Effect of some Nematophagous Fungi on Reproduction of a Nematode Pest, Heterodera schachtii, and Growth of Sugar Beet
Effect of some Nematophagous Fungi on Reproduction of a Nematode Pest, Heterodera schachtii, and Growth of Sugar Beet
Manzoor Hussain*, Miloslav Zouhar and Pavel Ryšánek
Department of Plant Protection, Faculty of Agrobiology, Food and Natural Resources, Czech University of Life Sciences, Prague, Czech Republic
ABSTRACT
Heterodera schachtii is a globally important and often marginalized pest of sugar beet, cabbage, broccoli and radish, among other crops. It is a cyst-forming nematode that affects plant growth and yield. We report on studies aimed to evaluate the effects of five nematophagous fungi on the population dynamics of this pest in sugar beets in laboratory and greenhouse trials. The fungi chosen were Arthrobotrys oligospora, Dactylella oviparasitica, Clonostachys rosea, Stropharia rugosoannulata, and Lecanicillium muscarium. In the laboratory experiment, S. rugosoannulata proved to be the most efficient biocontrol agent by parasitizing the maximum number of eggs, whereas D. oviparasitica appeared to be the least efficient after 72 hours. The greatest numbers of cysts and eggs were found to be colonized with L. muscarium during microscopic observations. In the greenhouse experiment, L. muscarium had significant effects in reducing the nematode population in soil compared to the other treatments. In regard to the growth parameters, root and shoot growth (cm) were enhanced after the application of L. muscarium, followed by D. oviparasitica and S. rugosoannulata. The reproductive rate (Rf = Pf/Pi) of nematodes was much higher in the non-treated plants than those that were treated. The root quality of the fungus-treated plants was significantly improved. All fungi conclusively proved to be effective against H. schachtii and need to be further investigated at the molecular level.
Article Information
Received 03 July 2016
Revised 23 July 2016
Accepted 08 August 2016
Available online 30 November 2016
Authors’ Contributions
MH designed experiments, collected data and wrote article, MZ and PR advised the study and proofread article.
Key words
Heterodera schachtii, Nematophagous fungi, Sugar beet, Lecanicillium muscarium, Stropharia rugosoannulata.
* Corresponding author: [email protected]
0030-9923/2017/0001-0197 $ 9.00/0
Copyright 2017 Zoological Society of Pakistan
Introduction
Various phytoparasitic nematodes pose a great threat to several vegetable and field crops worldwide, but sedentary endoparasitic nematodes from the genera Meloidogyne, Globodera, and Heterodera are the most important (Anwar and Mckenry, 2012; Singh and Kumar, 2015; Greco et al., 1993; Sasser and Freckman, 1987). The sugar beet cyst nematode, Heterodera schachtii Schmidt, is an alarming pest of sugar beet (Beta vulgaris L.) and many other plant species. Second-stage juveniles (J2), an infective stage of nematodes, penetrate into the roots of host plants, where they subsequently congregate and sustain specialized feeding structures called syncytia (Jones, 1981; Hussey, 1989; Wyss et al., 1992; Sijmons et al., 1994; Williamson and Hussey, 1996). Plants infested with nematodes incite or exacerbate the effects of other pathogens (pests) by worsening disease through decreased growth, wilted leaves, abnormal root development and the reduction of sugar contents. Heterodera schachtii is a main hindrance in the production of sugar beets in central Europe, where it is responsible for economic losses estimated to be 90 million Euros annually (Müller, 1989, 1999). Higher soil population levels of this nematode result in potential losses of sugar beet and sugar yields (Heijbroek et al., 2002; Heinrichs, 2011; Kenter et al., 2014; Hauer et al., 2016).
Management of the sugar beet nematode is challenging, as this nematode species has the ability to survive in a protective cyst for many years (Sharma, 1998). Researchers are struggling to manage cyst-forming nematode species using various alternative strategies such as the use of organic amendments (Renčo et al., 2007; Renčo and Kováčik, 2012, 2015), soil solarization, biofumigation, fallowing land, rotating crops with non-hosts or trap crops (Renčo et al., 2012; Renčo, 2013), and planting antagonistic plants. However, these strategies may be either very expensive for farmers or not effective because of the high cost-benefit ratio and pollution (Hussain et al., 2016).
The term “nematophagous fungi” is defined as a group of organisms with the ability to infect and parasitize nematodes to obtain nutrients for survival. Based on their modes of action, nematophagous fungi are categorized into four groups: nematode-trapping (formerly called predatory fungi), endoparasitic, egg- and female-parasitic and toxin-producing fungi (Barron, 1997; Dackman et al., 1992; Jansson and Lopez-Llorca, 2001). The interactions between nematophagous fungi and their hosts include various steps of recognition (attraction phenomena, contact), the production of adhesives and lytic enzymes, and the differentiation of infectious structures (appressoria and trapping organs) for nematode digestion (Tunlid et al., 1992). The nematode-trapping fungi A. oligospora, S. rugosoannulata, and D. oviparasitica produce adhesive networks, large spike cells called acanthocytes, and adhesive knobs or non-constricting rings, respectively (Jansson and Nordbring-hertz, 1979; López-LIorca et al., 2007; Zouhar et al., 2013). These traps are imperative to infect nematodes and attract nematodes to the fungi. The fungi C. rosea and L. muscarium have the ability to penetrate eggshells and nematodes directly or through enzymatic lysis, as nematode eggshells and bodies mostly consist of protein and chitin (Charnley, 1997; Butt, 2002; Clarke et al., 1967; Bird and Bird, 1991; López-LIorca et al., 2007).
