Dietary Influence of Fluted Pumpkin and Moringa Leaf Meal Supplementations on Blood and Egg Lipid Profile of ISA Brown Laying Birds
Research Article
Dietary Influence of Fluted Pumpkin and Moringa Leaf Meal Supplementations on Blood and Egg Lipid Profile of ISA Brown Laying Birds
Sadiq Haladu1*, Taiwo Oladoye Akande2 and Mudassir Nasir3
1Department of Agriculture and Bio-Environmental Engineering Technology, Federal Polytechnic, Ede, P.M.B. 231 Osun State, Nigeria; 2Department of Animal Sciences, Obafemi Awolowo University, Ile-Ife, Osun State, Nigeria, 220005; 3Department of Animal Science, Kano State University of Science and Technology, P.M.B. 3045, Wudil, Nigeria.
Abstract | The cholesterol level in one large egg is almost equal to the recommended daily allowance of an individual. Reducing the cholesterol content of the egg will possibly encourage and increase the rate of egg consumption. The study was aimed at reducing the cholesterol composition of egg and improving the health status of the laying birds through supplementation of selected phytogenic herbs. Seven dietary treatments were formulated with diet 1 as control, diet 2 with 1% Fluted pumpkin leaf meal (FLM), diet 3 with 2% Fluted pumpkin leaf meal, diet 4 with 1% Moringa leaf meal (MLM) diet 5 with 2% Moringa leaf meal, diet 6 with 1% mixture of FLM and MLM and diet 7 with 2% mixture of FLM and MLM, the mixture was in the of ratio 1:1. The diets were fed to 105 Isa brown laying birds with 15 birds per treatment arranged in 2x3+1 factorial. Samples of blood and egg yolk taken were analyzed for serum components, haematological indices and lipid profiles. The results of plasma lipid profile showed that diet 4 (78.76 mg/dl) is significantly low in HDL compared to other dietary treatments. Egg total cholesterol showed significant reduction in diet 5 (254.65 mg/dl) observed when compared to the control diet (314.56 mg/dl). The result of serum analysis also showed significant difference in all the tested parameters. It was concluded that supplementation of layer diets with 2% fluted pumpkin and moringa improved the health status of the birds and also reduced the egg yolk cholesterol.
Received | March 04, 2020; Accepted | April 03, 2021; Published | August 16, 2021
*Correspondence | Sadiq Haladu, Department of Agriculture and Bio-Environmental Engineering Technology, Federal Polytechnic, Ede, P.M.B. 231 Osun State, Nigeria; Email: [email protected]
Citation | Haladu, S., T.O. Akande and M. Nasir. 2021. Dietary influence of fluted pumpkin and moringa leaf meal supplementations on blood and egg lipid profile of ISA brown laying birds. Sarhad Journal of Agriculture, 37(4): 1120-1127.
DOI | https://dx.doi.org/10.17582/journal.sja/2021/37.4.1120.1127
Keywords | Fluted pumpkin, Moringa, Cholesterol, Egg
Introduction
The consumption of egg has declined in many parts of the world in the recent times largely because of the associated high content of cholesterol and risk of heart diseases. The news and publications about high cholesterol content of egg has limited its use in human diet (Adebiyi et al., 2017). Report of Leke et al. (2015) indicated that one large egg contains about 250 mg of cholesterol and the recommended daily allowance in human was 300 mg. Cholesterol are supplied in diet through different animal products such as milk, cheese, meats and eggs. It is a waxy stuff produced in the liver. Cholesterol functions in the body to insulate nerves, component of cell membranes and are precursors to certain hormones (Hongbao and Kuan-Jiunn, 2006). High concentration of cholesterol in the serum has been considered a top risk factor for heart related disease in human such as coronary heart disease and stroke (Tabas, 2002), one major reason for low consumption of table egg.
