95: 321-358. https://doi.org/10.1016/0003-2697(79)90738-3
Oti, E.E., 2002. Acute toxicity of cassava mill effluent to the African catfish fingerlings. J. aquat. Sci., 17: 31-34. https://doi.org/10.4314/jas.v17i1.19907
Pereira, L., Fernandes, M.N. and Martinez, C.B.R., 2013. Haematological and biochemical alterations in the Fish Prochilodus lineatus caused by the herbicide clomazone. Environ. Toxicol. Pharmacol., 36: 1–8. https://doi.org/10.1016/j.etap.2013.02.019
Prusty, A.K., Kohli, M.P.S. and Sahu, N.P., 2011. Effect of short term exposure tofenvalerate on biochemical and hematological responses in Labeo rohita (Hamilton) fingerling. Pestic. Biochem. Physiol., 100: 124-129. https://doi.org/10.1016/j.pestbp.2011.02.010
Reitman, S. and Frankel, S., 1957. A colorimetric method for the determination of serum glutamic oxalo acetic and glutamic pyruvic transaminase. Am. J. clin. Pathol., 766: 28-56.
Richter, B.D., Brain, D.P., Meldelson, M.A. and Master, L.L., 1997. Threats to imperiled fresh water fauna. Conserv. Biol., 11: 1081-1093. https://doi.org/10.1046/j.1523-1739.1997.96236.x
Sadauskas, H.H., Sakuragui, M.D. and Paulino, M.G., 2011. Using condition factor and blood variable biomarkers in fish to assess water quality. Environ. Monit. Assess., 181: 29-42. https://doi.org/10.1007/s10661-010-1810-z
Saravanan, M., Devi, U.K. and Malarvizhi, A., 2012. Effects of ibuprofen on hematological, biochemical and enzymological parameters of blood in Indian major carp Cirrhinus mrigala. Environ. Toxicol. Pharmacol., 34: 14-22. https://doi.org/10.1016/j.etap.2012.02.005
Shao, B. and Dong, M., 2012. DNA damage and oxidative stress induced by endosulfan exposure in Zebra (Danio rerio). Ecotoxicology, 21:1533-1540. https://doi.org/10.1007/s10646-012-0907-2
Sharma, S.K. and Krishna-Murti, C.R., 1968. Production of lipid peroxides by brain. J. Neurochem., 15: 147-149. https://doi.org/10.1111/j.1471-4159.1968.tb06187.x
Slaninova, M., Nagyova, B., Galova, E., Hendrychova, J., Bisova, K., Zachleder, V. and Vicek, D., 2000. Cell wall and cytoskeleton reorganization as the response to hypperostomatic stock in Saccharomyces cerevisiae. Arch. Microbiol., 173: 245-252. https://doi.org/10.1007/s002030000136
Smirnov, L.P., Sukhovskaya, I.V. and Nemova, N.N., 2005. Effects of environmental factors on low- molecular with peptides of fishes: A review. Russ. J. Ecol., 36: 41-47. https://doi.org/10.1007/s11184-005-0007-0
Tejada, S., Sureda, A. and Roca, C., 2007. Antioxidant response and oxidative damage in brain cortex after high of Pilo carpine. Brain Res. Bull., 71: 372-375. https://doi.org/10.1016/j.brainresbull.2006.10.005
Usman, K.A. and Knowles, C.O., 2001. Toxicity of pyrethroids and effects of synergists to larva and adult Helic overpazea, Spodoptera frugiperda and Agroisip silon (Lepidoptera, Noctuidae). J. Ent., 94: 868-873.
Velisek, J., Stesjskal, V., Kouril, J. and Svobodovo, Z., 2009. Comparison of the effects of four anesthetics on biochemical blood profiles of perch. Aquacult. Res., 40: 354–361. https://doi.org/10.1111/j.1365-2109.2008.02102.x
Yaji, A.J., Acute, J. and Onyinye, S.J., 2011. Effect of cypermethrin on behaviour and biochemical indices of freshwater fish, Oreochromis niloticus. Electron J. Environ. Agric. Fd. Chem., 10: 1927-1934.
Zhang, D.L., Hu, C.X. and Li, D.H... 2013. Lipid peroxidation and antioxidant responses in zebra fish brain induced by Aphanizonmenon flosaquae DC-1 aphantoxins. Aquat. Toxicol., 145: 250–256. https://doi.org/10.1016/j.aquatox.2013.10.011
To share on other social networks, click on any share button. What are these?










