Determination of Early Colonizer Urban Blowfly Species Using Small Bait Traps on a University Campus
Determination of Early Colonizer Urban Blowfly Species Using Small Bait Traps on a University Campus
Halide Nihal Açikgöz1*, Serdal Kenan Köse2 and Ali Açikgöz3
1Forensic Sciences Institute, Department of Forensic Biology, Ankara University, Ankara, Turkey
2Department of Biostatistics, Ankara University Medical School, Ankara-Turkey
3Havacılar Family Health Center, Yenimahalle, Ankara, Turkey
ABSTRACT
The aim of the present study was to identify the urban blowfly species having forensic importance on Ankara University Medical Faculty Cebeci Campus in Ankara. Eight small liver bait traps were used for catching flies. Chicken liver was used in the study to attract blowflies. Eggs and larvae of the flies that oviposited on the liver were raised and identified. Six different indices were used to determine diversity (Shannon-Wiener [H’], Simpson Dominance [Sd], Simpson Diversity [S], Margalef Species Richness Indices, Pielou’s Evenness [EH], and Simpson Resiprocal Indices). Calliphora vicina (Robineau-Desvoidy), Calliphora vomitoria (Linnaeus), Lucilia sericata (Meigen) and Lucilia richardsi (Collin) (Diptera: Calliphoridae) were identified. C. vicina was found in whole year in 2012 and 2015. All of the fly species identified have forensic importance. The highest taxa richness was observed in 2012, in May (307; 73.80%), while the lowest was in March (23; 5.53%). The highest taxa richness was observed in June (544; 21.92%), while the lowest was in January (6; 0.24%) in 2015. Macro and micro climatic factors and biodiversity of the fauna are significant for solving suspect entomological cases.
Article Information
Received 23 July 2016
Revised 18 August 2016
Accepted 30 August 2016
Available online 10 November 2016
Authors’ Contributions
AA evaluated and identified the entomological specimens. SKK performed statistical analysis. HNA collected entomological samples and supervised the work. All authors contributed to writting of manuscript.
Key words
Biodiversity, Calliphoridae, Diptera, Forensic entomology, Small bait trap.
* Corresponding author: nacikgoz@yahoo.com
0030-9923/2017/0001-0121 $ 9.00/0
Copyright 2017 Zoological Society of Pakistan
INTRODUCTION
Forensic entomology is the science enlightening forensic cases by examining the arthropods coming to corpses in the estimation of the minimum post-mortem interval (Amendt et al., 2004, 2007; Benecke, 2005; Goff, 2001; Marhoff et al., 2016). Different species feed on cadavers in different phases of decomposition depending on their biological structures. Behaving selective against decomposition stages, different insects are identified in different periods in many cadavers (Fremdt and Amendt, 2014). Blowflies reaching a corpse within the shortest time are generally used to estimate the post-mortem interval (Wallman, 2002). Forensic entomology provides information about the time of death and also help forensic entomologists to determine whether the body was removed from the crime scene, to establish a relationship between insects and the suspect, to help in child, elderly and disabled neglect and abuse cases. Species of necrophage insect families come to live in human and animal wounds and otherbody’s areas and cause myiasis. Flies which come to humans and animals belong to the Calliphoridae, Muscidae, Syrphidae, Phoridae, Piophilida, Sarcophagidae and Psychodidae families. While solving forensic cases, faunal evidence is used to predict the time of death, time of wound and place of primer crime scene. Determining fauna of primary sarcosaprophagous Diptera species which colonize corpses and using obtained knowledge in medico-legal investigations have economic benefits in addition to prevent loss of time. Availability of accurate data about local faunas of these species in a city will help both armed forces and legal authorities to solve cases more quickly.
Turkey is located in the Palearctic region including Europe, Asia and some parts of Africa (Konstantinov et al., 2009). Although located between Europe and Asia, the country has a geological structure different from the two continents. When considering the geological history of Anatolia and its ecological characteristics, it is the only country that shelter many species and is the center of many types of genes. It has a privileged position in terms of biodiversity due to having a place in a region where three continents are combined (Kence, 1987; Kışlalıoğlu and Berkes, 1987).
It is divided into seven biogeographical regions; namely, Marmara Region, Black Sea Region, Aegean Region, Central Anatolian Region, Eastern Anatolian Region, South Eastern Anatolian and Mediterranean Region (Fent et al., 2011; Gross, 2012). Kahraman et al. (2012) reported that Turkey always has four seasons in a year. The country usually experiences four seasons simultaneously due to its climatic and geographical characteristics. For these reasons, it has a rich biological diversity of insect fauna. Ankara is located in Central Anatolia and has a climate with heavily snowy and very cold and rainy winters, however, summers are very hot and dry. There is not a great deal of knowledge about insect faunas of Turkey and its neighboring countries (NBSAP, 2008). Multicenter studies from different countries and dissertations on comparisons of faunas in different countries will contribute to the available literature and provide guidance for further studies.