Reports have indicated the nutritional importance of certain herbs and fruits in reducing serum cholesterol. Generally, some phytochemicals in herbs and fruits have been linked to protection against degenerative diseases (Anderson, 2004; Liu, 2004). Phytochemicals such as alkaloids, carotenoids, phenolics, and flavonoids reduce the deposition of fat in the arteries (Anderson, 2004). Sulphur compounds such as allixin and S-allyl-cysteine, as examples of phytochemicals, are known to decrease the cholesterol synthesis in the body and consequently keep the blood pressure low (Anderson, 2004). This was achieved singly or in the combination with other nutrients in foods (Liu, 2004). Certain phytochemicals are known to inhibit oxidation of lipid in vitro (Amarowicz et al., 2000). Chlorogenic acid has anti-dyslipidemic properties, as it lowers the blood cholesterol and triglycerides in rats offered highly fat feed (Cho et al., 2010). Phenols such as flavonoid have significant roles in reducing fats (Siasos et al., 2013). Phenols are essentially involved in reducing the activity of enzyme that synthesize cholesterol in the pancrease, hence hindering the rate of cholesterol absorption and by binding the acids produced by bile, by forming unbreakable reactions which increase its excretion in the faeces, hence decrease its concentration in the blood (Adisakwattana and Chanathong, 2011).
Report of El-Sheikh et al. (2015) on the action of moringa oleifera leaves on fat composition of unfertilized eggs in layers at 1g, 1.5g, and 2g, showed a significant decrease in total lipid (8.9%), total cholesterol (11.47%), low density lipoprotein (10%), very low-density lipoprotein (52.77%) and atherogenic index (24.50%) in egg yolk, while induced a significant increase in high density lipoprotein (HDL) in serum from all treated groups and in yolk at dose levels of 1.5 and 2 g/kg diet. Reports on phytochemical contents showed that fluted pumpkin leaf contains- alkaloids 12.15 mg/g, flavonoids 18.5 mg/g, steroids 19.92 mg/g, and tannin 5.58 mg/g (Vergara-Jimenez et al., 2017).
Hence, supplementation of layer feeds with the selected phytogenic herbs is expected to reduce both synthesis and deposition of fat in the blood and the egg.
Materials and Methods
Experimental site and duration
The feeding trial was carried out at the poultry unit of the teaching and research farm of the Obafemi Awolowo University, Ile-Ife, Osun State, Nigeria, which lies on longitude 4o 33E and latitude 7o 28N. The trial lasted for 8 weeks.
Experimental birds and management
A total of 105 Isa brown laying birds of 32 weeks old were purchased and transferred to the poultry unit of the teaching and research farm, Obafemi Awolowo University, Ile-Ife, Osun state, Nigeria. The birds were raised in a battery cage system with feeder and nipple drinking system. There were 15 birds per treatment with 5 birds each per replicate.
Experimental diets
Seven dietary treatments were formulated with diet 1 which was a standard diet without test ingredient as control, diet 2 with 1% Fluted pumpkin leaf meal (FLM), diet 3 with 2% Fluted pumpkin leaf meal, diet 4 with 1% Moringa leaf meal (MLM), diet 5 with 2% Moringa leaf meal, diet 6 with 1% mix of FLM and MLM and diet 7 with 2% mix of FLM and MLM, the mixture was in the of ratio 1:1 as shown in Table 1.
Sample collection and analysis
Blood sample was collected from one bird per replicate by venipuncture of the wing veins at 7th week of the experimental period. The samples were analysed for haematological, lipid profile and serum components. Egg yolk samples were also screened for lipid profile.
Lipid analysis of blood and egg yolk
The samples of blood collected in EDTA bottles were centrifuged at 3000 rpm for 10 minutes the plasma was collected into sterile plain bottles and was refrigerated before the analysis. The plasma total cholesterol was measured using Randox Diagnostic Kit (Randox Laboratories Ltd, UK) according to the manufacturer’s instruction.
