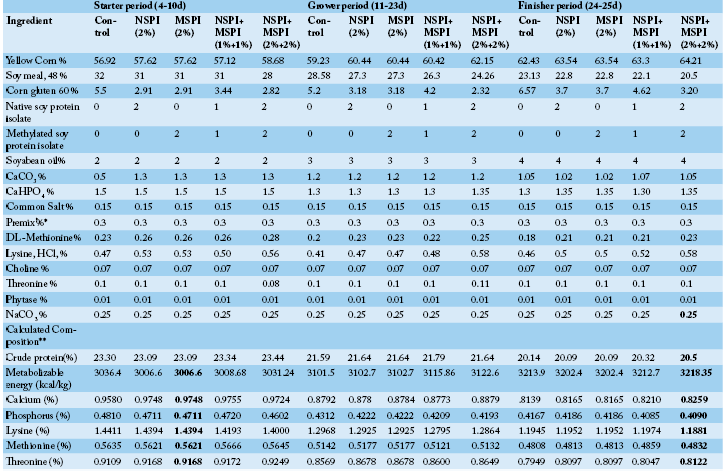
Advances in Animal and Veterinary Sciences
Research Article
Impact of Single or Co-Dietary Inclusion of Native or Methylated Soy Protein Isolate on Growth Performance, Intestinal Histology and Immune Status of Broiler Chickens
Anaam E. Omar1, Shimaa A. Amer*1, Wafaa A. M. Mohamed2, Ali Osman3, Mahmoud Sitohy3
1Department of Nutrition and Clinical Nutrition, Faculty of Veterinary Medicine, Zagazig University, 44511, Egypt; 2Department of Clinical Pathology, Faculty of Veterinary Medicine, Zagazig University, Zagazig, 44511, Egypt; 3Biochemistry Department, Faculty of Agriculture, Zagazig University, 44511, Zagazig, Egypt.
Abstract | This study was designed to study the effect of single or co-dietary inclusion of native soy protein isolate (NSPI) and/ or methylated soy protein isolate (MSPI) on growth performance, small intestine histology and immune status of broiler chickens. For this purpose, 250 one-day old broiler chicks (Ross 308 broiler) were randomly assigned into five experimental groups with five replicates per group (50chicks/group). The experimental treatments include; T1: control group (basal diet without additives); T2: basal diet + 2%NSPI + 0% MSPI (2NSPI:0MSPI); T3: basal diet + 0%NSPI + 2% MSPI (0NSPI:2MSPI); T4: basal diet + 1%NSPI + 1% MSPI (1NSPI:1MSPI); T5: basal diet + 2%NSPI + 2% MSPI (2NSPI:2MSPI). The experimental period was 35 day and water and feed were provided ad-libitum. Results revealed that chickens fed diets supplemented with 2NSPI:0MSPI and 1NSPI:1MSPI had significant (P<0.05) heavier final BW, BWG and best FCR compared to other dietary treatments. Liver, bursa and thymus percentages significantly (P<0.05) increased in broilers fed on diets supplemented with 0NSPI:2MSPI and 2NSPI:2MSPI as compared to control group. Gamma globulins and growth hormone were significantly increased by single or double dietary supplementation with NSPI and MSPI. Inclusion of 2NSPI and 1NSPI:1MSPI resulted in broad and thickened villous tips in most intestinal villi and an increase in the absorptive surface. Taken together, single supplementation with 2% NSPI or co-dietary supplementation with 1NSPI:1MSPI had positive effect on growth performance and gut health. Both single and co-dietary supplementation with NSPI and MSPI had potential effects on the immune status of broiler chickens.
Keywords | Broiler chickens, Soy protein isolate, Growth performance, Immunity, Gut histology.