The present work represents series of studies aiming to determine local insect faunas of Ankara and was directed towards identifying primary early blowfly species colonizing cadavers and having forensic importance in Cebeci, a district located in the center of Ankara.
MATERIALS AND METHODS
Series of field trials were conducted in March, May, September and December 2012. Later, other trials were carried out throughout the whole 2015 year.
Study area
The study setting is located in the center of Ankara city at coordinates of 39º56’02.66”N latitute and 32º53’06.91”E longitude. The campus is located at an altitude of 850 m. There are some departments of Medical Faculty. Two small parks for patients and their visitors, two canteens, three big cafeterias, a garage, a bank, a dormitory, a kindergarten, a department store, 10 cats, 2 dogs and several doves (Columbidae, Columbiformes) on the campus. It is surrounded by a neighborhood where families live, military buildings, government buildings, shopping centers, restaurants and a cenotaph for soldiers. An area on the campus where early blowflies would not be disturbed was selected as a study setting.
Trapping, preservation and identification
Eight small liver bait traps were used. These traps were easy to set in the study setting. In the middle of a 500 ml- plastic water bottle, two opposing holes were opened. On the bottom of the bottle, 150 g of chicken liver was placed and then the bottle was closed. This was to prevent rain or snow from entering the trap. At the end, arope was passed through the bottle. The traps were bound to the trees in the garden and placed on the floor at a distance of 10 m apart.
A “Daily Data Control Sheet” was prepared to record the times of examinations and ambient temperatures. Changes in the ambient temperature were recorded with a digital thermometer.
Daily hourly temperatures issued by Weather Underground, Inc. on http://turkish.wunderground.com and ambient temperatures measured on the campus of Forensic Sciences Institute during the study period were recorded. The traps were checked hourly during working hours (from 08:00 to 17:00) throughout March, May, September and December 2012 and whole year in 2015.
All traps were kept in their places during 2012 and 2015 studies. Adult flies coming to the traps were trapped daily. Only the eggs collected in March and December 2012 were reared in an incubator at a constant temperature of 25±2°C. The eggs and the larvae in the traps were left in place until they became adults. Adult flies were killed with ethyl acetate vapors.
The adults and larvae were identified under Leica S8APO trinocular stereo zoom research microscope in Forensic Entomology/Forensic Biology Laboratory at Forensic Sciences Institute, University of Ankara. During their identification, Szpila (2010), (2012) and Smith (1986), identification keys were used.
Statistical analysis
Bioidversity indices were used in the present study: Shannon-Wiener biodiversity index [H], Simpson Dominance Index [Sd], Simpson Diversity Index [S], Margalef Species Richness Index to specify the type of density, Pielou’s Evenness [EH] and Simpson Reciprocal Index.
Shannon-Wiener and Simpson diversity indices were used to determine the species diversity. For the assessment of dominance, Simpson Dominance Index was used. To determine the types of community-density relation Pielo’s-EvennesIndex was used.
The distribution of species richness and individuals dominance between species in an area in a given time periodis indicated by the Shannon-Wiener Index. Obtaining a high value of species richness shows a numerical equality between species.
Shannon-Wiener index was calculated using the following formula:
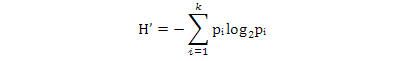
k, number of species; pi, proportion of the ith species from the total pool of species; ni, number of the ith species individual; N, total number of individuals.
Pielou’s Eveness Index shows that distribution of a number of individuals between species is homogeneous. When the index value is close to 1, the distribution is even. When it is closeto 0, distribution is uneven.
Pielou’s Eveness Index was calculated using the following formula:
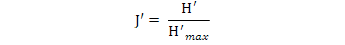
J′, J′ index value; H, Shannon-Wiener index; H′max, log2 S, (maximum diversity); S, species number.
Margalef Species Richness defines whether a region is rich and diverse in terms of species number. A high value of the index shows a richness in species.
Margalef species richness index was calculated as below:
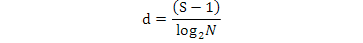
S, total number of species; N, total number of individuals.
Simpson Dominance Index (D) indicates whether a species is dominant in an area. Simpson Diversity Index (1/D) shows the distribution between individuals’ species. In terms of the species number.
Simpson Dominance Index, Simpson Diversity Index and Simpson Reciprocal Index were calculated using the following formulae:
Simpson dominance index (Simpson index):
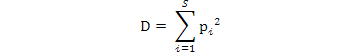
Simpson diversity index:
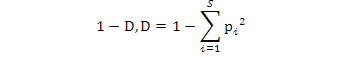
Simpson reciprocal index:
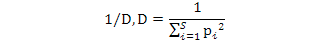
All descriptive statistics were determined using SPSS software 16 and BioDiversity Prosoftware for Ecology.
RESULTS
The focus of the present work is to determine and identify early blowfly species colonizing cadavers and having forensic importance in Cebeci, a district located in the centre of Ankara. The mean temperatures obtained are presented in Table I.
