Detection of Resistance in Lab and Field Collected Strains of Two Major Pests of Stored Grains against Deltamethrin and Phosphine
Detection of Resistance in Lab and Field Collected Strains of Two Major Pests of Stored Grains against Deltamethrin and Phosphine
Asim Munawar1,2*, Farooq Ahmad1, Aqsa Arshad1, Muhammad Ishaque Mastoi3 and Chengjuan Liang4
1Department of Entomology, University of Agriculture Faisalabad, Pakistan; 2Institute of Insect Sciences, College of Agriculture and Biotechnology, Zhejiang University, China; 3Plant Sciences Division, Pakistan Agricultural Research Council Islamabad, Pakistan; 4Institute of Applied Ecology, Fujian Agriculture and Forestry University, China.
Abstract | The lesser grain borer, Rhyzopertha dominica and red flour beetle, Tribolium castaneum are considered most destructive pests of storages commodities worldwide. Unfortunately, due to multiple applications of synthetic pyrethroids and fumigants some major pests of stored grains has develops resistance against them. Resistance mechanisms in R. dominica and T. castaneum against these control agents at different geographical locations was largely unknown. To overcome this gap our research was undertaken to evaluate the resistance mechanism in lab and field collected strains of R. dominica and T. castaneum against deltamethrin and phosphine. Results indicated significant difference between lab and field collected pests strains regarding their resistance mechanisms to deltamethrin as well as phosphine treatments. Field strains collected from various geographical locations were observed more resistant then lab strains. Calculated LC50 values was very less in field strains as compared to lab strains. Maximum mortality of lab and field collected strains of R. dominica and T. castaneum was observed against phosphine than against deltamethrin. Our results suggest that field strains experienced with different out door conditions was less susceptible as compared to lab strains which proved to be more susceptible and findings of this study could be helpful for effective management of these key pests keeping in mind the issue of resistance development.
Received | October 10, 2019; Accepted | January 13, 2020; Published | January 20, 2020
*Correspondence | Asim Munawar, Institute of Insect Sciences, College of Agriculture and Biotechnology, Zhejiang University China; Email: amento3762@hotmail.com; asim_munawar@zju.edu.cn
Citation | Munawar, A., F. Ahmad, A. Arshad, M.I. Mastoi and C. Liang. 2020. Detection of resistance in lab and field collected strains of two major pests of stored grains against deltamethrin and phosphine. Pakistan Journal of Agricultural Research, 33(1): 78-88.
DOI | http://dx.doi.org/10.17582/journal.pjar/2020/33.1.78.88
Keywords | Resistance, Strains, Deltamethrin, Phosphine, Mortality, Exposure
Introduction
World population is facing many important challenges for food safety as human population keep on increasing day by day with tremendous effects of food resources depletions (Tubeillo et al., 2007). During storages of grains mishandling along with other various environmental factors favors stored grains pests infestation (Upadhyay and Ahmad, 2011). Stored grains pests cause serious losses to storages commodities, by making grains unmarketable as well as uneatable due to decrease in nutritional values (Jood and Kapoor, 1994; Wavear and Subramanyam, 2000). Damaged grains loose their volume and average weight as well as seed germination badly (Nadeem et al., 2012).
Red flour beetle, T. castaneum and lesser grain borer, R. dominica are considered major and serious pests of storage commodities (Adedire, 2001). Both pests are a major cause of postharvest losses and contaminate or destroyed 5‒10 % food after harvest in the worldwide (Cao et al., 2002). Smallholder farmers experienced larger postharvest losses due to storage pests in developing countries with range 30‒80 % causing major threats to food security (Kitinoja et al., 2011; FAO and World Bank, 2011; Affognon et al., 2015). These pests main feed on kernels, contaminate grains with feces, webbing, exuviae, pests cadavers, and produces aflatoxins materials like uric acid, which promote micro floral growth causing nutritional losses and making food unfit for human consumption (Bhargava et al., 2007; Harris and Lind bland, 1976; Nighat et al., 2007; FAO and World Bank, 2011; Gustavasson et al., 2011; Flinn et al., 2010). Farmers who do not have access to modern grains storages techniques as well as facilities bears less grains loss to insect pasts during storages as compared to smallholders (Hodges et al., 2014). Introducing proper storage techniques that are socially as well as economically practical and sound to smallholder farmers can minimize the pest’s related losses during storages (Ali et al., 2012).
Mainly, small farmers in developing countries followed traditional storages techniques including pots, straw roofed storage structures, maize cribs, bamboo storage structures, underground storage, woven baskets, mud rhombus, storehouses or warehouse and bag storages (Barkholder and Faustini, 1991; FAO, 1994; Khan et al., 2010), as these techniques are cheapest, economically feasible and most importantly locally available (De Groote et al., 2013). Postharvest losses in bagged storages are prevalent than other storages techniques, while deltamethrin and phosphine are potentially affordable logical solution that can be favored to address the challenges in postharvest losses due to pests infestation (Costa, 2014; Anankware et al., 2014).
