Damaging Effect of Gall-Forming Insects (Diptera: Cecidomyiidae) on Castanopsis hystrix in China
Damaging Effect of Gall-Forming Insects (Diptera: Cecidomyiidae) on Castanopsis hystrix in China
Xia Lin Zheng1, Zhen Hua Xian1, Zhen De Yang2, Xiu Hao Yang3 and Wen Lu1,*
1 Guangxi Key Laboratory of Agric-Environment and Agric-Products Safety, College of Agriculture, Guangxi University, Nanning 530004, China
2College of Forestry, Guangxi University, Nanning 530004, China
3Department of Guangxi Forestry Pest Management, Nanning, 530028, China
ABSTRACT
Damaging effect of two species of gall midges (Diptera: Cecidomyiidae) have been investigated on Castanopsis hystrix Miq. (Fagales: Fagaceae) in Pubei County of Guangxi, China were investigated from December 2012 to December 2013. Jaapiella sp. damaged leaves, while Contarinia sp. damaged stems and leafstalks. The damage ratio and the index of Jaapiella sp. in December 2012, March, July and December 2013 were 9.7±2.0%, 14.8±1.9%, 24.1±1.5%, 12.3±0.6% and 25.6%, 31.3%, 35.6%, 26.2%, respectively, while those of Contarinia sp. were 33.2±3.4%, 38.4±4.3%, 43.2±3.1%, 31.6±3.5% and 29.6%, 30.9%, 36.0%, 30.9%, respectively. The results will provide essential information to develop strategies that can be used for their control.
Article Information
Received 02 November 2016
Revised 14 December 2016
Accepted 24 December 2016
Available online 02 August 2018
Authors’ Contributions
XLZ, ZHX and ZDY conceived and designed the study, and wrote the article. XHY identified the species. WL analyzed the data.
Key words
Castanopsis hystrix, Gall midge, Jaapiella sp., Contarinia sp., Damage ratio, Damage index.
DOI: http://dx.doi.org/10.17582/journal.pjz/2018.50.5.sc4
* Corresponding author: [email protected]
0030-9923/2018/0005-1975 $ 9.00/0
Copyright 2018 Zoological Society of Pakistan
Numerous microorganisms and arthropods interact intimately with plants and induce a specialized structure-galls. The cecidogenous parasites are especially common among insects with more than 13,000 known gall-forming species from several orders (Raman et al., 2005; Raman, 2012). Many hypotheses on the formation of insect-galls were summarized including nonadaptive, plant protection, mutual benefit, nutrition, microenvironment and enemy hypotheses (Price et al., 1987). Although the theoretical studies are helpful for understanding the interaction between plants and gall-forming insects, problems of practical crop protection are also important.
Castanopsis hystrix Miq. (Fagales: Fagaceae: Castanopsis) is one of the most important species of south subtropical evergreen broad leaved forest in southern provinces of China as well as Vietnam, Laos, Cambodia, Burma, Nepal, Bhutan, and India (Kayini and Pandey, 2010; Xu et al., 2013). These trees can be used for furniture, shipbuilding and military industry based on many merits, including fast growth, ironwood, stem straightness, strong adaptability, corrosion resistance, etc. (Xu et al., 2013). Therefore, C. hystrix became an important species of plantation. In China, the largest area of natural forest and plantation (approximately 1.3×105 km2) of C. hystrix is located at Pubei County of Guangxi Zhuang Autonomous Region (Gan and Liu, 2014). The number of pest species sharply increased with the expansion of the cultivated area of this tree. Among of the pest species, gall-forming insects are the most important. Understanding the species and damage status of gall-forming insects on C. hystrix will provide essential information to develop strategies that can be used for their control.
The objective of this study is to investigate (a) the species composition of gall-forming insects on C. hystrix and (b) the damage status of these insects.
Materials and methods
The study was conducted in Pubei County (22°16′ °N, 109°33′ °E) located at the south of Guangxi Zhuang Autonomous Region in China (annual-average air temperature 21.5 °C and precipitation 1,760 mm). The natural forests and plantations of C. hystrix in Pubei County are the largest in China so that the protection is important. By the end of 2012, few large plantations of C. hystrix were developed at Longmen, Beitong, Dacheng and Zhanghuang towns in Pubei County.