During 2012 studies, four fly species belonging to the Calliphoridae family were identified on Ankara University Medical Faculty Cebeci Campus in Ankara province. Out of four species, two belonged to subfamily Calliphorinae Calliphora vicina (Robineau-Desvoidy, 1830) and Calliphora vomitoria (Linnaeus, 1758) and two belonged to subfamily Luciliinae Lucilia sericata (Meigen, 1826) and Lucilia richardsi (Collin, 1926). Species from family Sarcophagidae (Diptera) and Dermestes sp. (Linnaeus, 1758) (Coleoptera: Dermestidae) were also identified. The seasonality of Calliphoridae species identified in 2012 surveys according to study periods is shown in Table II.
In September, in a very short time (about three minutes) after the liver trap was placed on the floor of the Institute’s garden, wasps which belong to Vespula germanica (Fabricius, 1793), Vespula vulgaris (Linnaeus, 1758) (Hymenoptera: Vespidae) swarmed around the liver. The 3rd instar larvae in the trap were collected to protect them against wasps’ attacks. For this reason, adult species could not be obtained.
In 2015, a total number of 2482 arthropods comprising of three orders and seven families were collected from all eight traps on the Cebeci Campus. Three fly species belonging to family Calliphoridae were identified out of three species, two belonged to subfamily Calliphorinae C. vicina (Robineau-Desvoidy, 1830) and Calliphora vomitoria (Linnaeus, 1758) and one belonged to subfamily Luciliinae Lucilia sericata (Meigen, 1826).
| Months | Meteorology Station |
Ambient Temperature |
| March, 2012 |
3.38+3.50 |
6.74+5.00 |
| May, 2012 |
19.64+2.61 |
24.13+3.25 |
| September, 2012 |
16.00+1.73 |
21.00+2.89 |
| December, 2012 |
0.40+4.22 |
1.00+4.18 |
| January, 2015 |
1.42 +4.26 |
1.50 +0.71 |
| February, 2015 |
3.50 +4.29 |
3.00 +1.41 |
| March, 2015 |
6.77 +2.94 |
8.50 +2.12 |
| April, 2015 |
8.33 +3.90 |
6.00 +2.83 |
| May, 2015 |
16.03 +3.17 |
17.00 +4.24 |
| June, 2015 |
18.37 +1.47 |
20.00 +1.41 |
| July, 2015 |
23.39 +2.25 |
28.50 +4.95 |
| August, 2015 |
23.87 +2.43 |
28.00 +2.83 |
| September, 2015 |
22.10 +1.65 |
20.50 +3.54 |
| October, 2015 |
13.87 +3.30 |
13.50 +0.71 |
| November, 2015 |
8.17 +1.91 |
8.00 +2.83 |
| December, 2015 |
-0.50 +6.36 |
0.50 +2.12 |
Table II.- The seasonality of Calliphoridae species identified in 2012 surveys according to study periods.
|
Name of species |
Mar. |
|
May |
|
Sept. |
|
Dec. |
|
Total |
|||||
| n | % |
|
n | % |
|
n | % |
|
n | % |
|
n | % | |
| C. vicina | 23 | 5.90 |
|
213 | 54.62 |
|
46 | 11.79 |
|
25 | 6.41 |
|
307 | 78.72 |
| C. vomitaria | 0 | 0.00 |
|
30 | 7.69 |
|
7 | 1.79 |
|
0 | 0.00 |
|
37 | 9.49 |
| L. sericata | 0 | 0.00 |
|
17 | 4.36 |
|
8 | 2.05 |
|
0 | 0.00 |
|
25 | 6.41 |
| L. richardsi | 0 | 0.00 |
|
21 | 5.38 |
|
0 | 0.00 |
|
0 | 0.00 |
|
21 | 5.38 |
| Total | 23 | 5.90 |
|
281 | 72.05 |
|
61 | 15.64 |
|
25 | 6.41 |
|
390 |
100.00 |
1Third instar larvae collected and identified.
Table III.- The seasonality of Calliphoridae species identified in 2015 study according to study period.