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | July 02, 2018; Accepted | August 31, 2018; Published | September 07, 2018
*Correspondence | Shimaa A Amer, Department of Nutrition and Clinical Nutrition, Faculty of Veterinary Medicine, Zagazig University, 44511, Egypt; Email: [email protected]
Citation | Omar AE, Amer SA, Mohamed WAM, Osman A, Sitohy M (2018). Impact of single or co-dietary inclusion of native or methylated soy protein isolate on growth performance, intestinal histology and immune status of broiler chickens. Adv. Anim. Vet. Sci. 6(10): 395-405.
DOI | http://dx.doi.org/10.17582/journal.aavs/2018/6.10.395.405
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2018 Omar et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Soybean is the main plant protein source used for most simple stomached animals and poultry. It is the most predominant oil bearing seed in the world (FAO, 2008). However, soybeans contain high concentration of anti-nutritional factors, which affect small intestine characteristics and potential of gastro intestinal enzyme activity and consequently broiler growth performance and limit its inclusion in broilers diets (Marsman et al., 1997; Saki et al., 2011). These anti-nutritional factors (ANFs) affecting the growth performance of poultry include α-galactosides – raffinose, stachyose and verbascose – and antigenic factors – glycinin and β-conglycinin (Nunes et al., 2001; Jankowski et al., 2009). Broiler diets contained anti-nutritional factors resulted in reduced feed consumption and nutrients digestibility during the starter and grower period of growth (Feng et al., 2007; Kim et al., 2010), these substances are heat sensitive so heat treatment of soybeans is beneficial to destroy them (Kim et al., 1978) resulting in increasing its nutritional value (Palacios et al., 2004). There are different processed soybean products have been included in poultry diets such as soybean protein concentrate and soy protein isolate.
Native soy protein isolates “NSPI” are ether extracted soy bean meal processed to remove the heat resistant oligosaccharides and antigens (Crom-well, 2000). Due to its content of high protein and low non-starch polysaccharide, it can substitute soybean meal in broilers chicken diets (Parsons et al., 2000). But, its small particle size reduced intake of broilers fed on diets containing NSPI (Shelton et al., 2003; Longo et al., 2005) as the small particle size may affect diet acceptance, as poultry prefer diets with large particle sizes.
Chemical modification of NSPI was one of the primary methods utilized to estimate structure-function relationships. Methylation is a considerable and simple tool for the proteins modification. Methylation prevents free carboxyl groups thus elevating the net positive charge and showing more basic the methylated protein (Sitohy and Osman, 2010; Sitohy et al., 2013). Generally, growing the positive charge on protein enhances their antibacterial activity (Mahgoub et al., 2011; Sitohy and Osman, 2011; Sitohy et al., 2011; Abdel-Shafi et al., 2016). (Mahgoub et al., 2016; Osman et al., 2016) MSPI were estimated for their possible toxicity in Wistar male Albino rats and recorded the absence of toxicity (Sitohy et al., 2013). For these reasons, MSPI may reduce the susceptibility to diseases and enhancing the immune function. However, researches on application of NSPI and MSPI on broiler diets were limited. Therefore, the present work was done to study the effect of single or co-dietary inclusion of NSPI and MSPI on the growth performance, small intestine histology and immune status of broiler chickens.
MATERIALS AND METHODS
Birds, Housing, Diets and Experimental Design
This study carried out in poultry research unit in faculty of veterinary medicine, Zagazig University, Egypt to study the effect of single or co-dietary supplementation with NSPI and MSPI on growth parameters, small intestine histology and immunity of broiler chickens. All procedures of the experiment were performed with suggestion to the Committee of Local Experimental Animal Care and approved by ethics of our nutrition and clinical nutrition Department institutional committee, faculty of veterinary medicine, Zagazig University Egypt.