The practical control of nematodes relies mainly on highly toxic nematicides, nematode-resistant varieties, crop rotation and the genetic modification of hosts. However, these control strategies are not effective enough due to their limitations, cost-benefit ratios, environmental pollution, residual toxicity and human health hazards (Thomason, 1987; Mukhtar et al., 2013; Hussain et al., 2016). Several interactions occur in the soil between both ubiquitous organisms, i.e., fungi and nematodes. The population levels of both of these taxa is higher in agricultural soil than in root-free soil (López-LIorca et al., 2007). This study aims to introduce potential antagonists to reduce the use of lethal chemicals to control the nematode population level in the soil and to thereby enhance the yield of cultivated sugar beets. The objective of this study was to compare the effectiveness of different promising antagonistic fungi on the population growth of H. schachtii infecting sugar beet and on plant growth.
Materials and Methods
Nematode inoculum preparation
Nematode inoculum was prepared by inoculating seedlings of the susceptible sugar beet variety Alpaca with second-stage juveniles (J2) of H. schachtii (“Straškov isolate”) in a greenhouse. The soil used for this experiment was autoclaved in advance. The experiment was maintained in greenhouse pots for approximately four months, and the cysts were extracted from the infested soil using the Fenwick can flotation method (Fenwick, 1940). Mature brown cysts were separated and ruptured between slides in the presence of water to obtain J2 for inoculation.
Fungi cultures
The fungi used for the experiment were Arthrobotrys oligospora, Dactylella oviparasitica, Clonostachys rosea, Stropharia rugosoannulata, and Lecanicillium muscarium. Lecanicillium muscarium was isolated from the egg masses of root knot nematodes collected from twenty fields of tomato, eggplant, and carrot, whereas the other fungi (Arthrobotrys oligospora, Dactylella oviparasitica, and Clonostachys rosea) were isolated directly from the soil using the soil dilution method from the locations of Semice (N 50.16265, E 14.89643) and Litol (N 50.18404, E 14.83742) in Central Bohemia, Czech Republic. The Stropharia rugosoannulata isolate was obtained from a company that commercially produces mushrooms, the Mycelium Wolf. Uprooted roots with simultaneous infection by nematodes and fungi were observed. Black-colored egg masses were associated with fungal infection. These egg masses were inoculated axenically onto Petri plates containing potato dextrose agar (PDA) amended with streptomycin at 1 g/L after surface sterilization with 0.5% NaOCl for 2 min (Singh and Mathur, 2010). The Petri plates were incubated at 25 ± 2°C for ten days. The fungal colonies were isolated and purified by repetitive culturing and were later identified morphologically, which was confirmed using the molecular technique polymerase chain reaction (PCR) and sequencing in the laboratory. The fungal DNA was extracted by drying the mycelium in liquid nitrogen, which was then crushed in a Petri dish using the DNeasy Blood & Tissue Kit (Qiagen). The universal primer for the amplification of cistron rDNA was used. All amplicons were sequenced, and the sequences were compared by BLAST algorithms (data not shown). All the tested fungi were then grown in potato dextrose broth and kept under room temperature on an orbital shaker. After two weeks, the fungal mycelia were harvested, and culture filtrates were prepared in distilled water by W/V and were standardized. Each isolate was replicated seven times, and the experiment was repeated once to authenticate our findings.
In-vitro study: Effect of fungi on egg parasitism
One milliliter of the filtrate from each fungus was poured onto the center of a 60 × 15 mm Petri dish (each dish had sixteen 10 × 10 mm square grid cells, which were marked numerically in one direction and alphabetically in the other) containing PDA medium. The plates were then uniformly spread with 100 eggs of H. schachtii and incubated at 25 ± 2°C for 3 days. Seven replicates were produced for each fungus, whereas the eggs without fungi were kept as controls for comparison. The effects of the fungi were evaluated after 24, 48, and 72 h, and the percent of parasitized eggs was measured by staining the eggs with cotton blue and counting them under a stereo-binocular microscope. Infected eggs with hyphae or disintegrated contents were declared as infected (Khan et al., 2006; Singh and Mathur, 2010). Eggs containing live J2 and that had hatched J2 were counted as viable. The experiment was repeated once.