| Species |
Months |
|||||||||||||
| Jan | Feb | Mar | Apr | May | Jue | Jul | Aug | Sept | Oct | Nov | Dec | Total | ||
| C. vicina |
n % |
6 0.24 |
65 2.62 |
68 2.74 |
22 0.89 |
334 13.47 |
226 9.11 |
132 5.32 |
67 2.70 |
20 0.81 |
27 1.09 |
43 1.3 |
7 0.3 |
1017 41.01 |
| C. vomi-toria |
n % |
0 0.00 |
0 0.00 |
3 0.12 |
18 0.73 |
16 0.65 |
35 1.41 |
24 0.97 |
38 1.53 |
28 1.13 |
14 0.56 |
0 0.0 |
0 0.0 |
178 7.10 |
| L. sericata |
n % |
0 0.00 |
0 0.00 |
0 0.00 |
4 0.16 |
100 4.03 |
65 2.62 |
102 4.11 |
183 7.38 |
38 1.53 |
5 0.20 |
2 0.1 |
0 0.0 |
499 20.12 |
| M. dom-estica |
n % |
0 0.00 |
0 0.00 |
0 0.00 |
0 0.00 |
15 0.60 |
25 1.01 |
15 0.60 |
40 1.61 |
23 0.93 |
19 0.77 |
0 0.0 |
0 0.0 |
137 5.52 |
| M. sta bulans |
n % |
0 0.00 |
0 0.00 |
0 0.00 |
0 0.00 |
0 0.00 |
0 0.00 |
17 0.69 |
0 0.00 |
0 0.00 |
0 0.00 |
0 0.0 |
0 0.0 |
17 0.69 |
| Antho myiidae |
n % |
0 0.00 |
0 0.00 |
0 0.00 |
1 0.04 |
10 0.40 |
42 1.69 |
39 1.57 |
58 2.34 |
0 0.00 |
0 0.00 |
0 0.0 |
0 0.0 |
150 6.05 |
| Sarco ph agidae |
n % |
0 0.00 |
0 0.00 |
0 0.00 |
5 0.20 |
56 2.26 |
63 2.54 |
34 1.37 |
50 2.02 |
28 1.13 |
15 0.60 |
0 0.0 |
0 0.0 |
251 10.12 |
| Histeri-dae |
n % |
0 0.00 |
0 0.00 |
0 0.00 |
0 0.00 |
0 0.00 |
25 1.01 |
0 0.00 |
0 0.00 |
0 0.00 |
0 0.00 |
0 0.0 |
0 0.0 |
25 1.01 |
| Derme s-tidae |
n % |
0 0.00 |
0 0.00 |
0 0.00 |
0 0.00 |
0 0.00 |
34 1.37 |
0 0.00 |
0 0.00 |
0 0.00 |
0 0.00 |
0 0.00 |
0 0.0 |
34 1.37 |
| Vesp idae |
n % |
0 0.00 |
0 0.00 |
0 0.00 |
0 0.00 |
4 0.16 |
29 1.17 |
56 2.26 |
27 1.09 |
42 1.69 |
16 0.65 |
0 0.0 |
0 0.00 |
174 7.02 |
| Total |
n % |
6 0.24 |
65 2.62 |
71 2.86 |
50 2.02 |
535 21.57 |
544 21.94 |
419 16.90 |
463 18.67 |
179 7.22 |
96 3.87 |
47 1.1 |
7 0.28 |
2482 100 |
Species from family Anthomyidae (Diptera), Musca domestica (Linnaeus, 1758), Muscina stabulans (Fallén, 1817) (Diptera: Muscidae), family Sarcophagidae (Diptera), Dermestes lardarius (Linnaeus, 1758) (Coleoptera: Dermestidae), family Histeridae (Coleoptera), family Vespidae (Hymenoptera) were also identified. As seen in Table III, the highest taxa richness was observed in June (544; 21.92 %), while the lowest was in January (6; 0.24%). Monthly values of biodiversity indices during trapping periods of 2012 and 2015 are shown in Tables IV and V.
Table IV.- Monthly values of biodiversity indices in 2012.
| Biodiversity indices |
Months |
|||
| Mar. | May | Sept. | Dec. | |
| Species number | 1 | 4 | 3 | 1 |
| Species individuals number | 23 | 281 | 61 | 25 |
| Biodiversity indices | ||||
| Shannon-Wiener [H’] | 0.000 | 1.172 | 1.050 | 0.000 |
| Simpson Dominance [Sd] | 1.000 | 0.595 | 0.599 | 1.000 |
| Simpson Diversity [S] | 0.000 | 0.405 | 0.401 | 0.000 |
| Margalef Species Richness Index | 0.000 | 0.369 | 0.337 | 0.000 |
| Density indices | ||||
| Pielou’s Evenness [EH] | 0.000 | 0.586 | 0.662 | 0.000 |
| Simpson Reciprocal Index (1/D) | 1.000 | 1.680 | 1.669 |
1.000 |
DISCUSSION
Our goal in this study is to answer one of the frequently asked questions in forensic cases, that is whether a corpse has been moved or not. Forensic entomologists answer this by investigating blowflies that have forensic importance. To contribute to the data needed to answer these questions, this study also focused on identifying fly species having forensic importance on Ankara University Medical Faculty Cebeci Campus, located in Ankara.
The diversity indices of insect communities describe their response to environmental factors. The number of species that defines the structure of the community and balanced distribution of individuals belonging to the species or the abundance of different species is the total number of individuals (Morris et al., 2014).
Diversity indices have the advantage of being independent of sample size and is dimensionless. The individuals number has a number of disadvantages due to being reduced to anonymous numbers. Time-dependent diversity variations are more informative than absolute diversity value.