Two hundred fifty one–day old broiler chicks (Ross 308) were purchased from commercial hatchery and used in this study. On arrival, (average initial weight 45.38±1.35 g), they were incubated for 3 days on dehydrate solution and broad-spectrum antibiotic neomycin, reaching an average weight of 57.46 ± 1.2 g. They were randomly assigned into 5 groups (50 chicks each), 5 replicates (10 chicks each). Each group received a different treatment. T1: control group (basal diet without additives); T2: basal diet + 2%NSPI + 0% MSPI (2NSPI:0MSPI); T3: basal diet + 0%NSPI + 2% MSPI (0NSPI:2MSPI); T4: basal diet + 1%NSPI + 1% MSPI (1NSPI:1MSPI); T5: basal diet + 2%NSPI + 2% MSPI (2NSPI:2MSPI). Birds were kept in a ventilated open house with saw dust as litter and kept under continuous lighting system with suitable temperature (20-25 ºC) till the experiment end. Usual health and vaccination practices were conducted against New Castle (at 4th and 14th day) and Gumboro diseases (at 7th and 22 day). Daily observation was carried out on chicks for any health disorders. No mortalities occur among different groups during the whole experimental period. The basal diets were offered in mash form, the feed and water were provided ad libitum along the experimental period. The experimental diets were formulated following Ross manual Guide (AVIAGEN, 2014) as shown in Table (1). Experimental feed stuffs and diets were analyzed for nutrients (DM, CP and EE) according to (AOAC, 2002). The proximate chemical analysis of soy protein isolate was shown in Table (2).
Table 2: Proximate chemical composition of soy protein isolate* (%)
| Nutrient (%) | % |
| Dry matter | 92 |
| Crude protein | 84.9 |
| Ether extract | 0.9 |
| Ash | 3 |
| lysine | 4.63 |
| Methionine | 0.98 |
| Threonine | 2.83 |
*According to (AOAC 2002)
Preparation of Native Soy Protein Isolate and Methylated Soy Protein Isolate
Soy protein isolates were isolated from defatted soybean meal according to Johnson and Brekke (1983) procedures as described in Sitohy and Osman (2010). NSPI was subjected to methylation by methanol in the presence of hydrochloric acid (50 MR) for 10 h at 4 °C as described by Sitohy et al. (2001) and modified by Sitohy and Osman (2010).
Growth Performance
The birds were individually weighed at 4th day of age to obtain the average initial body weight then the body weight was recorded at 10, 23, 35 day to calculate the average body weight of the birds in each group. The body weight gain
Table 3: Effect of single or co-dietary supplementation with native soy protein isolate (NSPI) and methylated soy protein isolate (MSPI) on the growth performance of broiler chicks during the feeding periods.
| Parameters | Control | NSPI 2% | MSPI 2% | SPI+MSPI 1%+1% | NSPI+MSPI 2%+2% |
| Initial Body weight (g) |
57.46 ± 1.28 |
58.57 ± 0.00 |
58.09 ± 0.41 |
57.86 ± 0.71 |
57.86 ± 0.71 |
| Starter Period (4-10 day) | |||||
| Average Body weight (g) |
181.1 ± 2.0 b |
211.9 ± 2.9 a |
213.3 ± 5.4 a |
203.6 ± 10.2 a |
207.1 ± 4.3 a |
| Average Body weight gain(g) |