Phosphine and deltamethrin are broad spectrum insecticides widely used to control storage insects pests (Zettler and Arthur, 2000; Kljajic and Peric, 2009). These are registered insecticide and fumigant as grain protectants in many parts of the world especially in USA (Dotson et al., 2010; Vayias et al., 2010). Deltamethrin is a synthetic pyrethorid derived from Chrysanthemum flowers (Bhanu et al., 2011). This pesticide can be metabolized by animals and plants and less bio-accumulate as well as less dangerous due to their low toxicity levels (Collins et al., 2017; WHO, 1990; Dotson et al., 2010). Phosphine is a colorless flammable toxic gas, denser than air, collected from low lying areas. Phosphine have oxidative reaction, target insect respiration system, and cause death due to suffocations (Collins et al., 2002; Wang et al., 2006).
Many previous studies confirmed that phosphine and deltamethrin are effective grains protectants (Hasan et al., 1996; Arthur, 1997; Benhalima et al., 2004; Kavallierators et al., 2015; Paudyal et al., 2016; Collin et al., 2017). Since last few decades, major insect pests of stored grains especially T. castaneum and R. dominica has developed resistance against fumigants as well as plant derived synthetic pyrethroids (Lorini and Galley, 1999; Opit et al., 2012; Paudyal et al., 2016; Shahid et al., 2019). However very few researches documented resistance level of T. castaneum and R. dominica against commonly used grain protectants i.e., deltamethrin and phosphine (Opit et al., 2012; Chen et al., 2013; Ahmad et al., 2013; Kock et al., 2015; Opit et al., 2016; Collin et al., 2017). Due to these consequences current research was focused to evaluate the resistance mechanism of field and lab collected strains of T. castaneum and R. dominica against deltamethrin and phosphine. We hope our research could provide a strong base to cope with resistance mechanisms in major insect pets of stored grains against selectively used pyrethroids and fumigants.
Materials and Methods
Current experiments were conducted at Grain Research Training and Storage Management Cell, University of Agriculture Faisalabad, Pakistan. Our research was subjected to evaluate resistance mechanism in lab and field collected strains of red flour beetle, T. castaneum and lesser grain borer, R. dominica against commonly used synthetic pyrethyroid deltamethrin and fumigant phosphine.
Collection of insects
Adults as well as immature of T. castaneum and R. dominica was collected from stores, godowns and markets located at different geographical positions of Faisalabad Pakistan. Collected strains were brought to laboratory for culturing homogeneous populations.
Rearing of insects
Sterilized pathogens free glass jars were used to kept collected population of both strains. Well cleaned sterilized wheat flour was used as diet for insects under study, and closed with muslin cloths to avoid entry of any other pathogen’s and insects. Rearing jars was placed in incubators (28 ± 2°C and R.H 65 ± 5%) and regularly monitored to check insect infestations. Around one hundred insects were added in each jar by assuming 50:50 male female ratio. Both strains were cultured in individually managed jars. Insects was allowed three days in each jars for mating and oviposition after which they were transferred to another new sterilized jars for further infestation to build up huge insect populations. Infested wheat flour and grains were transferred to another new jar to get different larval stages and adults. Both strains takes around forty days to become adults. Reared population was sieved out to collect equal size and aged adults for experiments tests.
Insecticides resistance
Deltamethrin with trade name “Guardian 1.5 EC” was obtained from Ali Akbar Group Pvt. Ltd. Deltamethrin was diluted in ethanol to make different concentrations (0.01, 0.02, 0.03, 0.04, 0.05 and 0.06 ppm). With the help of micro syringe, deltamethrin was applied on blotter papers and allowed to dry over time. Dry blotter paper was placed in petri dishes (100 x 15mm) in size. Wheat grains (5gm) were sterilized and transferred to each treated petri dishes. Twenty adults of each strain was inoculated in each petri dish. Control treatment received distilled water only. Mortality data was collected after 24, 48 and 72 hours of intervals. The exposure experiment was simultaneously undertaken for lab and field strains and replicated three times. Following formula was used to calculate corrected mortality:
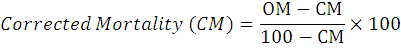
Where;
CM stands for Control mortality, OM for Observed mortality.
Phosphine resistance
To get fumigant gas Aluminum phosphide tablets manufactured by “Power Group” was obtained trading as Aluminum phosphide. Water displacement method was applied to release gas from tablets. A glass jars brought with two liter water capacity was half filled with water. On the bottom of jar, funnel was adjusted in a way the broad open section should be towards bottom. Plastic bottle with one liter capacity was filled with water. Aluminum phosphide tablets was placed between funnel as well as bottom of jar, after which the plastic bottle which was already filled with water was immediately was placed on the cross section of funnel. Produced phosphine gas started to accumulate on the bottom of plastic bottle, while water from this bottle was removed due to gas pressure. A rubber septum was placed on the bottom of bottle. Gas produced by tablets was taken from bottle through rubber septum with the help of micro syringe. Six concentration (0.006, 0.007, 0.008, 0.009, 0.010, and 0.011 ppm) was prepared from collected gas. Phosphine meter was used to measure gas concentrations. While control received distilled water only. For treatments desiccator was used as fumigant chamber, while twenty adults insect were confined into ventilated glass jars. Upper hole of desiccator was covered with rubber septum. Micro syringe was use to transferred gas into chamber. Both strains received same treatments. Mortality data was calculated after 24, 48 and 72 hours of intervals. Before taking mortality data insects was placed in fresh air for 24 hours to allow recovery or delayed mortality. To calculate resistance factor for both strains against phosphine and deltamethrin following formula was applied.