In the study that was conducted in 2012, when Shannon-Wiener Index was applied; H value was the highest in May representing 1.172, whereas a value of 0 was found in March and December. During these two months only C. vicina was observed. This may be due to low temperature
Table V.- Monthly values of biodiversity indices in 2015.
| Jan. | Feb. | Mar. | Apr. | May | June | July | Aug. | Sept. | Oct. | Nov. | Dec. | |
| Species number | 1 | 1 | 2 | 5 | 7 | 9 | 8 | 7 | 6 | 6 | 2 | 1 |
| Species inividuals number | 6 | 65 | 71 | 50 | 535 | 544 | 419 | 463 | 179 | 96 | 45 | 7 |
| Shannon-Wiener [H’] | 0.00 | 0.00 | 0.25 | 1.78 | 1.67 | 2.67 | 2.61 | 2.49 | 2.53 | 2.45 | 0.262 | 0.00 |
| Simpson Dominance [Sd] | 1.00 | 1.00 | 0.91 | 0.34 | 0.43 | 0.22 | 0.19 | 0.22 | 0.17 | 0.19 | 0.915 | 1.00 |
| Simpson Diversity [S] | 0.00 | 0.00 | 0.08 | 0.66 | 0.52 | 0.77 | 0.80 | 0.78 | 0.82 | 0.80 | 0.085 | 0.00 |
| Margalef Species Richness Index | 0.00 | 0.00 | 0.16 | 0.70 | 0.66 | 0.88 | 0.80 | 0.67 | 0.66 | 0.75 | 0.182 | 0.00 |
| Pielou’s Evenness [EH] | 0.00 | 0.00 | 0.25 | 0.77 | 0.59 | 0.84 | 0.87 | 0.88 | 0.98 | 0.94 | 0.262 | 0.00 |
| Simpson Resiprocal Index | 1.0 | 1.00 | 1.08 | 2.94 | 2.28 | 4.51 | 5.05 | 4.50 | 5.61 | 5.14 | 1.093 |
1 |
in March and December and development temperature of C. vicina eggs being between 2.0-3.5°C (Davies and Ratcliffe, 1994).
Pielous’ Regularity Index was found the lowest in March and December representing 0 and the highest in May and September representing, respectively, 0.586 and 0.662. This shows that the distribution of species is unregular in March and December and regular between May and September.
Margalef Index ranged from 0 to 0.369 in 2012. The average was found to be 0.177. The highest values were obtained in May (0.369) and September (0.337).
Simpson Dominance Index differed between 0.599 and 1, and Simpson Diversity Index ranged between 0 and 0.405.
Dominance Index in March and December was found to be 1 shows the dominance of C. vicina but less species diversity.
Simpson’s Diversity Index were found between 1 and 1.680. This low value indicates weak species richness. This may be due to the small number of samples data that could not be collected during March, May, September and December 2012 and all months of 2015.
The absence of sampling during March, April, September and December and during other months of the year caused a decrease in number of data.
In the surveys that was conducted in 2015, Shannon-Wiener Index (H’) was found between 0 and 2.677. The obtained value of 0 shows that diversity is low during January, February and December since only one species of C. vicina was observed. H value was the highest in June reaching 2.677. The index decreased gradually toward the end of the year with a value of 0.262 in November and reached 0 in December. Thus, diversity decreases during cold months and increases during the months where air temperature increases.
Pielous’ Eveness Index value was the lowest during January, February, and December representing 0, and reached the highest value between June and October. Overall, number of individuals in this month reached the highest value. This value shows irregularities in the distribution of the species when it is found to be close to 0 while it shows uniform distribution of the species when it is reported to be close to 1.
Margalef index value in January, February and December was 0, then rose to 0.163 in March and to 0.880 in June. The low values observed in January, February and December illustrate the dominance by the presence of only one species. The highest values in April, October and December explain the richness of species diversity. Parallelism in Shannon-Wiener and Pielous’ Eveness indices results that we found shows that both tests gave similar results.
Simpson dominance index value was 1 during January, February and December, 0.919 during March, and 0.915 in November. These results highlight the dominance of C. vicina species and reveals a low diversity. Variation of the value between 0.178 and 0.340 shows the presence of a much higher diversity.
Simpson diversity index during January, February, and December was found 0, and varied between 0.081 and 0.822 in the other months. Between April and October, species diversity is rich due to the relatively high temperature while diversity was lower in January, February, March, November and December. Shannon-Wiener Index results were parallel giving similar values.
Simpson Reciprocal Index was found between 1.000 and 5.616. Being between 1.000 and 1.093 due to lower the temperature in January, February, March, April, November and December, this index shows that the number of species is reduced. During May, June, July, August, September and October, it shows that number of species is higher.
As shown in Tables II and III, C. vicina was collected every month of the two studies periods. C. vicina is cosmopolitan species that arrives within the first four or seven minutes after death and begins to lay eggs on the carcass (Pellitero and Bordas, 2007). These observations are in agreement with our results since C. vicina arrived to the traps within four minutes during our surveys. In addition, the Table II shows that the highest taxa richness was observed in May (307; 73.80%), while the lowest was in March (23; 5.53%). As seen in Table III, the highest taxa richness was observed in June (544; 21.92%), while the lowest was in January (6; 0.24%).
