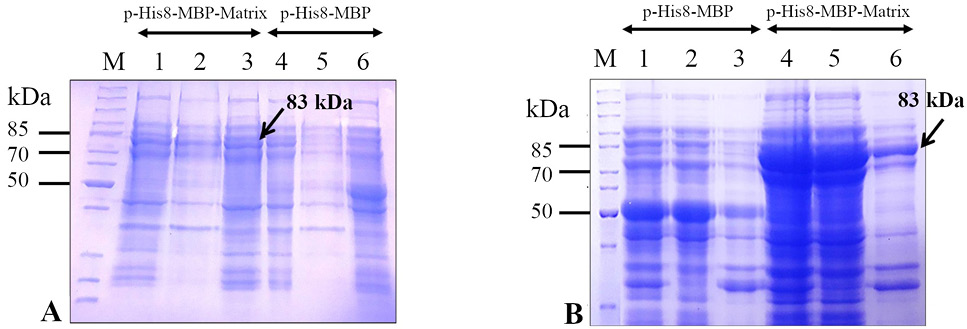
Expression analysis of the recombinant M (His8-MBP-Matrix) protein in Escherichia coli strains (BL21 (DE3) and Rosetta 2(DE3)) at 22 °C using 0.25 mM isopropyl β-D-1-thiogalactopyranoside (IPTG). Upon sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), a comparison of coomassie brilliant blue stained gels containing separated protein expressed in E. coli BL21 (DE3) (panel A) and E. coli Rosetta 2(DE3) (panel B) indicates that the target protein (His8-MBP-Matrix) is better expressed in E. coli Rosetta 2(DE3) cells (panel B) than in E. coli BL21 (DE3) cells (panel A) using the expression plasmid, pHis8-MBP-Matrix. The pHis8-MBP plasmid was used as the vector-only control. Lanes 1 and 4 depict the whole cell lysate, lanes 2 and 5 represent soluble fractions, and lane 3 and 6 show insoluble fractions. Arrows indicate the band corresponding to the target (His8-MBP-Matrix) protein (~ 83 kDa). Lane M depicts the mobility of a protein molecular weight marker for estimating the size of separated proteins.