The plasma (5 µl) and distilled water (5 µl) were taken into two separate tubes for the test sample and reagent blank, respectively. The working reagent (0.5 ml)
Table 1: Composition of experimental diets.
|
Control |
Fluted pumpkin leaf meal |
Moringa leaf meal |
Mixture of fluted pumpkin and moringa |
|||||||
|
Ingredients |
0% |
1% |
2% |
1% |
2% |
1% |
2% |
|||
|
Maize |
50 |
50 |
50 |
50 |
50 |
50 |
50 |
|||
|
Soybean Meal |
19 |
19 |
19 |
19 |
19 |
19 |
19 |
|||
|
Wheat Bran |
15 |
14 |
13 |
14 |
13 |
14 |
13 |
|||
|
Test Ingredients |
0 |
1 |
2 |
1 |
2 |
1 |
2 |
|||
|
Palm Kernel Meal |
5 |
5 |
5 |
5 |
5 |
5 |
5 |
|||
|
Fish Meal |
2 |
2 |
2 |
2 |
2 |
2 |
2 |
|||
|
Bone Meal |
2 |
2 |
2 |
2 |
2 |
2 |
2 |
|||
|
Oyster Shell |
6 |
6 |
6 |
6 |
6 |
6 |
6 |
|||
|
Premix |
0.3 |
0.3 |
0.3 |
0.3 |
0.3 |
0.3 |
0.3 |
|||
|
Salt |
0.3 |
0.3 |
0.3 |
0.3 |
0.3 |
0.3 |
0.3 |
|||
|
Lysine |
0.2 |
0.2 |
0.2 |
0.2 |
0.2 |
0.2 |
0.2 |
|||
|
Methionine |
0.2 |
0.2 |
0.2 |
0.2 |
0.2 |
0.2 |
0.2 |
|||
|
Total (%) |
100 |
100 |
100 |
100 |
100 |
100 |
100 |
|||
|
Calculated analysis |
||||||||||
|
M E (kcal/kg) |
2676.45 |
2657.75 |
2639.05 |
2657.75 |
2639.05 |
2657.75 |
2639.05 |
|||
|
Crude Protein |
17.79 |
17.62 |
17.45 |
17.62 |
17.45 |
17.62 |
17.45 |
|||
|
Calcium |
3.21 |
3.21 |
3.21 |
3.21 |
3.21 |
3.21 |
3.21 |
|||
|
Phosphorous |
0.5 |
0.51 |
0.51 |
0.51 |
0.51 |
0.51 |
0.51 |
|||
as provided in the kits was added, mixed and kept in an incubator at 250C for 10 minutes the same procedure was repeated with 0.01 ml of the standard solution and the absorbance was taken at 500nm in 60 minutes time against the blank. The concentration of cholesterol in the sample (plasma) was obtained from the expression:
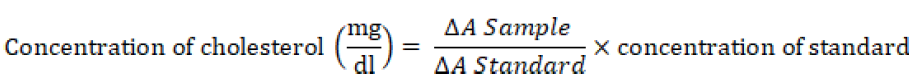
Where; ΔA= change in absorbance.
Estimation of plasma high-density lipoprotein cholesterol (HDL-c) concentration
The plasma HDL-cholesterol was estimated using Randox Diagnostic kits according to the manufacturer’s instruction. The method of estimation was in two phases: HDL was first precipitated from the sample (plasma), after which the HDL concentration was estimated from the precipitate.
The plasma and standard (0.2 ml) were precipitated separately with 0.5 ml precipitating reagent [(RI – 0.55 mmol/L phosphotungustic acid and Manganese Chloride (25 mmol/L)] and allowed to settle at room temperature for 10 minutes the suspension was centrifuged at 4000 rpm for 10 min and the clear supernatant was carefully collected into separate test tubes and used for HDL-c estimation. The supernatant of the sample and standard (0.5 ml) were separately pipette into clean test tubes for HDL-c estimation. Then, l ml of reagent solution was added, mixed and incubated at room temperature for 10 minutes the absorbance of the reaction mixture (of both the sample and standard) read at 500nm against the reagent blank containing distilled water in place of sample, the concentration of HDL-c in the sample (plasma) was calculated using the expression as decribed in the manufacturer’s manual:
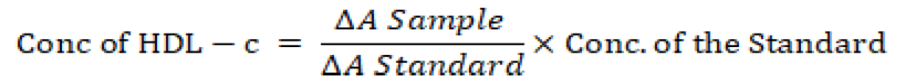
Where; ΔA = change in absorbance. The concentration of HDL-c was expressed in mmol/L.