In a study conducted by Khoobdel et al. (2008) in Iran, which is located in the Palearctic region similar to Turkey, C. vicina was found to be active throughout the year. In addition, Wallman (2002) from the Australian region reported that C. vicina has spread to the world and appears throughout the year. Also, Prado e Castro et al. (2010) in a study in Portugal which is also located in the Palearctic region, observed that C. vicina is a cosmopolitan species. Similarly, in our work C. vicina was identified among collected specimens throughout the study period in 2012 and 2015.
Predominancy of C. vicina in this study can be explained by the ability of this species to continue living under low temperatures. Results of the present study are consistent with those of Faucherre et al. (1999) who reported a case in which C. vicina was able to fly and oviposit under extreme conditions. The present study also revealed that C. vicina continued laying eggs and growing although the average air temperature was 6.74± 5.00°C in March and 1.00± 4.18°C in December. In fact, Marchenko (2001) reported that sub-development temperatures were 2°C for C. vicina, 3°C for Calliphora vomitoria and 9°C for Lucilia sericata. Nonetheless, an unexpected decrease in the temperature in March and December led to freezing of eggsand thus to death of the larvae. Therefore, the eggs were reared in an incubator at a constant temperature of 25±2°C.
In the present study, Lucilia sericata was found in May. These observations are in agreement with those reported by Lord (1990), Campobasso et al. (2001), Sevgili et al. (2004) and Sabanoğlu and Sert (2010). Sevgili et al. (2004) in their study in Şanlıurfa, Turkey found Lucilia sericata between May and October and C. vicina in May, September and October but Calliphora vomitoria only in September. As seen in Table II, Lucilia sericata was found in May and September, C. vicina throughout the year and Calliphora vomitaria in both May and September. Sabanoğlu and Sert (2010) at Beytepe Campus, Ankara province, identified C. vicina, Calliphora vomitoria, Phaenicia sericata (syn. Lucilia sericata) and Chrysomya albiceps. Karatepe et al. (2005) stated that the flies coming to the meat left outdoor after “Kurban Bayramı” (feast of sacrifice, a celebration held in Islamic countries) were mostly C. vicina and Muscina stabulans (Fallen, 1817). Likewise, in the present study, as could be seen in Tables II and III, C. vicina was identified in the pieces of liver in each month of the two studies periods. Fly species that first arrive to cadavers in nature are blowflies species (blue and green bottle flies) belonging to Calliphoridae family. It is also reported in the literature that C. vicina is the species that first comes to cadavers and is quite high in number (Byrd and Castner, 2010). In a study on the fly fauna conducted in the rubbish dumps of the towns of Mamak and Sincan, in Ankara, Güler (2000) collected Lucilia cuprina (Wiedemann, 1830) between June and September and Lucilia sericata between April and October. Şaki and Özer (1999) investigated the species that cause external myiasis in Elazig. Eight species were identified including Lucilia sericata, Lucilia caesar, C. vicina, Calliphora vomitoria, Chrysomya albiceps, Wohlfahrtia magnifica, Sarcophaga haemorrhoidalis and Sarcophaga carnaria. Human myiasis caused by Sarcophaga, Wohlfahrtia, Calliphora and Lucilia species play an important role (Kokcam and Saki, 2005). The holoarctic species Lucilia sericata is considered as a common species in the World and causes myiasis in animals (Eren et al., 2010). In wound myiasis, female flies belonging often to Calliphoridae family leave their eggs or first-instar larvae on neglected wounds and cause tissue destruction (Karakuş et al., 2015; Kaya et al., 2014; Kılıç et al., 2011).
In the present study, Vespula germanica (Fabricius, 1793) and Vespula vulgaris (Linnaeus, 1758) attracted to the liver trap and prevented the flies from laying their eggs on the liver. In parallel with the results of the study by Foil and Hogsette (1994), wasps were observed to capture eggs, larvae and adult flies and divide them into pieces in the present study. Vespidae family, extremely active in July and August, was also found to appear in autumn. Archer and Elgar (2003) determined that blowflies came in the decomposition process in Victoria, Australia, and reported that wasps consumed the meat quickly and attacked the blowflies in the meat. Moretti et al. (2011) in a study noted that species of blowflies could not be determined since premortem wounds were remarkably disrupted due to wasp attacks after death, which is comparable with the results of the present study. In fact, blowflies were not able to lay eggs due to wasp attacks (Archer and Elgar, 2003).
CONCLUSION
The goal of the present study is to determine local faunas of Ankara and identify early blowfly species colonizing cadavers and having forensic importance in Cebeci, a district located in the centre of Ankara. Data from forensic entomologic studies from Turkey has been increasing. The results obtained from the present study will contribute to this accumulation of information. Understanding the insect fauna in different locations of Ankara will also contribute to this. Forensic entomology studies mostly aim to estimate the time of death and specify the place of death. These studies usually help us to obtain reliable and rapid results in consideration of the local fauna. It suggests that animal models should be increased in Turkey to identify the insect fauna for forensic purposes. In further studies, faunas of other locations in Ankara will be revealed.