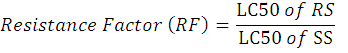
Where;
RS stands for Resistant strain, SS for Susceptible strain, LC for Lethal concentration.
Statistical analysis
Collected data was subjected to probit analysis to determine resistance factor (RF) and LC50 of susceptible strains. Mortality was observed at 24, 48 and 72 hours of intervals. Mortality data were analyzed to determine concentration that kill 50% of insect’s population. This concentration will be referred as LC50. Poloplus software was used to conduct probit analysis (LeOra Software, 2005). Mortality data received at different concentration was analyzed by Analysis of Variance (ANOVA). When results were significant, the comparisons among means was carried out by Tucky HSD test at 5% significance level.
Results and Discussion
The present study was focused to evaluate resistance mechanism of field and lab strains against two commonly used insecticides. Deltamethrin and phosphine has been used for many year for the control of storages insect’s pests especially T. castaneum and R. dominica. Factorial analysis was applied to compare the effect of both tested insecticides against currently studied two major insect pests of stored grains.
Our results conclude that field collected strains showed maximum resistance to deltamethrin and phosphine as compare with lab strains. Lesser grain borer, R. dominica was observed more resistant than red flour beetle, T. castaneum. This showed that how geographical positions of localities and factors affect the biology as well as insect behavior against control strategies. Lab strain normally have less experience to outgoing factors which make them unable to resist against insecticides and fumigants. Mostly in field we cannot apply phosphine against storage insect’s pests due to its gaseous form, but deltamethrin and plant derived pyrethroids can successfully be applied. Most importantly in fields or other big commodities storage holders directly or indirectly depends on synthetic pyrethroids to secure their food against insects pests, but phosphine have a little use like these localities. Lab strains due to repeated application of insecticides and fumigants develop less resistance as field strains (Tables 1, 2).
Table 1: Factorial analysis for mortality of field and lab strains of T. castaneum and R. dominica against different concentrations of deltamethrin (P < 0.05; df: degree of freedom; n=20).
| Source | df | Tribolium castaneum | df | Rhyzopertha dominica | ||
| F | P | F | P | |||
| Concentration | 5 | 190.936** | 0.0042 | 5 | 81.018** | 0.0013 |
| Exposure time | 2 | 231.910** | 0.0031 | 2 | 122.662** | 0.0021 |
| Concentration × Exposure time | 10 | 4.156* | 0.0328 | 10 |
0.510ns |
0.8718 |
| Error | 36 | ― | — | 36 | ― | — |
| Total | 53 | — | — | 53 | — | — |
Table 2: Factorial analysis for mortality of field and lab strains of T. castaneum and R. dominica against different concentrations of phosphine (P < 0.05; df: degree of freedom; n=20).
| Source | df | Tribolium castaneum | df | Rhyzopertha dominica | ||
| F | P | F | P | |||
| Concentration | 5 | 136.063** | 0.0089 | 5 | 158.621** | 0.0062 |
| Exposure time | 2 | 150.671** | 0.0033 | 2 | 284.257** | 0.0031 |
| Concentration × Exposure time | 10 | 11.269* | 0.0016 | 10 | 7.020* | 0.0145 |
| Error | 36 | ― | — | 36 | ― | — |
| Total | 53 | — | — | 53 | — | — |
Different concentration of insecticides and fumigants was applied to check the mortality rate of both tested strains. Our results showed that deltamethrin and phosphine cause maximum mortality as compared to field strains, but undoubtly phosphine cause maximum mortality of T. castaneum as compared to deltamethrin (Figure 1). Mortality of T. castaneum gradually increased as concentrations was incresed. Maximum mortality rate was observed at 0.06 against deltamethrin.
Basically synthetic pyrethroids and fumigants become unable to control major insects pests of stored products due to their repeated applications. Now a days synthetic pyrethroids and fumigants become essential components of storage products pests managments systems. However resistence of major pests of stored grains to insecticides and fumigants posing a great threat to their viability. Even in some areas multiples resistance mechanism becomes so high leaving no alternative protactant options for storagers. Main reason is that grain protecting chemicals are a rare sources, but the ability to manage or to minimize the impact of resistance is basic priority. Our reslts showed that mortality rate of R. dominica was also somewhat same with T. castaneum against pyrethroid, deltamethrina and fumigants. But unfortunately it was observed that R. dominica field and lab strains showed little moratality and appeared most resistance to our treatments (Figure 2). As compared to T. castaneum, R. dominica not much common, in case of damages to grains. Tribolium castenum have multiple taste of pyrethroids and fumigants and dvelop resistant higher as compared to other pests species.
Moratlity directly depends on concentration, as concentration increases mortality was also increased. By the way in the fields we have less damages caused by storage pests, as compared to storages godowns. Under storage conditions some pests hide themselves within the walls or hiding places and not exposed to our treatments, which make them more resistant to pyrethroids and fumigants. Even we can kill complete population from a specific storage area, but due their speedy oviposition rate its difficult to make them out of space for loger period wothout application of pyrethroids and fumigants.