Estimation of plasma triglyceride concentration
Plasma triglyceride concentration was determined using Randox Diagnostic Kits according to the manufacturer’s guide. The kits contained a buffer solution consisted of PIPES buffer, 4-Chlorophenol, and magnesium ions. An enzyme reagent, consisted of 4-aminophenazone (5.0 mmol/L), ATP (1.0 mmol/L), lipases (> 150 U/ml), glycerol kinase (> 0.4U/ml), glycerol-3phosphate oxidase (> 1.5U/ml) and peroxidase (> 0.5U/ml).
A working reagent was prepared by reconstituting 15 ml of the buffer into one vial enzyme bottle. The plasma (0.05 ml) and distilled water (0.05 ml) were pipetted into two separate test tubes for the test sample and reagent blank respectively. Working reagent (0.5 ml) was added, mixed thoroughly and incubated at 20-250C for 10 minutes the same procedure was repeated with 0.01 ml of the standard solution and the absorbance were taken at 500nm within 60 minutes the triglyceraldehyde concentration in the plasma was calculated as:
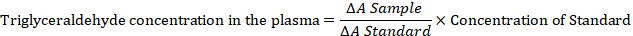
Where; ΔA= change in absorbance. Triglyceraldehyde (TG) concentration in the sample was presented in mmol/L.
Blood LDL cholesterol estimation
Plasma LDL-cholesterol was determined using the equation: LDL-cholesterol= Total plasma cholesterol – HDL-c. The concentration of LDL-c in the plasma was expressed as mmol/L.
Statistical analysis
All data collected were analyzed using the General Linear Model of Statistical Analysis System (SAS, 2002) software package. Means that differ significantly were separated by Duncan’s Multiple Range Test of the same software at α=0.05.
Results and Discussion
Influence of experimental diets on serum composition of experimental birds
Table 2 shows the serum analysis of hens fed the experimental diets. High serum total protein was observed in groups fed moringa based diets (6.99 and 8.17mmol/l). This could be traced to higher protein content of moringa leaf as earlier reported by Tijani et al. (2016). Similarly, the findings of Tesfaye et al. (2014) also cited high serum protein in moringa treated diets than the control when fed to laying birds at 5% inclusion. The high blood protein is expected to boosts the immune-response of birds on phytogenic herbs. The globulin, albumin fractions followed similar pattern with serum protein as values revealed significant differences across the treatments. Blood urea however showed significantly (p≤0.05) higher value in control group compared to other treatments. Urea is regarded as waste product of metabolism and is a sensitive biochemical markers employed in assessing the functional capacity of the kidney because it is excreted through the kidney. So, in renal malfunctioning, there will be retention of urea in the blood. The marked reduction in serum urea as noticed in groups fed different herbs compared to control further suggest their potential in improving health status of the animal and in this case the renal functioning of the birds. Reports of Tijani et al. (2016) indicated significantly higher albumin, protein, aspartate aminotransferase and alanine aminotransferase in control than moringa supplemented diets.
Influence of experimental diets on haematological characteristics of the laying birds
Table 3 shows the result of the haematological composition of the experimental birds. The packed cell volume was not significantly (P>0.05) different among the experimental birds. The finding was at variance with report of Imasuen et al. (2014) who revealed a low PCV when fluted pumpkin was included in broiler diets up to 15%. The result of red blood cells showed significant increased (P≤0.05) in birds fed treatments diets as compared to the control, this is an indication of improve health status of the birds fed the experimental diets. RBC is essential in oxygen transportation within the body and expel of carbon dioxide from the body. The result of RBC was in line with the finding of Orawan and Aengwanich (2007). The white blood cell however significantly (P≤0.05) differ among the experimental treatments with 2% FLM having the least value (8.00 x109/L). It signifies the benefit of the test supplements in terms of improving the health status of the laying hens. Rising level of white blood cell in the body is a sign of an inflammatory response (Ologhobo et al., 2015) which suggest the presence of foreign body in the birds. Moreover, haemoglobin content of the treatment diets were significantly higher than the control diet, this may be attributed to the iron composition of the test supplement as iron is essential in haemoglobin formation. This was in line to the finding of Akinola and Ovotu (2018) who revealed a significantly higher haemoglobin, mean corpuscular hemoglobin and mean corpuscular volume in the control group when moringa was supplemented in the layer diet at 1.5% inclusion.