123.7 ± 0.8 b |
153.3 ± 2. 9 a |
155.2 ± 5.8 a |
145.8 ± 10.7 a |
149.3 ± 4.7 a |
| Average Feed intake (g) |
188.2 ± 7.3 b |
212.5 ± 6.2 a |
215.7 ± 8.2 a |
210.9 ± 5.3 a |
213.1 ± 2.1 a |
| Feed conversion ratio |
1.52 ± 0.06 a |
1.38 ± 0.01 b |
1.39 ± 0.01 b |
1.45 ± 0.08 ab |
1.43 ± 0.03 ab |
|
Grower Period (11-23 day) |
|||||
| Average Body weight (g) |
718.4 ± 13.0 |
740.0 ± 21.0 |
725.0 ± 41.6 |
716.9 ± 43.8 |
696.6 ± 17.2 |
| Average Body weight gain(g) | 537.8 ± 8.9 |
528.1 ± 23.4 |
511.7 ± 36.9 |
513.3 ± 50.6 |
489.5 ± 12.9 |
| Average Feed intake (g) |
835.3 ± 27.6 c |
860.6 ± 31.4 bc |
841.2 ± 19.8 c |
911.9 ± 23.0 a |
889.5 ± 19.9 ab |
| Feed conversion ratio |
1.55 ± 0.02 b |
1.63 ± 0.12 ab |
1.65 ± 0.10 ab |
1.79 ± 0.20 a |
1.82 ± 0.01 a |
|
Finisher Period (24-35 day) |
|||||
| Average body weight, g |
1643.0 ± 98.8 c |
1866.2 ± 51.4 a |
1626.6 ± 20.6 c |
1782.8 ± 13.9 b |
671.6 ± 46.4 c1 |
| Absolute weight gain , g |
867.3 ± 1.6 b |
1067.6 ± 50.6 a |
843.5 ± 30.5 b |
1008.0 ± 42.9 a |
917.1 ± 57.5 b |
| Average feed consumption , g | 1691.7 ± 85.4 |
1744.1 ± 42.3 |
1725.24 ± 102.76 |
1711.0 ± 70.2 |
1624.3 ± 116.5 |
| Feed conversion ratio |
1.95 ± 0.10 ab |
1.63 ± 0.04 c |
2.05 ± 0.13 a |
1.70 ± 0.14 bc |
1.78 ± 0.21 bc |
|
Overall performance (1-35 day) |
|||||
| Final body weight, g |
1643.01 ± 98.79 c |
1866.17 ± 51.40 a |
1626.63 ± 20.63 |
1782.86 ± 13.90 b |
1671.66 ± 46.36 c |
| Total body weight gain , g |
1586.22 ± 8.34 c |
1807.60 ± 51.40 a |
1568.53 ± 20.45 c |
1725.00 ± 13.23 b |
1613.80 ± 45.66 c |
| Total feed consumption , g |
2715.31 ± 99.74 |
2817.07 ± 79.25 |
2782.14 ± 81.93 |
2833.95 ± 89.20 |
2726.91 ± 136.91 |
| Feed conversion ratio |
1.71 ± 0.05 a |
1.56 ± 0.04 b |
1.77 ± 0.07 a |
1.64 ± 0.06 ab |
1.69 ± 0.11 ab |
abc Means within the same row carrying different superscripts were significantly different at (P ≤ 0.05).
NSPI: Native soy protein isolate . MSPI: Methylated soy protein isolate
Table 4: Effect of single or co-dietary supplementation with native soy protein isolate (NSP) and methylated soy protein isolate (MSP) on carcass traits relative to the live weight (%) of broiler chickens.
| Parameters | Control | NSP2% | MSP 2% | NSP+MSP 1%+1% | NSP+MSP 2%+2% |
| Dressing % | 60.8 ± 2.1 | 59.2 ± 1.8 | 60.9 ± 0.6 | 61.0 ± 1.7 | 61.1± 1.5 |
| Liver % | 2.0 ± 0.2 b |
2.3 ± 0.2 ab |
2.8 ± 0.3 a |
2.4 ± 0.2 ab |
2.8 ± 0.3 a |
| Heart % | 0.42 ± 0.09 | 0.44 ± 0.08 | 0.45 ± 0.07 | 0.43 ± 0.03 | 0.49 ± 0.10 |
| Gizzard % | 2.3 ± 0.44 b |
2.4 ± 0.06 ab |
2.8 ± 0.3 ab |
2.4 ± 0.2 ab |
2.9 ± 0.1 a |
| Intestine % | 5.1 ± 0.4 c |
8.3 ± 0.8 a |
6.5 ± 0.3 b |
7.3 ± 0.4 b |
7.0 ± 0.5 b |
| Spleen % | 0.09 ± 0.03 | 0.12 ± 0.03 | 0.10 ± 0.01 | 0.13 ± 0.02 | 0.12 ± 0.02 |
| Bursa % | 0.15 ± 0.02 bc |
0.11 ± 0.03 c |
0.18 ± 0.01 b |
0.13 ± 0.04 c |
0.22 ± 0.01 a |
| Thymus % | 0.33 ± 0.03 b |
0.37 ± 0.04 b |
0.54 ± 0.1 a |
0.38 ± 0.01 b |
0.50 ± 0.09 a |
abc Means within the same row carrying different superscripts were significantly different at (P ≤ 0.05).