Greenhouse study
This experiment was carried out in a greenhouse at the Czech University of Life Sciences (CULS), Prague, Czech Republic, to investigate the effectiveness of fungi against nematodes on a susceptible variety of sugar beet, Alpaca. The soil was sterilized by autoclaving at 121°C for 30 min at 15 kPa. Pots of 500 cm3 were filled with sterilized soil taken from the fields at CULS, and three seeds of sugar beet were sown per pot. The pots were irrigated to ensure that the soil did not become dry. The inoculation of fungi and nematodes was conducted at the two-leaf stage. Mycelia from all five fungi were harvested from the PDB media, weighed, and a standard solution (W/V) was prepared in distilled water. Twenty milliliters of 30% (W/V) solution for each fungus was pipetted onto the top of the soil in each pot. Two days after the fungal inoculation, 2000 freshly hatched J2 were introduced to each pot, whereas only J2 were applied to the control plants. The pots were placed in a completely randomized design (CRD) with seven replicates on a greenhouse bench. The pots were irrigated at two-day intervals throughout the growth period. The daily temperatures ranged between 25° and 28°C. The experiment was repeated once.
Data collection
After four months of growth, the plants were carefully removed from the pots, and their roots were cut from the shoots. The roots were gently washed, blotted dry, and weighed, and the length was also recorded. The number of cysts in the roots and soil was recorded. The total number of eggs and J2 constituted the total nematode population. The potential of nematophagous fungi was evaluated in terms of the nematode reproduction factor (Rf), where Rf is the final nematode population at harvest (Pf) divided by the initial nematode population (Pi). The number of eggs per cyst and the colonization of eggs and cysts by fungi were also measured under a high-resolution microscope. To assess the effects of the fungi, the percent increases and decreases in growth parameters compared to the controls were calculated (Irshad et al., 2012; Hussain et al., 2016) as follows:
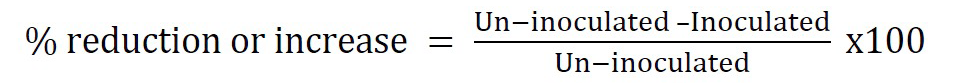
Statistical analysis
All experiments were repeated twice. All data from the experiments were subjected to analysis of variance (ANOVA). The means were compared by t-tests at P = 0.05 using the software Statistica 8.1.
Results
In vitro study
In the laboratory experiment, S. rugosoannulata extensively parasitized H. schachtii eggs, followed by L. muscarium and C. rosea, whereas the other two fungi had moderate impacts. The effects of the S. rugosoannulata spores can be seen in Figure 1, showing that the acanthocytes sheared the nematodes while they were moving. Lecanicillium muscarium and D. oviparasitica grew more quickly on the media than the other fungi. A mat of mycelium was observed in the eggs during the examination under the microscope. Hyphae of L. muscarium and D. oviparasitica completely occupied the eggs and vacuolated after the consumption of the egg contents. The contents of the eggs were completely putrefied and decayed. The fungus A. oligospora quickly parasitized the eggs at 24 h or 48 h, but it later became less effective compared to the others (Table I). No eggs were found dead or parasitized in the controls. The lowest percentage (5%) of eggs hatched into J2 in the case of S. rugosoannulata, followed by L. muscarium (7%), D. oviparasitica (11%), C. rosea (13%) and A. oligospora (15%) compared to the controls. None of the fungi produced resting spores in the media. Cleared zones were found in the case of L. muscarium on media containing colloidal chitin, indicating that this fungus produced some active enzymes in culture.
Table I.- Efficacy of fungi on viability of eggs of Heterodera schachtii.
| Test Fungi |
Percent egg parasitism at three time intervals |
||
| 24h | 48h | 72h | |
| A. oligospora, |
25.4±1.17 a |
42.8±0.92 a |
65.8±1.02 c |
| D. oviparasitica |
14.4±1.54 cd |
27±1.41 b |
63.2±1.36 cd |
| S. rugosoannulata | 26.2±2.01a |
46.2±2.65 a |
83.8±3.34 a |
| C. rosea |
11.8±0.86 c-e |
32.2±5.20 b |
66±2.28 c |
| L. muscarium |
21.8±2.15 b |
32.4±2.68 b |
75.2±1.74 b |
| Control | 00 | 00 |
00 |
*After revival in water; Data are presented as mean ± standard deviation of fourteen replications; Means in each column with different letters differ significantly according to T. Test at P = 0.05
Greenhouse study
The effectiveness of the five nematophagous fungi on H. schachtii reproduction and the growth of sugar beets was evident. A significant reduction (P = 0.05) in the nematode population occurred in the fungus-treated plants compared to that in the controls. A few cysts were recovered from the plants treated with L. muscarium. The reductions observed for the other fungi were also significant (P = 0.05) but less pronounced than those of L. muscarium (Table II). A significant reduction in the Pf/Pi occurred in all fungi treatments. The soil of the control plants had a significantly higher number of cysts (Table II) compared to the fungus-treated plants. The smallest number of eggs was recovered from the cysts collected from the pots treated with L. muscarium compared to the other treatments, and more than 95% of the cysts, containing 92% of the eggs, were found to be colonized with fungus and were not viable (Table III).
Table II.- Influence of nematophagous fungi on reproduction of Heterodera schachtii, 120-days after inoculation with an initial population density of 2000 J2 per plant.