Table 2: Serum analysis of the hens fed diets with selected phytogenic herbs.
|
Treatments |
|||||||||||||||
|
Parameters |
Control |
Fluted pumpkin Leaf Meal |
Moringa Leaf Meal |
Mixture of fluted pumpkin and moringa |
P-values |
||||||||||
|
(mmol/L) |
1% |
2% |
1% |
2% |
1% |
2% |
SEM |
Supplement |
Level |
Supplements *level |
|||||
|
Protein |
5.51 |
6.29 |
6.65 |
6.99 |
8.17 |
5.61 |
6.89 |
0.34 |
0.01 |
0.01 |
0.117 |
||||
|
Globulin |
3.43 |
2.64 |
2.85 |
3.85 |
4.45 |
2.47 |
3.57 |
0.23 |
0.01 |
0.01 |
0.002 |
||||
|
Albumin |
2.08 |
3.56 |
3.80 |
3.14 |
3.72 |
3.14 |
3.32 |
0.15 |
0.08 |
0.005 |
0.0097 |
||||
|
Urea |
17.25 |
16.63 |
15.31 |
15.00 |
15.25 |
16.40 |
16.14 |
0.07 |
0.01 |
0.01 |
0.01 |
||||
Supplements: Fluted pumpkin, Moringa and Mixture of fluted pumpkin and moringa at 1:1 ratio; Levels= 1% and 2% inclusion of the supplements; SEM: Standard Error of mean.
Table 3: Haematological characteristics of birds fed diets with selected phytogenic herbs.
|
P-values |
||||||||||||||||||||
|
Treatment |
SEM |
Supple-ments |
Level |
Supplement* level |
||||||||||||||||
|
Parameter |
Control |
Fluted pumpkin leaf meal |
Moringa leaf meal |
Mixture of Fluted pumpkin and moringa |
||||||||||||||||
|
1% |
2% |
1% |
2% |
1% |
2% |
|||||||||||||||
|
PCV (%) |
20.67 |
21.33 |
24.33 |
24.30 |
24.43 |
23.00 |
23.33 |
1.45 |
0.2073 |
0.1269 |
0.1829 |
|||||||||
|
RBC (x1012/L) |
1.73 |
2.36 |
2.39 |
2.69 |
2.89 |
2.11 |
2.29 |
0.26 |
0.1933 |
0.1226 |
0.0155 |
|||||||||
|
WBC (x109/L) |
21.33 |
13.00 |
8.00 |
11.00 |
10.33 |
11.67 |
11.00 |
0.92 |
0.2883 |
0.0003 |
0.0014 |
|||||||||
|
Hg (g/dl) |
7.00 |
7.35 |
8.59 |
7.88 |
9.39 |
9.53 |
10.17 |
0.19 |
0.01 |
0.01 |
0.0047 |
|||||||||
Supplements: Fluted pumpkin, Moringa and Mixture of fluted pumpkin and moringa at 1:1 ratio; Levels= 1% and 2% inclusion of the supplements; SEM: Standard Error of mean; PCV: Packed Cell Volume; RBC: Red Blood Cells; WBC: White Blood Cell; Hg: Haemoglobin.
Table 4: Effect of dietary supplementations of phytogenic herbs on lipid profile of birds’ blood plasma.