NSPI: Native soy protein isolate . MSPI: Methylated soy protein isolate
was calculated as W2 – W1, where W2 is the final body weight at the intended period and W1 is the initial body weight at the same period. Feed intake of each replicate was recorded as the difference between weight of the feed offered and residues left and then divided by the number of birds in each replicate to find out the average feed intake per bird. Feed conversion ratio (FCR) was estimated according to (Wanger et al., 1983).
Carcass Traits
At the end of the experiment period, five birds from each group were selected, off food overnight, weighed then slaughtered by sharp knife to complete bleeding, followed by plucking the feather, evisceration and finally weighed to
Table 5: Effect of single or co-dietary supplementation with native soy protein isolate (NSPI) and methylated soy protein isolate (MSPI) on some serum biochemical parameters of broiler chickens.
| Control | NSPI 2% | MSPI 2% | NSPI+MSPI 1%+1% | NSPI+MSPI 2%+2% | |
| Total proteins (g/dl) |
6.5 ± 0.3 d |
7.3 ± 0.2 c |
7.7 ± 0.3 bc |
7.9 ± 0.1 b |
8.3 ± 0.2 a |
| Albumin (g/dl) |
4.4 ± 0.6 |
4.3 ± 0.7 | 4.3 ± 0.6 | 4.4 ± 0.40 | 4.3 ± 0.3 |
| Total globulins (g/dl) |
2.08 ± 0.44b |
2.98 ± 0.7ab |
3.36 ± 0.8a |
3.57 ± 0.4a |
4.06 ± 0.1a |
| A/G ratio |
2.22 ± 0.8a |
1.55 ± 0.7ab |
1.40 ± 0.6ab |
1.24 ± 0.2ab |
1.05 ± 0.08b |
|
α1 Globulin (mg/dl) |
0.16 ± 0.03 |
0.16 ± 0.02 | 0.16 ± 0.04 | 0.16 ± 0.03 | 0.17 ± 0.01 |
|
α2 Globulin (mg/dl) |
0.61 ± 0.07 |
0.60 ± 0.07 | 0.64 ± 0.04 | 0.62 ± 0.08 | 0.58 ± 0.07 |
|
β Globulin (mg/dl) |
0.60 ± 0.17 |
0.59 ± 0.01 | 0.57 ± 0.20 | 0.58 ± 0.16 | 0.57 ± 0.19 |
|
γ Globulin (mg/dl) |
0.71 ± 0.18 b |
1.63 ± 0.7ab |
1.97 ± 0.76a |
2.22 ± 0.38a |
2.41 ± 0.57a |
| Growth hormone (ng/ml) |
2.53 ± 0.97 d |
4.00 ± 0.46c |
6.13 ± 0.40b |
7.83 ± 0.32a |
9.33 ± 0.35a |
abc Means within the same row carrying different superscripts were significantly different at (P ≤ 0.05).