Mortality rate of both strains was also monitored after different time intervals. After 24 hour of treatment both strain showed non-significant results. Both treatments cause somewhat equal mortality with a little difference. But overall after 24 hour of exposure phosphine recorded maximum mortality rate, and proved more resistant to pests. As time periods increases we observed significant differences of mortality of both tested pests. Maximum mortality rate was recorded in lab strains against both T. castaneum and R. dominica. Field collected strains observed more resistant to treatments after 48 hour of exposures.
Our study showed interesting results how field’s strains develops themselves against pyrethroids, insecticides and fumigants. In fact, lab strains should show more resistant due to multiple faces toward insecticides and fumigants application. Lab strains T. castaneum and R. dominica showed (64.588±3.51) and (60.781±3.89) mean mortality against deltamethrin which was less as compared to phosphine. Concentration and time intervals are both most important factors considered for the control of storages pests. In our study we observed that both pests showed less mortality rate at initial hours, as time period was increased mortality was start to increase gradually. Field strains showed maximum (37.769 ± 4) and (40.886 ± 4.22) mortality rates of T. castaneum and R. dominica against deltamethrin while in case of phosphine it was (32.328 ± 3) and (44.148 ± 4.10) respectively. In big storages there is always chances to escape for pests as well as fumigants, due to these conditions these pests are less intact with treatments as compare to small holder’s godowns. Phosphine have oxidative reaction due to which it causes maximum mortalities, while parathyroid, deltamethrin kill pests which come in contact or feed treated grains. As overall results
Table 3: Mean mortality (%±SE) of field and lab strains of T. castaneum and R. dominica subsequent time intervals. Means sharing different letters are significantly different at 5 % level (P < 0.05 Tucky HSD test, n=20).
| Field strains | ||||
| Exposure time (h) | Tribolium castaneum | Rhyzopertha dominica | ||
| Deltamethrin | Phosphine | Deltamethrin | Phosphine | |
| 24 | 13.565± 2.53 C | 17.234 ± 2.34 C | 11.414 ± 2.37 C | 15.233± 2.12 C |
| 48 | 25.864 ± 3.49 B | 34.989 ± 4.85 B | 19.868 ± 2.64 B | 27.193 ± 3.52 B |
| 72 | 37.769 ± 4 A | 46.886 ± 4.22 A | 32.328 ± 3 A | 40.148 ± 4.10 A |
| Lab strains | ||||
| 24 | 26.289 ± 3.04 C | 31.707 ± 2.50 C | 21.027 ± 3.57 B | 29.036 ± 2.41 C |
| 48 | 44.508 ± 4.32 B | 53.093 ± 4.55 B | 41.802 ± 3.08 B | 48.653 ± 4.19 B |
| 72 | 59.588 ± 3.51 A | 65.781 ± 3.89 A | 52.264 ± 4.50 A | 61.662 ± 5.11 A |
R. dominica was observed less tolerant to phosphine and deltamethrin, while T. castaneum was found more tolerant with higher developed resistance (Table 3).
A log probit analysis was applied to determine the LC50 values of treated field and lab strains against deltamethrin and phosphine. Strains was exposed to different time intervals (24, 48 and 72h). Our results reported that T. castaneum and R. dominica showed less resistance factor as compares to phosphine treatments which presented highest. Field strains showed very less LC50 values as compared to lab strains. Pests exposed to phosphine concentration at various time intervals showed highest LC50 values. Resistance factor and LC50 values was continuously increased as time increased. Lab strains showed highest LC50 values as compared to field strains. Pests treated with phosphine showed high LC50 as compared to deltamethrin.
Red flour beetle, T. castaneum was appeared to more resistant than lesser grain borer, R. dominica. Pyrethroids commonly less used for the control of storages pests, while phosphine usages are more. In fact, due to multiple and continuous application of phosphine it should show less resistant. Reason is that phosphine act as oxidative control and cause mortality by suffocation, while parathyroid, deltamethrin cause mortality only when insect feed them. Main reason was to expose them at different time is to confirm best time approach to manage them. Lab strains was appeared most susceptible as filed strains which were most resistant. Results confirm that T. castaneum was 3.9 and 1.9 times fold resistant then R. dominica against deltamethrin and phosphine respectively. Pest’s adjustments to environments fluctuation make them stronger as well more resistant against insecticides and fumigants, which was observed in field collected strain, while lab strain was unable to resist as like field strains to coped with deltamethrin and phosphine (Tables 4, 5).
Table 4: Log probit analysis for field and lab strains of T. castaneum and R. dominica to calculate the LC50 and resistance factor at 95 % fiducial limit against deltamethrin after subsequent time intervals.
| Tribolium castaneum | |||||
| Strains | Exposure time (h) | Treatment |
LC50 |
95 % fiducial limit | RF |
| LS | 24 | Del. | 0.2920 | 0.0240‒0.0319 | 0.2570 |
| FS | 24 | Del. | 0.2041 | 0.0116‒0.0202 | |
| LS | 48 | Del. | 0.5088 | 0.0346‒0.0482 | 0.8998 |
| FS | 48 | Del. | 0.4108 | 0.0559‒0.0805 | |
| LS | 72 | Del. | 1.7274 | 0.0425‒0.0562 | 1.1414 |
| FS | 72 | Del. | 0.8390 | 0.0756‒0.1511 | |
| Rhyzopertha dominica | |||||
| LS | 24 | Del. | 0.1023 | 0.0264‒0.0169 | 0.7814 |
| FS | 24 | Del. | 0.1999 | 0.0212‒0.0287 | |
| LS | 48 | Del. | 0.2873 | 0.0487‒0.0581 | 1.4343 |
| FS | 48 | Del. | 0.3123 | 0.0303‒0.0449 | |
| LS | 72 | Del. | 0.3998 | 0.0843‒0.2511 | 1.8893 |
| FS | 72 | Del. | 0.2929 | 0.0716‒0.1532 | |
LS: Lab strains; FS: Field strains; Del: Deltamethrin; RF: Resistance factor; LC: Lethal concentration.