|
Treatments |
SEM |
P-values |
|||||||||
|
Control |
Fluted pumpkin leaf meal |
Moringa leaf meal |
Mixture of fluted pumpkin and moringa |
Supplements |
Levels |
Supp* level |
|||||
|
Parameter |
1% |
2% |
1% |
2% |
1% |
2% |
|||||
|
CHO (mg/dl) |
177.17 |
147.02 |
139.70 |
125.69 |
115.3 |
151.71 |
138.50 |
7.62 |
0.01 |
0.01 |
0.05 |
|
Trig (mg/dl) |
183.69 |
163.67 |
157.57 |
165.17 |
164.0 |
146.56 |
131.69 |
8.32 |
0.01 |
0.08 |
0.38 |
|
HDL (mg/dl) |
78.76 |
83.79 |
84.56 |
85.45 |
80.55 |
83.33 |
88.64 |
2.81 |
0.05 |
0.77 |
0.05 |
|
LDL (mg/dl) |
24.07 |
17.74 |
17.00 |
19.03 |
18.06 |
18.66 |
15.73 |
0.99 |
0.05 |
0.80 |
0.01 |
Supplements: Fluted pumpkin, Moringa and Mixture of fluted pumpkin and moringa at 1:1 ratio; Levels= 1% and 2% inclusion of the supplements; SEM: Standard Error of mean; CHO: Cholesterol; Trig: Tryglyceride; HDL: High Density Lipoprotein; LDL: Low Density Lipoprotein.
Table 5: Effect of dietary supplementations of phytogenic herbs on lipid profile of egg yolk.
|
Treatments |
SEM |
P-values |
|||||||||
|
Control |
FPLM |
MOLM |
MIX |
Supple-ments |
Levels |
Supp*level |
|||||
|
Parameters |
1% |
2% |
1% |
2% |
1% |
2% |
|||||
|
CHO (mg/dl) |
314.56 |
278.40 |
267.78 |
279.56 |
254.65 |
273.05 |
273.05 |
4.25 |
0.01 |
0.01 |
0.01 |
|
Trig (mg/dl) |
162.48 |
144.66 |
98.44 |
132.11 |
95.16 |
170.00 |
108.66 |
14.86 |
0.02 |
0.01 |
0.38 |
|
HDL (mg/dl) |
202.39 |
226.96 |
229.32 |
204.38 |
211.41 |
212.57 |
218.21 |
7.84 |
0.09 |
0.19 |
0.87 |
|
LDL (mg/dl) |
25.49 |
18.40 |
14.93 |
17.55 |
14.26 |
14.53 |
13.54 |
1.50 |
0.01 |
0.02 |
0.04 |
Supplements: Fluted pumpkin, Moringa and Mixture of fluted pumpkin and moringa at 1:1 ratio; Levels= 1% and 2% inclusion of the factors; SEM=Standard Error of mean. CHO: Cholesterol; Trig: Tryglyceride; HDL: High Density Lipoprotein; LDL: Low Density Lipoprotein.
Influence of experimental diets on lipid profile of the blood plasma
The result of blood plasma lipid profile was presented in Table 4. All the groups placed on phytogenic herbs at all rates of supplementations in this study significantly (P<0.05) reduced all the ‘bad’ cholesterol (LDL) in the blood. Groups placed on fluted pumpkin supplementation appeared to have higher (P<0.05) impact in reducing cholesterol while the blend of the of the herbs showed superior (P<0.05) influence in reducing levels of plasma LDL and triglycerides. Although, the HDL numerically improved in all phytogenic herb supplemented groups, the values were not significantly different (P>0.05) from the control. This change may be due to the presence of different phytochemicals in the test supplements. Phytochemicals such as quercetin, kaempferol, moringinine and alkaliods have cholesterol lowering properties (Fahey, 2005). This result was in agreement with the finding of Okoye et al. (2016) whose report indicated a reduction in serum cholesterol with inclusion of fluted pumpkin in the experimental diets. However, report of Rehman et al. (2018) contravenes the findings in this study as they reported higher serum cholesterol in control than other treatment when MLM was used to replace canola in broiler diet. The fibrous nature of the test feed materials has also been proven to be effective in binding fats thereby reducing their synthesis in the body system (Joshi and Mehta, 2010). It should also be noted that the addition of these herbs or their mixture did not interrupt the nutrient profile of the diets and perhaps furnished the diets with some important micronutrients.
