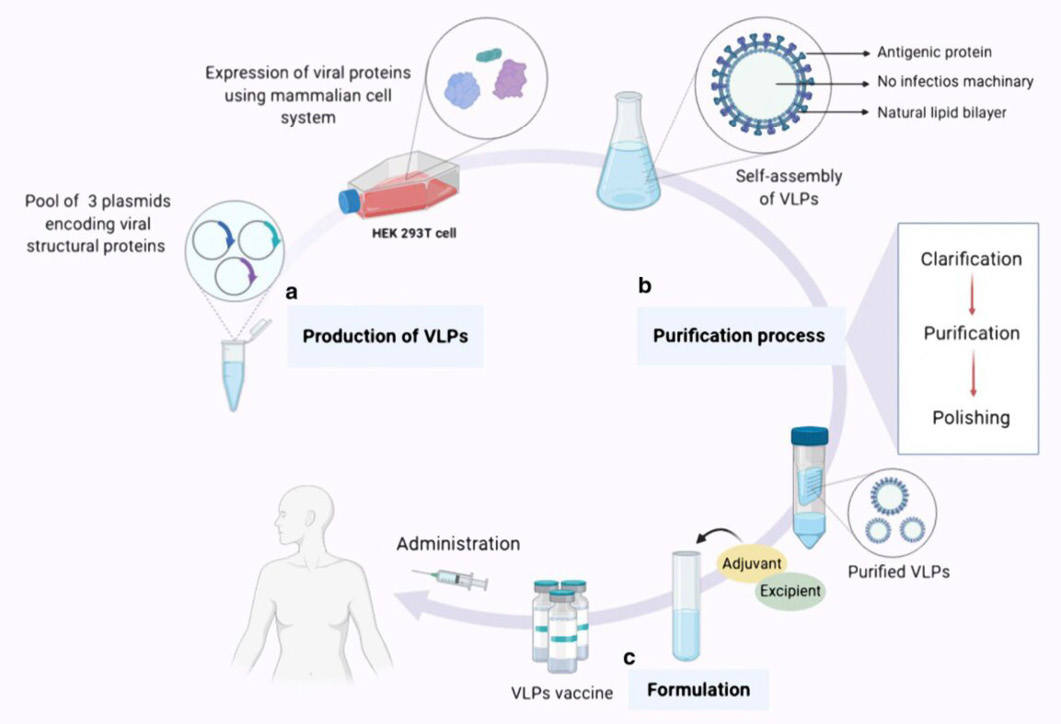
An overview of the expression, purification, and formulation of vaccines based on VLPs. There are typically three steps involved in producing a vaccination based on VLPs. The first step is called “Production,” and it entails cloning the viral structural genes of interest and then expressing the viral proteins that have the potential to self-assemble in an appropriate expression platform (in this case, the HEK293T cell line, a mammalian expression system). The final product of this process is a collection of VLPs in the form of noninfectious particles. b Purification phase, which includes downstream processes such as clarification, purification, and refining, culminates in the acquisition of intact purified VLPs devoid of any residual host detritus. c. The formulation stage, during which adjuvants as well as supplementary ingredients are incorporated into the vaccine formulation to attain a final product that is not only secure but also efficient and effective for vaccination purposes.