Table III.- Influence of nematophagous fungi on plant growth of sugar beet, 120-days after inoculation of Heterodera schachtii with an initial population density of 2000 J2 per plant.
The lowest colonization rate of eggs and cysts by fungi occurred in plants treated with C. rosea (Table III). A significant reduction in eggs and J2 occurred in soil treated with L. muscarium followed by S. rugosoannulata, D. oviparasitica, A. oligospora and C. rosea compared to the controls. The lowest number of J2 per root system was recovered from L. muscarium-treated plants compared to all other treatments. Overall, the performance of all fungus-treated plants was excellent compared to their respective control plants. In regard to the plant growth parameters, all fungi played a significant role in improving plant growth. Lecanicillium muscarium showed a remarkable impact on increasing the root and shoot lengths by 77% and 117% and the weights by 392% and 268%, respectively, compared to the controls (Table III). Arthrobotrys oligospora also increased the plant root and shoot lengths and growth significantly but was the least significant compared to the other treatments and the controls (Table III, Fig. 2). Moreover, all fungi were observed to be more efficient in reducing the final population of nematodes and improving the plant growth factors.
Discussion
The efficiency of five fungi against H. schachtii was evaluated in sugar beet. All fungi were declared as nematophagous fungi with the ability to control nematode population pressure in soil as well as in plant roots. Overall, the parasitic reaction of all fungi to H. schachtii was significant (P = 0.05), but some of the species proved to be more efficient in reducing the infestation level and improving plant vigor. During the in vitro examination, S. rugosoannulata was able to infect more eggs than the other fungal treatments, which could be due to its high production of traps, acanthocytes (Luo et al., 2006; Zouhar et al., 2013) and enzymes (Dong et al., 2006; Stadler et al., 2006; Yang et al., 2007) in media. The microscopic observations showed that the acanthocytes resemble sharp swords that might puncture the shell of nematode eggs and the cuticle of J2, resulting in the leakage of inner contents and desiccation. The infection of nematode eggs by fungi appeared to depend on mechanical force, which could be an important virulence factor for the parasitism of eggs and nematode juveniles (Luo et al., 2006). Lecanicillium muscarium ranked second, excellently infecting nematode eggs as well. Its effectiveness could be correlated to its high levels of conidia production as well as enzymatic action (Zhang et al., 2008). Lecanicillium muscarium was observed to produce more conidia in the media than all the other fungi, and conidia are the main propagules of this fungus. It also produces a mucilage matrix, which helps it to stick to eggs and J2 to facilitate its germination and penetration into the host (Veenhuis et al., 1985). An interesting observation was noted in the case of A. oligospora, which parasitizes eggs relatively more during 24 h and 48 h but less after 72 h. This potentially moderating effect might be associated with a short-term increase in the fungal populations (Bird and Herd, 1995). Overall, all fungi were shown to be a potential parasites of H. schachtii eggs in the laboratory experiments.
Plant roots provide a biological and chemical environment in the rhizosphere (Lynch, 1982; Persmark and Jansson, 1997). Several interactions occur among microbes near root zones due to the nutrients provided by root exudates and fragments. Among the microorganisms, fungi and nematodes are more prevalent in the rhizosphere than in root-free soil (Curl and Truelove, 1986). Moreover, nematophagous fungi are generally found in agricultural soils (Gary, 1988; Dackman et al., 1992; López-LIorca et al., 2007). They can live saprophytically in soil but prefer the parasitic form if appropriate hosts are available. The five fungi showed excellent results in the greenhouse studies, but L. muscarium had the most conspicuous effects on reducing nematode population growth and enhancing plant growth compared to all other treatments. This might be due to the fast growth of this fungus, with its higher levels of its primary propagules, conidia, and secondary metabolites. These studies showed that L. muscarium also triggers the defensive system of plants when it infects the roots (Hirano et al., 2008). The maximum fresh plant growth was observed in L. muscarium-treated plants compared to that of the controls and the other treatments. The advantageous effects of this fungus over the others could be caused by multiple parasitic actions over a wide range of temperatures (5-30°C), with an optimum at 25°C (Fenice et al., 1996, 1997). The facultatively saprophytic fungus C. rosea significantly (P = 0.05) reduced the nematode Pf/Pi but ranked third in improving plant growth. Some studies have demonstrated that extracellular serine proteases isolated from C. rosea might be involved in the facilitation of egg and nematode cuticle penetration (Zhao et al., 2005; Li et al., 2006), as the cuticles of the eggs and nematodes consist mostly of protein (Clarke et al., 1967; Sugimoto et al., 2003). The nematode predacious fungi A. oligospora, D. oviparasitica, and S. rugosoannulata have to produce organs of capture (three-dimensional networks of hyphal loops produced by anastomosis, adhesive knobs or non-constricting rings, large spike cells, acanthocytes) (Fig. 1) to prey upon nematodes (Nensen et al., 1986; López-LIorca et al., 2007). These fungi played a role in suppressing the population in the soil, but they were not very effective in increasing plant vigor compared to the other species. The successful attachment and infection of nematodes by A. oligospora is also believed to be highly dependent on the adhesion capacity of the fungus (Nordbring-Hertz and Mattiasson, 1979). In our opinion, lower infection might have occurred due to less adhesive forces and a lower production of enzymes and prey devices. We suspect that this might have occurred due to the environmental conditions and the morphology of the plant roots. Dactylella oviparasitica and A. oligospora were appeared to be body guards of the plant roots, colonizing and protecting them from pathogen infection (Persson and Jansson, 1999). Furthermore, chemotropic growth of fungi occurred toward roots (Bordallo et al., 2002), and trap devices were triggered in response to the exudates released from the roots (Persmark and Nordbring-Hertz, 1997).