Acknowledgment
We would like to thank Nursel Duransoy (MA), native-like speaker of English specializing in English Language Teaching at Yeditepe University, for revision of the language of this article.
Conflict of interest statement
We declare that we have no conflict of interest.
REFERENCES
Amendt, J., Campobasso, C., Gaudry, E., Reiter, C., LeBlanc, H. and Hall, M., 2007. Best practice in forensic entomology--standards and guidelines. Int. J. Legal Med., 121: 90-104. http://dx.doi.org/10.1007/s00414-006-0086-x
Amendt, J., Krettek, R. and Zehner, R., 2004. Forensic entomology. Naturwissenschaften. 91: 51-65. http://dx.doi.org/10.1007/s00114-003-0493-5
Archer, M. and Elgar, M., 2003. Effects of decomposition on carcass attendance in a guild of carrion-breeding flies. Med. Vet. Ent., 17: 263-271. http://dx.doi.org/10.1046/j.1365-2915.2003.00430.x
Archer, M. and Elgar, M. 2003. Yearly activity patterns in southern Victoria (Australia) of seasonally active carrion insects. Foren. Sci. Int., 132: 173-176. http://dx.doi.org/10.1016/S0379-0738(03)00034-3
Benecke, M., 2005. Arthropods and corpses. Foren. Pathol. Rev., 2: 207-240. http://dx.doi.org/10.1385/1-59259-872-2:207
Byrd, J. and Castner, J., 2010. Insects of forensic importance. In: Forensic entomology: The utility of arthropods in legal investigations (eds. J. Byrd and J. Castner). CRC Press LLC, USA. pp. 41-111.
Campobasso, C., Di Vella, G. and Introna, F., 2001. Factors affecting decomposition and diptera colonization. Foren. Sci. Int., 120: 18-27. http://dx.doi.org/10.1016/S0379-0738(01)00411-X
Davies, L, and Ratcliffe, G., 1994. Developmental rates of some pre-adult stages in blowflies with reference to low temperatures. Med. Vet. Entomol. 8:245-254.
Eren, H., Aypak, S., Ural, K. and Seven, F., 2010. Lucilia sericata (diptera: Calliphoridae) larvalarına bağlı kedide ocular ve köpekte travmatik myiasis olguları. Kafkas Univ. Vet. Fak. Derg., 16: 883-886.
Faucherre, J., Cherix, D. and Wyss, C., 1999. Behavior of Calliphora vicina (Diptera, Calliphoridae) under extreme conditions. J. Insect Behav., 12: 687-690. http://dx.doi.org/10.1023/A:1020931804597
Fent, M., Kment, P., Çamur-Elipek, B. and Kırgız, T., 2011. Annotated catalogue of Enicocephalomorpha, Dipsocoromorpha, Nepomorpha, Gerromorpha, and Leptopodomorpha (Hemiptera: Heteroptera) of Turkey, with new records. Magnolia Press, New Zealand. pp. 1-84.
Foil, L. and Hogsette, J., 1994. Biology and control of tabanids, stable flies and horn flies. Rev. Sci. Tech. Off. İnt. Epiz., 13: 1125-1158.
Fremdt, H. and Amendt, J., 2014. Species composition of forensically important blow flies (Diptera: Calliphoridae) and flesh flies (Diptera: Sarcophagidae) through space and time. Foren. Sci. Int., 236: 1-9. http://dx.doi.org/10.1016/j.forsciint.2013.12.010
Goff, M., 2001. A fly for the prosecution: How insect evidence helps solve crimes? Harvard University Press, England. pp. 22.
Gross, M., 2012. Turkey’s biodiversity at the crossroads. Curr. Biol., 22: 503-505. http://dx.doi.org/10.1016/j.cub.2012.07.064
Güler, Ş., 2000. Flies fauna in Ankara, Mamak and Sincan rubbish dump. Master, Ankara Üniversitesi.
Kahraman, A., Onder, M. and Ceyhan, E., 2012. The importance of bioconservation and biodiversity in turkey. Int. J. Biosci. Biochem. Bioinform., 2: 95-99. http://dx.doi.org/10.7763/IJBBB.2012.V2.79
Karakuş, M., Ünver, A., Turgay, N., Töz, S. and Özbel, Y., 2015. Ege üniversitesi hastanesi’nde yatmakta olan bir hastada nazal miyaz. Nasal myiasis in a patient hospitalized at ege university hospital. Ege Tıp. Dergisi., 54: 36-38.
Karatepe, M., Yağcı, Ş., Karatepe, B. and Karaer, Z., 2005. The remains of cattle slaughtered in the open fields provided the growth medium for the Myiasis fly. Turk. Parazitol. Derg., 29: 271-274.