This was a first time study that explaining the resistance mechanisms of lab and field collected strains against parathyroid and fumigants. Different concentration of deltamethrin and phosphine was applied against T. castaneum and R. dominica. Pests was exposed to different time intervals 24, 48 and 72 h to determine their resistance factor as well best mortality duration. Basically resistance is the avoidance of biotic as well abiotic factors (Collin et al., 2017) which was determined in our current research. Due to multiple and frequently applications of pyrethroids and fumigants storages pests develops more resistance as previous experience (Opit et al., 2016). Geographical position of a locality have a great impact to change insect physiology to resist them against biotic and abiotic factor (Holloway et al., 2016). By keeping in mind these views and implications current research was focused to determine the resistance level in field and lab collected strains against commonly used deltamethrin and phosphine.
Table 5: Log probit analysis for field and lab strains of T. castaneum and R. dominica to calculate the LC50 and resistance factor at 95 % fiducial limit against phosphine after subsequent time intervals.
| Tribolium castaneum | |||||
| Strains | Exposure time (h) | Treatment |
LC50 |
95 % fiducial limit | RF |
| LS | 24 | Phos. | 0.7460 | 0.0301‒0.0234 | 0.2124 |
| FS | 24 | Phos. | 0.4671 | 0.0218‒0.0223 | |
| LS | 48 | Phos. | 0.8366 | 0.0583‒0.0309 | 0.5811 |
| FS | 48 | Phos. | 0.6292 | 0.0396‒0.0405 | |
| LS | 72 | Phos. | 0.9518 | 0.0833‒0.0591 | 0.9142 |
| FS | 72 | Phos. | 0.7292 | 0.0690‒0.0401 | |
| Rhyzopertha dominica | |||||
| LS | 24 | Phos. | 0.4405 | 0.0123‒0.0152 | 0.3686 |
| FS | 24 | Phos. | 0.2956 | 0.0108‒0.0191 | |
| LS | 48 | Phos. | 0.6163 | 0.0283‒0.0392 | 1.129 |
| FS | 48 | Phos. | 0.3646 | 0.0199‒0.0196 | |
| LS | 72 | Phos. | 0.7281 | 0.0470‒0.0377 | 1.6194 |
| FS | 72 | Phos. | 0.4813 | 0.0386‒0.0295 | |
LS: Lab strains; FS: Field strains; Del: Deltamethrin; RF: Resistance factor; LC: Lethal concentration.
In our first parameter both strains were exposed to different concentration of deltamethrin and phosphine to determine their vulnerability and mortality rate. Statically analyzed results show that T. castaneum lab strain showed maximum mortality rates while in field strains it was minimum against deltamethrin and phosphine treatments. But as compared to deltamethrin and phosphine, deltamethrin was observed less resistant while phosphine was found more resistant against these two tested pests. Our results are same with Winks (1982) who did similar type of experiment and get same type of results. Kock et al. (2015) carried out an experiment to check the resistance of T. castaneum against phosphine and get significant results which are correlated with our study. Ahmad et al. (2013) determines the resistance level in T. castaneum and R. dominica against phosphine. Chen et al. (2013) carried an investigation to find out the susceptibility of R. dominica against deltamethrin, and get similar observation as we recorded. In our study we observed that the mortality rate was differed as time was increased. After 24 h of exposure no significant difference was achieved in mortality rates of T. castaneum and R. dominica against deltamethrin and phosphine. At 48 and 72 h of exposure a significant difference was observed. Phosphine caused maximum mortalities in field and lab strains against T. castaneum and R. dominica and was observed more resistant, but deltamethrin recorded less mortality which was observed less resistant. Shahid et al. (2019) did same experiments and found similar results to our research. Khaleqazzaman and Khanom (2006) did an experiment to evaluate the mortality of T. castaneum against cypermethrin at different time intervals. There results showed that mortality rate was increased as time was increased. Zettler and Cuperus (1990) conducted an experiment to check the resistance mechanism of T. castaneum and R. dominica against malathion, chlorpyriphos, diclhorvas and phosphine. There results concluded that both pests T. castaneum and R. dominica showed resistance against their treatment but phosphine was observed more resistant then others. In our results we observed that the deltamethrin was most susceptible to pests as compared to phosphine (Saxena and Sinha, 1995). Lorini et al. (2007) observed that R. dominica was more susceptible to deltamethrin and their young ones. In our results the LC50 values was calculated to determine the resistance factor of field and lab collected strains against deltamethrin and phosphine. Our results concluded that phosphine exposed strains give high LC50 value as compare to deltamethrin against T. castaneum and R. dominica. We want to compere our results with Shahid et al. (2019); Andric et al. (2010); Vojoudi et al. (2012) and Velki et al. (2014) who performed same type of experiments and observed results related to our study. There finding conclude that Phosphine exposed pests give higher LC50 values as compared to deltamethrin. Storage pest’s resistance to insecticides and fumigants has been becomes big challenge for researchers to find an alternative to overcome this issue. Pest’s species found at different localities or geographical positions present various types of resistance levels against insecticides and fumigants. By keeping in mind these challenges current research was focused to evaluate resistant in field and lab strains. This was the first time research which describes this research gaps. We hope our study will provide a great understanding for researcher to overcome resistance developing issues in major insect pests of stored grains.