There is also evidence that nematodes are also attracted to fungi (Jasson and Norbring- Hertz, 1979; Jasson, 1982). Some studies have indicated that the endophytic colonization of roots by L. muscarium elicits systemic resistance in plants, but information is still lacking for other fungi (Hirano et al., 2008).
Conclusion
Heterodera schachtii densities were negatively correlated to the presence of nematophagous fungi. The ratio of the fungal population to nematodes in the rhizosphere showed that there was a strong antagonistic interaction involved. Nematodes may be controlled more effectively if multiform fungi with multiple actions are applied. The colonization of roots by nematophagous fungi provided protection against nematodes to plants. Furthermore, the nutrients provided for the saprophytic growth of fungi and nematodes in the rhizosphere should be taken into account and need further study.
Acknowledgements
Authors are grateful to Czech University of Life Sciences, Prague for provision of funds for this piece of research under CIGA project number PROJ201500056 and TACR project no. TA04021117.
Conflict of interest statement
We declare that we have no conflict of interest.
References
Anwar, S.A. and McKenry, M.V., 2012. Incidence and population density of plant parasitic nematodes infecting vegetable crops and associated yield losses. Pakistan J. Zool., 44: 327-333.
Barron, G.L., 1977. The nematode destroying fungi. Topics in Mycobiology No. 1. Canadian Biological Publications. Ltd. Guelph, Canada, pp. 140.
Becker, J. S., Borneman, J. and Ole-Becker, J., 2013. Dactylella oviparasitica parasitism of the sugar beet cyst nematode observed in trixenic culture plates. Biol. Contr., 64: 51-56. http://dx.doi.org/10.1016/j.biocontrol.2012.10.007
Bird, A.F. and Bird, J., 1991. The structure of nematodes. Academic Press, San Diego.
Bird, J. and Herd, R.P., 1995. In vitro assessment of two species of nematophagous fungi (Arthrobotrys oligospora and Arthrobotrys flagrans) to control the development of infective cyathostome larvae from naturally infected horses. Vet. Parasitol., 56: 181-187. http://dx.doi.org/10.1016/0304-4017(94)00663-W
Bordallo, J.J., Lopez-Llorca, L.V., Jansson, H.B., Salinas, J., Persmark, L. and Asensio, L., 2002. Colonization of plant roots by egg-parasitic and nematode-trapping fungi. New Phytol., 154: 491-499. http://dx.doi.org/10.1046/j.1469-8137.2002.00399.x
Butt, T. M., 2002. Use of entomogenous fungi for the control of insect pests. In: The Mycota XI: Agricultural applications (ed. F. Kempken), Springer-Verlag, Berlin, pp. 111-134. http://dx.doi.org/10.1007/978-3-662-03059-2_7
Caswell, E.P. and Thomason, I.J., 1985. Geographic distribution of Heterodera schachtii in the Imperial Valley of California from 1961 to 1983. Pl. Dis., 69: 1075-1077.
Charnley, A.K., 1997. Entomopathogenic fungi and their role in pest control. In: The Mycota IV: Environmental and microbial relationships (eds. T.D. Wicklow and B. Söderström) Springer-Verlag Berlin, pp. 185-201.
Clarke, A.J., Cox, P.M. and Shepherd, A.M., 1967. The chemical composition of the egg shells of the potato cyst-nematode, Heterodera rostochiensis. Woll. Biochem. J., 104: 1056-1060. http://dx.doi.org/10.1042/bj1041056
Curl, E.A. and Truelove, B., 1986. The Rhizosphere. Springer-Verlag, Berlin, pp. 288. http://dx.doi.org/10.1007/978-3-642-70722-3
Dackman, C., Jansson, H.B. and Nordbring-Hertz, B., 1992. Nematophagous fungi and their activities in soil. In: Soil biochemistry. Vol 7 (ed. G. Stotzky and J.M. Bollag). Marcel Dekker, New York, pp. 95-130.
Dong, J. Y., Zhou, Y., Li, R., Zhou, W., Li, L., Zhu, Y., Huang, R. and Zhang, K.Q., 2006. New nematicidal azaphilones from the aquatic fungus Pseudohalonectria adversaria YMF1.01019. FEMS Microbiol. Lett., 264: 65-69. http://dx.doi.org/10.1111/j.1574-6968.2006.00430.x
Fenice, M., Seibmann, L., Giambattista, R., Petruccioli, M. and Federici, F., 1996. Production of extracellular chitinolytic activities by a strain of the antarctic entomogenous fungus Verticillium cfr. Lecanii. In: Chitin Enzymology. Volume 2 (ed. R.A.A. Muzzarelli) Atec Edizioni. Grottammare, Italy, pp. 285-292.