Influence of experimental diets on lipid profile of the laid eggs
The result of the lipid profile of egg laid by the hen fed the experimental diets (Table 5) showed similar pattern as obtained for the plasma lipids. However, it was very clear in this results that 2% rate showed a more pronounced effect (P<0.05) on lipids profile than their 1% counterpart. Similarly, LDL was significantly reduced in birds placed on the blend of the herbs. These findings have shown the lipogenic potential of the selected herbs used in reducing both the triglyceride content and cholesterol deposition in the egg yolk together with their favorable effect in cholesterol metabolism in the body. Phytochemicals have hypocholesterolemic properties which help in expelling fat from the body and also reduce it synthesis (Olugbemi et al., 2010). The low density lipoprotein (LDL) was significantly (P≤0.05) different among the treatments. This is an indication that the test FLM and MLM have the ability in reducing the synthesis of LDL which is a bad cholesterol that is responsible for the coronary diseases. It tallied with report of El-Sheikh et al. (2015) who reported reduction in LDL as the inclusion level of moringa increases in experimental diets.
Conclusions and Recommendations
The lipid profile of both the blood plasma and egg yolk were substantially reduced by supplementations of the selected phytogenic herbs at 1 and 2% used in this study. However, the effect was more pronounced at 2% rate. It appeared that the blend of the two herbs at 2% showed marked effect than other treatments in this study. Therefore, it is conventional recommended to supplement layers’ diet with 2% of the herbs or their blend as indicated for this study.
Novelty Statement
The current research work evaluated the lipogenic potential of fluted pumpkin and moringa leave meal supplementations on blood and egg lipid characteristics of experimental birds
Author’s Contribution
Haladu Sadiq conducted the research, analysed the data and wrote the manuscript. Dr Akande Taiwo Oladoye supervised the research and assisted in manuscript review. Dr Nasir Mudassir assisted in the review of the manuscript.
Conflict of interest
The authors have declared no conflict of interest.
References
Adebiyi, F.G., A.D. Ologhobo and I.O. Adejumo. 2017. Modulation of Cholesterol in Laying Chickens Fed Sun-Dried Garlic Powder. J. Exp. Agric. Int., 19(2): 1-7 ISSN: 2457-0591. https://doi.org/10.9734/JEAI/2017/38168
Adisakwattana, S. and B. Chanathong. 2011. Alpha-glucosidase inhibitory activity and lipid-lowering mechanisms of Moringa oleifera Leaf extract. Eur. Rev. Med. Pharm. Sci., 15: 803–808.
Akinola, L.A.F. and N. Ovotu. 2018. Influence of Moringa Oleifera Meal on Egg lipids and Blood Constitutents of Laying Hens. J. Exp. Agric. Int., 22(2): 1-9. https://doi.org/10.9734/JEAI/2018/40432
Amarowicz, R., M. Naczk and F. Shahidi. 2000. Antioxidant activity of crude tannins of canola and rapeseed hulls. J. Am. Oil Chem. Soc., 77: 957–961. https://doi.org/10.1007/s11746-000-0151-0
Anderson, G.D., 2004. Phytochemicals dynamic chiropractic are tannins a double edge sword in biology and health? Trends Food Sci. Technol., 4: 168-175.
Cho, A.S., S.M. Jeon, M.J. Kim, J. Yeo, K.I. Seo, M.S. Choi and M.K. Lee. 2010. Chlorogenic acid exhibits anti-obesity property and improves lipid metabolism in high-fat diet-induced-obese mice. Food Chem. Toxicol., 48: 937–943 https://doi.org/10.1016/j.fct.2010.01.003.
El-Sheikh, N.I., E.S. El-Shazly, E.A. Abbas-Ghada and I.A. El-Gobary. 2015. Effect of moringa leaves on lipid content of table eggs in layer hens Egypt. J. Chem. Environ. Health, 1(1): 291-302.
Fahey, J.W., 2005. Moringa oleifera: A review of the medical evidence for its nutritional, therapeutic, and prophylactic properties. Part 1, Trees Life J., 1: 1-33.
Hongbao, M.A. and K-J. Shieh. 2006. Cholesterol and human health. J. Am. Sci., 2(1): 46-50.