Conclusions and Recommendations
Best approaches to minimize resistance levels in stored products insects’ pests is to know their geographical locations, control methods and storage techniques. Our research was based on geographical locations of storages pests. We checked resistance levels in lab and field collected strains of two major pests of stored grains against largely applied synthetic pyrethroid and fumigant. Field collected strains were observed more resistant to deltamethrin and phosphine as compared to lab strains. Our research was based on new approach, nobody before this made any attempt to evaluate resistance in major insect pests of stored grains collected from different geographical positions as well as their comparisons with lab rearing strains. We hope this research could be helpful for improvements in storages insect’s pest management’s strategies.
Acknowledgments
We are thankful to Dr. Farooq Ahmad for their guide and helpful comments throughout the research. We are thankful to Dr. Eric Siaw Ntiri and Dr. Muhammad Ishaque Mastoi for review, suggestions and improvements in this manuscript. We are thankful to other researchers cited in this article for their help practically, theoretically as well as technically.
Author’s Contributions
All the authors cited in this research article been allowed to participate equally. Dr. Eric Siaw Ntiri help in data analysis. Mr. Asim Munawar conducted experiments and wrote the manuscript. Ms. Aqsa Arshad helps in data collection as well as helps in collection of pest’s population strains from different cities. Ms. Chengjuan Liang help to formate references. Dr. Farooq Ahmad supervise this project. This research was reviewed by many researchers having experience in relevant research area.
Funding resources
This research was based on partial funding. Pesticides (deltamethrin and phosphine) was sponsored by pesticide company Ali Akbar Group Pakistan Limited. Wheat flour, grains and others handling materials was bought by lab funds. Travelling expenditures was sponsored by research supervisor. Other expenditures was self-supported, no further assistance was made by any organization and university.
Conflicts of interest
All the authors cited in this article declared no conflict of interest.
References
Affognon, H., C. Mutungi, P. Sanginga and C. Borgemeister. 2015. Unpacking postharvest losses in sub-Saharan Africa: A meta-analysis. World Dev. 66: 49- 68. https://doi.org/10.1016/j.worlddev.2014.08.002
Ali, M.S., A.M.Y.M. Khandoker, M.A. Afroz and A.K.F.H. Bhuiyan. 2012. Ovarian response to different dose levels of follicle stimulating hormone (FSH) in different genotypes of Bangladeshi cattle. J. Anim. Sci. 25: 52-58. https://doi.org/10.5713/ajas.2011.11167
Andric, G., G. Kljajic, I. Peric and M.G. Prazic. 2010. Susceptibility of red flour beetle Tribolium castaneum (Herbst) populations from Serbia to contact insecticides. Julius. Kuhn. Archiv. 425: 869-873.
Arthur, F.H. 1997. Differential effectiveness of deltamethrin dust on plywood, concrete, and tile surfaces against three stored-product beetles. J. Stored Prod. Res. 33: 167- 173. https://doi.org/10.1016/S0022-474X(96)00041-0
Barkholder, W.E. and D.L. Faustini.1991. Biological method of survey and control. In: Ecology and Management of Food Industry pest, AOAC: 361-372. Bell CH, 2000. Fumigation in the 21st century. Crop Prot. 19: 563- 569. https://doi.org/10.1016/S0261-2194(00)00073-9
Benhalima, H., M.Q. Chaudhry, K.A. Mills and N.R. Price. 2004. Phosphine resistance in stored product insects collected from various grain storage facilities in Morocco. J. Stored Prod. Res. 40: 241-249. https://doi.org/10.1016/S0022-474X(03)00012-2
Bhargava, M.C., R.K. Choudhary and P.C. Jain. 2007. Advances in management of stored grain pests, In Jain P.C. Bhargava M.C. (eds.), Entomology: Novel approaches. New India Publ. Agency, New Delhi. pp. 425-451.