To find out the species composition and damage status of gall-inducing insects on C. hystrix, sampling surveys were conducted in the Maojia village (109.25′ °E, 22.09′ °N), Zhongnan village (109.25′ °E, 22.08′ °N) and Liutonglu village (109.26′ °E, 22.10′ °N), in December 2012, March, July and December 2013, respectively. Three sampling sites were established in each village, and the area of each site was 10,000 m2. Five-plot sampling model was adopted, and the area of each plot was 500 m2. A total of 200 trees was investigated in each plot. Morphological characteristics of insect-galls and damage symptoms of gall-inducing insects were taken with a digital camera (Sony HX60, Sony Corporation, Tokyo, Japan). The species of gall-inducing insects, the number of damaged trees and the damaged degree of each tree were recorded. The damage was divided into five levels (Table I). The classification critera of each degree of damage were (a) the number of insect-galls per meter of stem and (b) the number of damaged leaves per 100 leaves. Both criteria were correlated and enabled unequivocal distinguishing of the degree of damage in all observed cases.
Table I.- Damage degree of gall-induced insects on Castanopsis hystrix.
|
Damage degree |
No. of insect galls/m of stem |
No. of damaged leaves/100 leaves |
|
0 |
0 |
0 |
|
I |
1-5 |
10-20 |
|
II |
6-10 |
21-30 |
|
III |
11-15 |
30-40 |
|
IV |
>15 |
>40 |
The damage ratio and the index of gall-inducing insects were calculated by the following formulas (Mensah et al., 2005; Zhang et al., 2010):
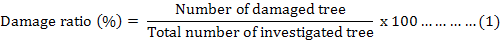

Where, DI means damage index, n is the number of damaged trees in the classification criterion as showed in Table I, N is the total number of investigated trees, Vi is the scale value, Va is the maximal scale value, respectively.
Statistical analysis was performed using SPSS 16.0 (SPSS, Chicago, IL, USA). The damage ratios of gall-inducing insects on C. hystrix among different months were compared using one-way ANOVA followed by Tukey’s test for multiple comparisons. Proportion data were arcsin transformed prior to analysis. A level of P < 0.05 significance was accepted in all statistical analyses.
Results
Two species of gall-inducing insects were found on C. hystrix, which belongs to Cecidomyiidae (Diptera). Jaapiella sp. only damaged the leaves where it formed the cone-shaped galls. Gall formation began soon after the eggs were laid. Oviposition site gradually became henna coloured and where it formed a circle of 5-8 mm diameter (Fig. 1A). At this place began to form a cone-shaped gall whose colour changed from green to crimson (Fig. 1B). Subsequently, epidermis of the cone-shaped gall slightly intumesced and the color became crimson (Fig. 1C). Finally, the cone-shaped gall formed that inner is a hollow space which is the nest of Jaapiella sp. (Fig. 1D). Each gall has one chamber and one larva, and the larval color was pink (Fig. 1E). The top of cone-shaped gall had a hole after the adults emerged (Fig. 1D), which could be the emergence hole.
Contarinia sp. mainly damaged the old stems, young shoots and leafstalks. Adults laid eggs on the old stems or bifurcations of branches (Fig. 2A, B). The oviposition site slowly expanded and formed a wholly enclosed gall (the same as the trunk tumor). There were some galls around the stem. Others only located at the oviposition site (Fig. 2C). A total of 5-10 galls allocated on the stem per meter (Fig. 2C). Each gall had 3-7 larvae, and each larva owned an ellipse chamber (Fig. 2D). Larval color was faint yellow (Fig. 2E).In one gall, each chamber was not communicated. Generally, larvae bited a gap as emergence hole when they were ready to pupate (Fig. 2F). On the young shoots, it would slowly die after attacked by Contarinia sp. (Fig. 2G, H). On the leafstalk, it would defoliate after attacked by Contarinia sp. Although, 1-3 galls were usually found on the leafstalk, only one gall located on the leafstalk was the most common.
During the period of investigation, the larvae and pupae were always found in galls. In March 2013, a few emergence holes appeared on galls tagged in December
2012. Up to July 2013, many emergence holes were found on galls marked in December 2012 and March 2013.
Damage of Jaapiella sp. significantly varied with the course of the season (F=15.668, df=3,11, P < 0.01) (Fig. 3A). The damage ratio was the lowest (9.7 ± 2.0%) in December, and the highest (24.1 ± 1.5%) in July. Although the variation in damage ratio of the Contarinia sp. had similar tendency with Jaapiella sp., seasonal differences were not significant (F = 2.118, df = 3,11, P = 0.176) (Fig. 3B). The damage index of Jaapiella sp. and Contarinia sp. were 25.6%, 31.3%, 35.6%, 26.2%, and 29.6%, 30.9%, 36.0%, 30.9% in December 2012, March, July and December 2013, respectively.