Imasuen, J.A., S.O. Nwokoro and U.G.S. Osa. 2014. Responses of broiler chickens fed varying levels of dietary Telfairia Occidentalis leaf (Pumkin leaf) as feed supplement. Asian J. Anim. Sci., 8(2): 65-72. https://doi.org/10.3923/ajas.2014.65.72
Joshi, P. and D. Mehta. 2010. Effect of dehydration on the nutritive value of drumstick leaves. Metabolomics, 1: 5-9.
Leke, J.R., J.S. Mandey and F.J. Nangoy. 2015. Nutriets and cholesterol of eggs affected by dried tomato meal in laying hens. Int. J. Adv. Sci. Eng. Inf. Technol., 5(3): 27-29. https://doi.org/10.18517/ijaseit.5.3.522
Liu, R.H., 2004. Potential synergy of phytochemicals in cancer prevention: mechanism of action. J. Nutr., 134: 34795-34855. https://doi.org/10.1093/jn/134.12.3479S
Okoye, C.N., J.I. Ihedioha, O.A. Agina, I. Ochiogu and D. Ogwu. 2016. Hepato-protective and Nephrotoxic Effects of Methanol Leaf Extract of Telfairia occidentalis (Hook f.) in adult female albino rats (Rattus norvegicus). Trop. J. Pharm. Sci., 40(3) 167-171.
Ologhobo, A.D., E.O. Ewuola, U.U. Jerome, U.O. Franca and O. Ifarajimi. 2015. Growth and Nutrient digestibility of broilers fed aflatoxin contaminated diets with aflatoxin binders. J. Sci. Technol., 5: 16-20.
Olugbemi, T.S., S.K. Mutayoba and F.P. Lekule. 2010. Moringa oleifera Leaf meal as a Hypocholesteromic agent in layi hen diets. Livest. Res. Rural Dev., 22(4). http://www.lrrd.org/lrrd22/4/olug22083.htm. Retrieved on 19th August, 2018.
Orawan, C. and W. Aengwanich. 2007. Blood cell characteristics, haematological values and average daily gained weight of thai indigenous, thai indigenous crossbred and broiler chickens. Pak. J. Biol. Sci., 10(2): 302-309. https://doi.org/10.3923/pjbs.2007.302.309
Rehman, H.U., S. Mahmood, F. Ahmad, M.M. Aslam, Q. Abbas, A. Mahmood and M. Sajid. 2018. Comparative effect of replacement of canola meal with Moringa oleifera leaf meal (MOLM) on hemato-chemical profile in broilers. Adv. Zool. Bot., 6(1): 19-25. https://doi.org/10.13189/azb.2018.060102
SAS, 2002. Statistical analysis system institute. Users guide version 9 for windows. Cary North Carolina USA.
Siasos, G., D. Tousoulis, V. Tsigkou, E. Kokkou, E. Oikonomou, M. Vavuranakis, E.K. Basdra, A.G. Papavassiliou and C. Stefanadis 2013. Flavonoids in atherosclerosis: An overview of their mechanisms of action. Curr. Med. Chem., 20: 2641–2660. https://doi.org/10.2174/0929867311320210003
Tabas, I., 2002. Cholesterol in health and disease. J. Clin. Invest., 110: 583-590. https://doi.org/10.1172/JCI0216381
Tesfaye, E.B., G.M. Animut, M.L. Urge and T.A. Dessie. 2014. Cassava root chips and Moringa oleifera leaf meal as alternative feed ingredients in the layer ration 1. J. Poult. Resour., 23: 614–624. https://doi.org/10.3382/japr.2013-00920
Tijani, L.A., A.M. Akanji, K. Agbalaya and M. Onigemo. 2016. Comparative effects of graded levels of moringa leaf meal on haematological and serum biochemical profile of broiler chickens. J. Agric. Sci., 11(3): 137-146. https://doi.org/10.4038/jas.v11i3.8167
Vergara-Jimenez, M., M.M. Almatrafi and M.L. Fernandez. 2017. Review: Bioactive components in Moringa oleifera leaves protect against chronic disease. Antioxidants, 6(91): 1-12. https://doi.org/10.3390/antiox6040091
To share on other social networks, click on any share button. What are these?