Cao, D., D. Pimental and K. Hart. 2002. Postharvest crop losses (insects and mites). In Pimental D. Encyclopdia of pest management. Marcel Dekker, Inc., USDA. https://doi.org/10.1201/NOE0824706326.ch299
Chen, C. and M. Chen. 2013. Susceptibility of field populations of the lesser grain borer Rhyzopertha dominica (F.) to deltamethrin and spinosad on paddy rice in Taiwan. J. Stored Prod. Res. 55: 124-127. https://doi.org/10.1016/j.jspr.2013.10.001
Collins, P.J., G.J, Daglish, M. Bengston, T.M. Lambkin and H. Pavic. 2002. Genetics of resistance to phosphine in Rhyzopertha dominica (Coleoptera: Bostrichidae). J. Econm. Entomol. 95: 862-869. https://doi.org/10.1603/0022-0493-95.4.862
Collins, P.J., M.G. Falk, M.K. Nayak, R.N. Emery and J.C. Holloway. 2017. Monitoring resistance to phosphine in lesser grain borer, Rhyzopertha dominica, in Australia: A national analysis of trends, storage types and geography in relation to resistance detections. J. Stored Prod. Res. 70: 25-36. https://doi.org/10.1016/j.jspr.2016.10.006
Costa, S.J. 2014. Reducing food losses in Sub-Saharan Africa: An ‘action research’ evaluation trial from Uganda and Burkina Faso. UN World Food Programme Kampala, Uganda. https://documents.wfp.org/stellent/groups/public/documents/special_initiatives/WFP265205.pdf
De Groote, H., S.C. Kimenju, P. Likhayo, F. Kanampiu, T. Tefera and J. Hellin. 2013. Effectiveness of hermetic systems in controlling maize storage pests in Kenya. J. Stored Prod. Res. 53: 27- 36. https://doi.org/10.1016/j.jspr.2013.01.001
Dotson, D., E. Scollon and M. Collantes. 2010. Deltamethrin: Human health assessment a coping document in support of registration review. DP Barcode D368592, dated 2/17/2010. Office of Prevention, Pesticides and Toxic Substances. U.S. Environ. Prot. Agency, Washington, DC. https://www.regulations.gov/docket?D=EPA-HQ-OPP-2009-0637.
Flinn, P.W., D.W. Hagstrum, C. Reed, T.W. Phillips. 2010. Insect population dynamics in commercial grain elevators. J. Stored Prod. Res. 46: 43-47.
Food and Agriculture Organization, World Bank. 2011. Missing food: The case of post-harvest grain losses in Sub-Saharan Africa. Int. Bank Reconstr. Dev. World Bank, Food Agric. Org. Rep. No. 60371-AFR. p. 116.
Food and Agriculture Organization. 1994. Grain storage techniques: Evolution and trends in developing countries. FAO, Agric. Ser. Bull. No: 109. FAO, Rome. Italy. ISBN: 92-5-1 03456-7.
Gustavasson, J., C. Cederberg and U. Sonesson. 2011. Global food losses and food waste: Extent causes and prevention. Rome, Food Agric. Organ. (FAO) U. N. http://www.fao.org/3/a-i2697e.pdf.
Hasan, M., F. Ahmad, K. Ashraf and M. Ahmad. 1996. Field detection of resistance to various insecticide in Tribolium castaneum collected from Multan and Bahawalpur. Pak. Entomol. 18: 76-77.
Herron, G.A., 1990. Resistance to grain protectants and phosphine in coleopterons pests of grain stored on farms in New South Wales. J. Aust. Entomol. Soc. 29: 183-189. https://doi.org/10.1111/j.1440-6055.1990.tb00345.x
Hodges, R., M. Bernard and F. Rembold. 2014. APHLIS- Postharvest cereal losses in Sub-Saharan Africa, their estimation, assessment, and reduction. Publ. Office Eur. Union, Luxembourg. https://doi.org/10.2788/19466.
Holloway, J.C., M.G. Falk, R.N. Emery, P.J. Collins and M.K. Nayak. 2016. Resistance to phosphine in Sitophilus oryzae in Australia: A national analysis of trends and frequencies over time and geographical spread. J. Stored Prod. Res. 69: 129-137. https://doi.org/10.1016/j.jspr.2016.07.004
Jood, S. and A.C. Kapoor. 1994. Vitamins contents of cereal grains as affected by storage and insect infestation. Pl. Food Hum. Nutr. 46: 237-243. https://doi.org/10.1007/BF01088996
Kavallieratos, N.G., C.G. Athanassiou and F.H. Arthur. 2015. Efficacy of deltamethrin against stored-product beetles at short exposure intervals or on a partially treated rice mass. J. Econ. Entomol. 108: 1416-1421. https://doi.org/10.1093/jee/tov060
Khalequzzaman, M. and M. Khanom. 2006. Effects of cypermethrin alone and in combination with leaf and seed extracts of neem against adult Tribolium castaneum (Herbst). Univ. J. Zool. Rajshahi Univ. 25: 45-49. https://doi.org/10.3329/ujzru.v25i0.326
Kitinoja, L., S. Saran, S.K. Roy and A. Kader. 2011. Postharvest technology for developing countries: Challenges and opportunities in research, outreach and advocacy. J. Sci. Food Agric. 91: 597- 603. https://doi.org/10.1002/jsfa.4295