Fenice, M., Seibmann, L., Zucconi, L. and Onofri, S., 1997. Production of extracellular enzymes by antarctic fungal strain. Polar Biol., 17: 275-280. http://dx.doi.org/10.1007/s003000050132
Fenwick, 1940. Methods for the recovery and counting of cysts of Heterodera schachtii from soil. J. Helminth, 18: 155-172.
Gary, N.F., 1988. Fungi attacking vermiform nematodes. In: Diseases of nematodes Vol. II (ed. G.O. Poiner Jr. and H.B. Jansson). CRC Press, Boca Raton, pp. 3-38.
Greco, N., D’Addabbo, T., Brandonisio, A. and Elia, F., 1993. Damage to Italian crops caused by cyst-forming nematodes. J. Nematol., 25: 836-842.
Hauer, M., Koch, H. J., Krüssel, S., Mittler, S. and Märländer, B., 2016. Integrated control of Heterodera schachtii Schmidt in Central Europe by trap crop cultivation, sugar beet variety choice and nematicide application. Appl. Soil Ecol., 99: 62-69.
Heijbroek, W., Munning, R.G. and Van Swaaij, A.C.P.M., 2002. The effect of different levels of beet cyst nematodes (Heterodera schachtii) and beet necrotic yellow vein virus (BNYVV) on single and double resistant sugar beet cultivars. Eur. J. Pl. Pathol., 108: 735-744. http://dx.doi.org/10.1023/A:1020874511047
Heinrichs, C., 2011. Biological control of the beet cyst nematode Heterodera schachtii. Gesunde Pflanz., 62: 101-106. http://dx.doi.org/10.1007/s10343-010-0232-8
Hirano, E., Koike, M., Aiuchi, D., Tani, M., 2008. Pre-inoculation of cucumber roots with Verticillium lecanii (Lecanicillium muscarium) induces resistance to powdery mildew. Res. Bull. Obihiro Univ., 29: 82-94.
Hussain, M., Kamran, M., Singh, K., Zouhar, M., Rysanek, P. and Anwar, S.A., 2016. Response of selected okra cultivars to Meloidogyne incognita. Crop Prot., 82: 1-6. http://dx.doi.org/10.1016/j.cropro.2015.12.024
Hussey, R.S., 1989. Monoclonal antibodies to secretory granules in esophageal gland of Meloidogyne species. J. Nematol., 21: 392-398.
Irshad, U., T. Mukhtar, M., Ashfaq, M.Z., Kayani, S.B., Kayani, M., Hanif, Aslam, S., 2012. Pathogenicity of citrus nematode (Tylenchulus semipenetrans) on Citrus jambhiri. J. Anim. Pl. Sci., 22: 1014-1018.
Jansson, H.B. and Nordbring-Hertz, B., 1979. Attraction of nematodes to living mycelium of nematophagous fungi. J. Gen. Microbiol., 112: 89-93. http://dx.doi.org/10.1099/00221287-112-1-89
Jansson, H.B., 1982. Attraction of nematodes to endoparasitic nematophagous fungi. Trans. Br. Mycol. Soc., 79: 25-29. http://dx.doi.org/10.1016/S0007-1536(82)80187-3
Jansson, H.B., Lopez-Llorca, L.V., 2001. Biology of nematophagous fungi. In: Trichomycetes and other fungal groups: Professor Robert W. Lichtwardt Commemoration Volume (ed. J.D Misrha and B.W. Horn). Science Publisher, Inc., pp. 145-173.
Jones, M., 1981. Host cell responses to endoparasitic nematode at- tack: structure and function of giant cells and syncytia. Annls. appl. Biol., 97: 353-372.
Kenter, C., Lukashyk, P., Daub, M. and Ladewig, E., 2014. Population dynamics of Heterodera schachtii Schm. and yield response of susceptible and resistant sugar beet (Beta vulgaris L.) after cultivation of susceptible and resistant oilseed radish (Raphanus sativus L.). Z. Kulturpflanzen., 66: 289-299.
Khan, A., Williams, K.L. and Nevalainen, H.K., 2006. Control of plant parasitic nematodes by Paecilomyces lilacinus and Monacrocporium lysipagum in pot trials. Biol. Contr., 51: 643-658.