Discussion
Two species of gall-inducing insects, belonging to Jaapiella sp. and Contarinia sp. (Diptera) but their precise species identification needs further study. Both species had a multivoltine life cycle and overwintered as larval or pupal stage. However, biology and ecology of the two species are unclear. Caged experiment in the field should be made to study the biological characteristics and population dynamics of both species. Galls should be regularly dissected to establish the development of particular stages and number of generations.
The family of Cecidomyiidae has subfamilies of Lestremiinae, Porricondylinae and Cecidomyiinae. Species of the first two subfamilies are primitive and feed on fungi in decaying organic matter. The Cecidomyiinae (3,850 described species) represents about 80% of all known cecidomyiid species. Some of these are fungus feeders, but most are plant feeders or predators (Stone and Schönrogge, 2003). Two genera of Cecidomyiinae were studied in this study. Jaapiella sp. only damaged leaves while Contarinia sp. damaged the perennial stems, young shoots and leafstalk. The external morphology and inner structure of the galls of both species are completely different. Of the hypotheses that have been advanced for the adaptive significance of gall induction (Cornell, 1983; Price et al., 1987), three are relevant to attempts to explain gall morphology: the nutrition hypothesis, microenvironment hypothesis and enemy hypothesis (Abrahamson and Weiss, 1997; Harris et al., 2003; Stone and Schönrogge, 2003). Gall provides nutrition to the larvae since its tissues are rich in soluble amino acids and sugars. In gall, it can enhance the protein synthesis by cells and transport of nutrients from the vascular system of the plant (Rohfritsch, 1992). We suppose that the galls induced by Jaapiella sp. and Contarinia sp. supported the nutrition hypothesis. Mechanism of gall forming should be further studied.
Acknowledgements
We are grateful to numerous staffs of Pubei Forestry Bureau for their assistance on the investigation of insect-galls species in the field and also thank Huan Chen (The University of Warwick) for her advice on the language. This work was supported by the National Natural Science Foundation of China (31300549 and 31560212), Guangxi Natural Science Foundation (2013GXNSFBA019111), and the Project Sponsored by the Scientific Research Foundation of Guangxi University (XBZ160068).
Conflict of interest statement
The authors have declared that no competing interests exist.
References
Abrahamson, W.G. and Weiss, A.E., 1997. Evolutionary ecology across three trophic levels: goldenrods, gall makers and natural enemies. Princeton University Press, Princeton, USA, pp. 456.
Cornell, H.V., 1983. Am. Midl. Nat., 110: 225–234. https://doi.org/10.2307/2425263
Gan, J.W. and Liu, D.F., 2014. For. Guangxi, 2: 21.
Harris, M.O., Stuart, J.J., Mohan, M., Nair, S., Lamb, R.J. and Rohfritsch, O., 2003. Ann. Rev. Ent., 48: 549–577. https://doi.org/10.1146/annurev.ento.48.091801.112559
Kayini, A. and Pandey, R.R., 2010. J. Am. Sci., 6: 118–124.
Mensah, C., Difonzo, C., Nelson, R.L. and Wang, D., 2005. Crop Sci., 45: 2228–2233. https://doi.org/10.2135/cropsci2004.0680
Price, P.W., Fernandes, G.W. and Waring, G.L., 1987. Environ. Ent., 16: 15–24. https://doi.org/10.1093/ee/16.1.15
Raman, A., 2012. J. Pl. Interact., 7: 29–44. https://doi.org/10.1080/17429145.2011.630847
Raman, A., Schaefer, C.W. and Withers, T.M., 2005. Biology, ecology and evolution of gall-inducing arthropods. Science Publisher Ltd., Hampshire, UK, pp. 817.
Rohfritsch, O., 1992. Patterns in gall development. In: Biology of insect-induced galls (eds. J. Shorthouse and O. Rohfritsch). Oxford University Press, New York, UK, pp. 60–86.
Stone, G.N. and Schönrogge, K., 2003. Trends Ecol. Evol., 18: 512–522. https://doi.org/10.1016/S0169-5347(03)00247-7
Xu, B., Zhang, F.Q., Pan, W. and Liu, Y.C., 2013. Sci. Silv. Sin., 49: 162–166.
Zhang, G.R., Gu, C.H. and Wang, D.C., 2010. Theor. appl. Genet., 120: 1183–1191. https://doi.org/10.1007/s00122-009-1245-5
To share on other social networks, click on any share button. What are these?