Kaya, F., Orkun, Ö., Çakmak, A., İnkaya, A. and Ergüven, S., 2014. Cutanous myiasis caused by sarcophaga spp. larvae in a diabetic patient. Mikrobiyol. Bul., 48: 356-361. http://dx.doi.org/10.5578/mb.7107
Kence, A., 1987. Biological diversity in Turkey. Environmental Problems Foundation, Turkey. pp. 235.
Khoobdel, M., Jonaidi, N. and Rashti, M., 2008. Blowfly and flesh fly (Diptera: Cyclorrhpha) fauna in Tehran, Iran). J. Ent., 5: 185-192. http://dx.doi.org/10.3923/je.2008.185.192
Kılıç, K., Arslan, M. and Kara, M., 2011. A postoperative wound myiasis caused by Lucilia sericata (Diptera: Calliphoridae) in a woman in Kars. Turk. Parazitol. Derg., 35: 43-46. http://dx.doi.org/10.5152/tpd.2011.11
Kışlalıoğlu, M. and Berkes, F., 1987. Biological diversity. Environmental Problems Foundation, Turkey. pp. 122.
Kokcam, I. and Saki, C., 2005. A case of cutaneous myiasis caused by Wohlfahrtia magnifica. J. Dermatol., 32: 459-463. http://dx.doi.org/10.1111/j.1346-8138.2005.tb00780.x
Konstantinov, A., Korotyaev, B. and Volkovitsh, M., 2009. Insect biodiversity in the Palearctic region. Blackwell Publishing Ltd. http://dx.doi.org/10.1002/9781444308211.ch7
Lord, W. (Ed.), 1990. Case histories of the use of insects in investigations. In: Entomology and death; a procedural guide. Joyce’s Print Shop Inc., Clemson.
Marchenko, M.I., 2001. Medicolegal relevance of cadaver entomofauna for the determination of the time of death. Foren. Sci. Int., 120: 89-109. http://dx.doi.org/10.1016/S0379-0738(01)00416-9
Marhoff, S., Fahey, P., Forbes, S. and Green, H., 2016. Estimating post-mortem interval using accumulated degree-days and a degree of decomposition index in Australia: A validation study. Aust. J. Foren. Sci., 48: 24-36. http://dx.doi.org/10.1080/00450618.2015.1021378
Moretti, T.C., Giannotti, E., Thyssen, P., Solis, D. and Godoy, W., 2011. Bait and habitat preferences, and temporal variability of social wasps (Hymenoptera: Vespidae) attracted to vertebrate carrion. J. med. Ent., 48: 1069-1075.
Morris, E.K., Caruso, T., Buscot, F., Fischer, M., Hancock, C., Maier, T.S., Meiners, T., Müller, C., Obermaier, E., Prati, D., Socher, S.A., Sonnemann, I., Wäschke, N., Wubet, T., Wurst, S. and Rillig, M.C., 2014. Choosing and using diversity indices: Insights for ecological applications from the german biodiversity exploratories. Ecol. Evolut., 4: 3514-3524. http://dx.doi.org/10.1002/ece3.1155
NBSAP, 2008. The national biodiversity strategy and action plan. Tasarım Ofset Ankara. pp. 20. http://www.ormansu.gov.tr/
Prado e Castro, C., Arnaldos, M. and García, Y.M., 2010. Additions to the Calliphoridae (Diptera) fauna from Portugal, with description of new records. Boln. Asoc. esp. Ent., 33: 425-437.
Sabanoğlu, B. and Sert, O., 2010. Determination of Calliphoridae (Diptera) fauna and seasonal distribution on carrion in Ankara province. J. Foren. Sci., 55: 1003-1007. http://dx.doi.org/10.1111/j.1556-4029.2010.01366.x
Sevgili, M., Şaki, C. and Özkutlu, Z., 2004. External myiasis in the Şanlıurfa Province: The distribution of flies. Türk. Parazitol. Derg., 28: 150-153.
Smith, K., 1986. A manual of forensic entomology. University Printing House, Oxford. pp. 1-102.
Szpila, K., 2010. Key for the identification of third instars of European blowflies (Diptera: Calliphoridae) of forensic importance. In: Current concepts in forensic entomology (eds. J. Amendt, C. Campobasso, M. Goff and M. Grassberger). Springer. pp. 43-56.
Szpila, K., 2012. Key for identification of European and Mediterranean blowflies (Diptera, Calliphoridae) of medical and veterinary importance - adult flies. In: Forensic entomology, an introduction (ed. D. Gennard). Willey-Blackwell. pp. 77-81.
Şaki, E. and Özer, E., 1999. Morphology and development of several external myiasis larvae recorded in Elazığ. Turk. J. Vet. Anim. Sci., 23: 723-731.
Wallman, J.F., 2002. Winged evidence: Forensic identification of blowflies. Aust. J. Foren. Sci., 34: 73-79. http://dx.doi.org/10.1080/00450610209410839
To share on other social networks, click on any share button. What are these?