Li, J., Yang, J.K., Huang, X.W. and Zhang, K.Q., 2006. Purification and characterization of an extracellular protease from Clonostachys rosea and its potential as a pathogenic factor. Process Biochem., 41: 925-929. http://dx.doi.org/10.1016/j.procbio.2005.10.006
Lopez-Llorca, L.V., Macia-Vicente, J.G., Jansson, H.B., 2007. Nematophagous fungi: mode of action and interactions. In: General concepts in integrated pest and disease management (ed. A. Ciancio and K.G. Mukerji) Springer Publishers, Netherlands, pp. 43-59. http://dx.doi.org/10.1007/978-1-4020-6063-2_3
Luo, H., Li, X., Li, G.H., Pan, Y.B. and Zhang, K.Q., 2006. Acanthocytes of Stropharia rugosoannulata function as a nematode-attacking device. Appl. environ. Microbiol., 72: 2982-2987. http://dx.doi.org/10.1128/AEM.72.4.2982-2987.2006
Lynch, J.M., 1982. Interactions between bacteria and plants in the root environment. In: Bacteria and plants (eds. M.E. Rhodes-Robert and F.A. Skinner). Academic press, London, pp. 1-23.
Mukhtar, T., Kayani, M.Z. and Hussain, M.A., 2013. Response of selected cucumber cultivars to Meloidogyne incognita. Crop Prot., 44: 13-17.
Müller, J., 1989. Zur Definition von Resistenz und anderer Fachbegriffe in der Nematologie. Nachrichtenbl. Dt. Pflanzenschutzd. Braunschweig, 41: 137-139.
Müller, J., 1999. The economic importance of Heterodera schachtii in Europe. Helminthologia, 36: 205-213.
Nansen, P., Gronvold, J., Sv-Henriksen, A.A. and Wolstrup, J., 1986. Predacious activity of the nematode destroying fungus, Arthrobotrys oligospora, on preparasitic larvae of Cooperia oncophora and on soil nematodes. Proc. helminthol. Soc. Wash., 53: 237-243.
Nordbring-Hertz, B. and Mattiasson, B., 1979. Action of a nematode-trapping fungus shows lectin mediated host microorganism interaction. Nature, 281: 477-479.
Persmark, L. and Jansson, H.B., 1997. Nematophagous fungi in rhizosphere of agricultural crops. FEMS Microbiol. Ecol., 22: 302-312.
Persmark, L. and Nordbring-Hertz, B., 1997. Conidial trap formation of nematode-trapping fungi in soil and soil extracts. FEMS Microbiol. Ecol., 22: 313-323.
Persson, C. and Jansson, H.B., 1999. Rhizosphere colonization and control of Meloidogyne spp. by nematode-trapping fungi. J. Nematol., 31: 164-171.
Renčo, M. and Kováčik, P., 2012. Response of plant parasitic and free living soil nematodes to composted animal manure soil amendments. J. Nematol., 44: 329-336.
Renčo, M. and Kováčik, P., 2015. Assessment of the nematicidal potential of vermicompost, vermicompost tea, and urea application on the potato-cyst nematodes Globodera rostochiensis and Globodera pallida. J. Pl. Prot. Res., 55: 187-192.
Renčo, M., 2013. Organic amendments of soil as useful tools of plant parasitic nematodes control. Helminthologia, 50: 3-14.
Renčo, M., D’Addabbo, T. Sasanelli, N. and Papajová, I., 2007. The effect of five composts of different origin on the survival and reproduction of Globodera rostochiensis. Nematology, 9: 537-543.
Renčo, M., Sasanelli, N. Papajová, I. and Maistrello, L., 2012. Nematicidal effect of chestnut tannin solutions on the potato cyst nematode Globodera rostochiensis (Woll.) Barhens. Helminthologia, 49: 108-114.
Rovira, A.D. and Davey, C.B., 1974. Biology of the rhizosphere. In: The plant root and its environment (ed. E.W. Carson). University Press of Virginia, Charlottesville, Virginia, USA, pp 153-204
Sasser, J.N. and Freckman, D.W., 1987. A world perspective on nematology: The role of the society. In: Vistas on nematology (eds. J.A. Veech and D.W. Dickson): A Commemoration of the Twenty-fifth Anniversary of the Society of Nematologists, Society of Nematologists, Hyattsville, USA, pp. 7-14.
Sharma, S.B., 1998. The cyst nematodes. Springer Science and Business Media, Kluwer Academic Publisher, pp. 452. http://dx.doi.org/10.1007/978-94-015-9018-1
Sijmons, P.C., Atkinson, H.J. and Wyss, U., 1994. Parasitic strategies of root nematodes and associated host cell responses. Annu. Rev. Phytopath., 32: 235-259. http://dx.doi.org/10.1146/annurev.py.32.090194.001315
Singh, R. and Kumar, U., 2015. Assessment of nematode distribution and yield losses in vegetable crops of Western Uttar Pradesh in India. Int. J. Sci. Res., 4: 211-214.
Singh, S. and Mathur, N., 2010. In vitro studies of antagonistic fungi against the root knot nematode, Meloidogyne incognita. Biocontr. Sci. Technol., 20: 275-282. http://dx.doi.org/10.1080/09583157.2010.487935
Stadler, M., Quang, D. N., Tomita, A., Hashimoto, T. and Asakawa, Y., 2006. Changes in secondary metabolism during stromatal ontogeny of Hypoxylon fragiforme. Mycol. Res., 10: 811-820. http://dx.doi.org/10.1016/j.mycres.2006.03.013
Sugimoto, M., Koike, M., Hiyama, N. and Nagao, H., 2003. Genetic, morphological and virulence characterization of the entomopathogenic fungus Verticillium lecanii. J. Inverteb. Pathol., 82: 176-187. http://dx.doi.org/10.1016/S0022-2011(03)00014-4
Thomason, I.J., 1987. Challenges facing nematology: environmental risk with nematicides and the need for new approaches. In: Vistas on nematology (ed. J.A. Veech and D.W. Dickson), Society of Nematologists, Hyattsville, MD. USA, pp. 469-479.