Kljajic, P. and I. Peric. 2009. Residual effects of deltamethrin and malathion on different populations of Sitophilus granaries (L.) on treated wheat grains. J. Stored Prod. Res. 45: 45- 48. https://doi.org/10.1016/j.jspr.2008.07.004
Kock, E., D. Schlipalius, R. kaur, A. Tuck, P. Ebert, P. Collins and A. Yilmaz. 2015. Determining phosphine resistance in rust red flour beetle Tribolium castaneum (Herbest.) (Coleoptera:Tenebrionidae) Population from Turkey. Turk. J. Entomol. 39: 129-136. https://doi.org/10.16970/ted.17464
Lorini, I., P.J. Collins, G.J. Daglish, M.K. Nayak and H. Pavic. 2007. Detection and characterization of strong resistance to phosphine in Brazilian Rhyzopertha dominica (F.) (Coleoptera: Bostrychidae). Pest Manage. Sci. 63: 358-364. https://doi.org/10.1002/ps.1344
Lorini, I. and D.J. Galley. 1999. Deltamethrin resistance in Rhyzopertha dominica (F.) (Coleoptera: Bostrichidea), a pest of stored grain in Brazil. J. Stored Prod. Res. 35: 37-45. https://doi.org/10.1016/S0022-474X(98)00028-9
Nadeem, M., J. Iqbal, M. K. Khattak and M. A. Shahzad. 2012. Management of Tribolium castaneum (Hbst.) (Coleoptera: Tenebrionidae) Using Neem (Azadirachta indica A. Juss) and Tumha (Citrullus colocynthis) (L.). Pak. J. Zool. 44: 325-1331. https://www.zsp.com.pk/pdf44/1325-1331%20_20_%20PJZ-744-11%20neem+and+tumha+paper.pdf
Opit, G.P., T.W. Phillips, M.J. Aikins and M.M. Hasan. 2012. Phosphine resistance in Tribolium castaneum and Rhyzopertha dominica from stored wheat in Oklahoma. J. Econ. Entomol. 105: 1107-1114. https://doi.org/10.1603/EC12064
Opit, G.P., E, Thomas, T.E. Phillips and M.E. Payton. 2016. Effectiveness of sulfuryl fluoride fumigation for the control of phosphine-resistant grain insects infesting stored wheat. J. Econ. Entomol. 109: 930-941. https://doi.org/10.1093/jee/tov395
Paudyal, S., G.P. Opit, F.H. Arthur, G.V. Bingham and S.G. Gautam. 2016. Contact toxicity of deltamethrin against Tribolium castaneum (Coleoptera: Tenebrionidae), Sitophilus oryzae (Coleoptera: Curculionidae), and Rhyzopertha dominica (Coleoptera: Bostrichidae) adults. J. Eco. Entomol. 109(4): 1936-1942. https://doi.org/10.1093/jee/tow107
Shahid, M., F. Farooq, H. Mansoor, S. Muhammad, A.S. Muhammad, N.U. Unsar, S. Muhammad, A.Q. Mirza, A. Furqan, R. Abdur, M.S. Rao, J. Muhammad, H. Muhammad and I. Muhammad. 2019. Resistance in Tribolium castaneum (Hbst.) against phosphine and deltamethrin. J. Sci. Agric. 1(3): 46-50.
Tubeillo, F.N. and S.M.S Howden. 2007. Crop and pasture response to climate change. PNAS. 104: 19686-19690. https://doi.org/10.1073/pnas.0701728104
Vayias, B.J., N.G. Kavallieratos, C.G. Athanassiou and G. Tatsi. 2010. Insecticidal action of the combined use of spinosad and deltamethrin against three stored product pests in two stored hear-wheat varieties. In: Carvalho, M.O., Fields, Proc. 10th Int. Working Conf. Stored Prod. Prot., Estoril, Portugal, Julius Kühn Archiv Nr. 425. pp. 921- 924. https://doi.org/10.5073/jka.2010.425.223
Velki, M., I. Plavsin, J. Dragojevic and B.K. Hackenberger. 2014. Toxicity and repellency of dimethoate, pirimiphos-methyl and deltamethrin against Tribolium castanem (Herbst) using different exposure methods. J. Stored Prod. Res. 59: 36- 41. https://doi.org/10.1016/j.jspr.2014.04.005
Vojoudi, S., M. Saber, V. Mahdavi, H. Golshan and Z. Abedi. 2012. Efficacy of some insecticides against red flour beetle, Tribolium castaneum (Herbst.) (Coleoptera: Tenebrionidae) adults exposed on glass, ceramic tile, plastic and paper disc surfaces. J. Life Sci. 6: 405-410.
Wang, D., P.J. Collins and X. Gao. 2006. Optimizing indoor phosphine fumigation of paddy rice bag-stacks under sheeting for control of resistant insects. J. Stored Prod. Res. 42: 207-217. https://doi.org/10.1016/j.jspr.2005.02.001
Winks, R.G., 1982. The Toxicity of phosphine to adults of Tribolium castaneum (Herbst): Time as a response factor. J. Stored Prod. Res. 18: 159-169. https://doi.org/10.1016/0022-474X(82)90026-1
Zettler, J.L. and F.H. Arthur. 2000. Chemical control of stored product insects with fumigants and residual treatments. Crop Prot. 19(8-10): 577-582. https://doi.org/10.1016/S0261-2194(00)00075-2
Zettler, J.L. and G.W. Cuperus. 1990. Pesticide resistance in Tribolium castaneum (Coleoptera: Tenebrionidae) and Rhyzopertha dominica (Coleoptera: Bostrichidae) in wheat. J. Econ. Entomol. 83: 1677-1681. https://doi.org/10.1093/jee/83.5.1677
To share on other social networks, click on any share button. What are these?