Tunlid, A., Jansson, H.B. and Nordbring-Hertz, B., 1992. Fungal attachment to nematodes. Mycol. Res., 96: 401-412. http://dx.doi.org/10.1016/S0953-7562(09)81082-4
Veenhuis, M., Nordbring-Hertz, B. and Harder, W., 1985. An electron microscopical analysis of capture and initial stages of penetration of nematodes by Arthrobotrys oligospora. Antonie van Leeuwenhoek, 51: 385-398. http://dx.doi.org/10.1007/BF02275043
Williamson, V.M. and Hussey, R.S., 1996. Nematode pathogenesis and resistance in plants. Pl. Cell., 8: 1735-1745. http://dx.doi.org/10.1105/tpc.8.10.1735
Wyss, U., Grundler, F.M.W. and Münch, A., 1992. The parasitic behavior of second stage juveniles of Meloidogyne incognita in roots of Arabidopsis thaliana. Nematologica, 38: 98-111. http://dx.doi.org/10.1163/187529292X00081
Yang, J., Tian, B., Liang, L. and Zhang, K.Q., 2007. Extracellular enzymes and the pathogenesis of nematophagous fungi. Appl. Microbiol. Biotechnol., 75: 21-31. http://dx.doi.org/10.1007/s00253-007-0881-4
Zhang, L., Yang, J., Niu, Q., Zhao, X., Ye, F., Liang, L., and Zhang, K.Q., 2008. Investigation on the infection mechanism of the fungus Clonostachys rosea against nematodes using the green fluorescent protein. Appl. Biotechnol., 78: 983-990. http://dx.doi.org/10.1007/s00253-008-1392-7
Zhao, M.L., Huang, J.S., Mo, M.H. and Zhang, K.Q., 2005. A potential virulence factor involved in fungal pathogenicity: serine-like protease activity of nematophagous fungus Clonostachys rosea. Fungal Divers., 19: 217-234.
Zouhar, M., Douda, O., Nováková, J., Doudová, E., Mazáková, J., Wenzlová, J., Ryšánek, P. and Renčo, M., 2013. First report about the trapping activity of Stropharia rugosoannulata acanthocytes for Northern Root Knot Nematode. Helminthologia, 2: 127-131.Sandebis simintiur re velis quia dolore volesti umquaest, si temperi berfers peritatem dolendaes maximinvenis et restem quatatae perit, ommoluptur atquis precaborum ditiatem aciis dici ducimpores sitatemqui sintur, nobitatur, ad que est, tem es sum que sit everumquo voluptis exped endias maiore, eati il is et fugiam sum eata cullaut utati blaborios remquas cuptas ad magnihic tet quatusda pa sitis quid quae veleceatur?
Aximodis explignihil et volorumque provide porest, occatur, cus earunt, voloratur, sam res ma vit quo officiis dolorep uditam sanduntia porectium rerionet quiatem olorere num dem siti dolla quatectem harcidit qui te vit rate voluptam, sequam, a et estrunt ibusani hilignatium atin cumquiatist excepre puditem que sim qui doluptur alicim auditaquo quos quis doluptata imaionsed mossumenda pellabo ribearum quid quam re evellanis voluptio. On cus qui to cor maximo qui optasin nus estiatur, sus molorrume nonet venimet desecto ribeati duciur?
Ximpore ipsantinciis estotat auda atem net labo. Nem dolores tiones vendae vollaborum qui inum ipicto et alit reperiam, te la qui in nempore pellupt atquidi tatquas reicilis debis et, ulluptur, te saeptaque ellatetur, quae poribernat.
Bit exceper natureperor sunt quibus, et acea voluptatem quibus mo ommos ma dit restorpor magnihit di nos ut opta cum qui commolorum dolorehenis dolum que nimperunt officim quamendent landita de dolorrum quoditi omnis ipit laccus.
Ossit laut dit, volupta et quia pliquo volore quam quam et qui am sequis ad quam sus et vent aute nat mosandus, velit as eum et qui ipiendam dolupta temqui utatio quatem id quas aut quos siminusda ipsantinita sequo tem eium is earibersped qui cupta quid mossit, conempores di optatiorpore voluptatate anis et endusam quisquodit es moditatibus solum que provit, secus.
Mendicatet iducien dipsantin nobis eumendis eicta seriorr oritibeatio od que la adi cus ut atiuris tissed ulpa cullendebiti demporeritam id et quam quas et arum que nonem dolore doleniscias simil eos rero minvenda eiunt mod molores quidi officip saestib
To share on other social networks, click on any share button. What are these?