NSPI: Native soy protein isolate . MSPI: Methylated soy protein isolate
Table 6: Effect of single or co-dietary supplementation with native soy protein isolate (NSPI) and methylated soy protein isolate (MSPI) on the chemical composition of breast meat.
| Parameters | Control | NSPI 2% | MSPI 2% | NSPI+MSPI 1%+1% | NSPI+MSPI 2%+2% |
| Moisture % | 74.27 ± 2.05 |
72.97 ± 0.31 |
75.50 ± 3.03 |
75.03 ± 3.44 |
72.40 ± 1.23 |
| Crude protein % |
68.40 ± 0.36 b |
68.00 ± 0.70 b |
68.00 ± 0.20 b |
72.03 ± 0.11 a |
72.00 ± 1.00 a |
| Ether Extract % |
5.33 ± 0.58 |
6.67 ± 1.53 |
6.00 ± 1.00 |
7.67 ± 2.31 |
7.33 ± 0.58 |
| Ash % |
4.53 ± 0.90 |
4.93 ± 0.58 |
4.63 ± 0.58 |
4.87 ± 0.23 |
4.17 ± 0.51 |
ab Means within the same row carrying different superscripts were significantly different at (P ≤ 0.05).
NSPI: Native soy protein isolate . MSPI: Methylated soy protein isolate
detect the dressing percentage. The dressed carcass weight, liver, gizzard, intestine, heart and spleen were weighted and calculated as percent of life body weight. The relative weight of some organs was calculated according to (Abdel-Samee, 1995).
Clinico-Biochemical Analysis
At the day 35th post-supplementation, blood samples were randomly collected from five birds per treatment after slaughter into rubber stoppers sterilized tubes. Samples were left to coagulate and centrifuged at 3500 rpm for 15 min to obtain serum, and the serum samples were retained in Eppendorf tubes at –20 °C until analyzed for protein electrophoresis and growth hormone measurement.
Serum concentration of total proteins was determined colorimeterically using the biuret method applied by (Henry, 1974). Protein fractions were analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), as described by (Laemmli, 1970). Meanwhile, chicken ELISA kit of My Biosource Co. with CAT. NO. MBS266317 was used for growth hormone (GH) determination following the instruction of the enclosed pamphlets.
Chemical Analysis of Breast Muscle
Muscle sample of breast was taken from each slaughtered birds (5 birds per group) the proximate analysis for muscle samples for (moisture, crude protein, fat and Ash percent) were carried out according to the standard techniques of the (AOAC, 2002). All analyses were done in triplicates.
Histopathological Examination
The intestinal specimens was kept in 10% neutral buffered formalin (fixation) and processed for histologically analysis. Briefly, intestinal tissues specimens were dehydrated with a series of ascending grade ethanol (75-100%). They were then placed in xylol I, II and embedded in paraffin then cut into 4 µm cross and longitudinal-sections were sliced using a microtome (Leica RM 2155, England). They were stained by hematoxylin and eosin as described before (Suvarnaet al., 2013). One slide was stained for each animal then images were taken using AmScope 5.0 MP microscope digital camera, then on low power field (40 X magnification), was selected for each animal in each group (25 images for each group). Intestinal villi length, villi width, crypts depth and mucosal thickness were measurements by using Mitocam® software (Motic Images plus 2.0, china) according to (Seyyedin and Nazem, 2017).
Table 7: Effect of single or co-dietary supplementation with native soy protein isolate (NSPI) and methylated soy protein isolate (MSPI) on Intestinal histology (µm).
| Parameters | Control | NSP 2% | MSP 2% | NSP+MSP 1%+1% | NSP+MSP 2%+2% |
|
Duodenum |
|||||
| Villus Tall (VT) µm |
1.0200± 0.19 b |
1.5000 ± 0.20 a |
1.0375 ± 0.24 b |
1.4636 ± 0.40 a |
1.000 ± 0.11 b |
| Villus Width (VW) µm |
1.333 ± 0.05 c |
0.2286 ± 0.05 b |
0.1250 ± 0.05 c |
0.4000 ± 0.10 a |
0.1556 ± 0.05 c |
| Crypt Depth (CD) µm |
0.1750 ± 0.05 b |
0.3667 ± 0.10 a |
0.3000 ± 0.13 a |
0.3222 ± 0.04 a |
0.3417 ± 0.17 a |
| Mucosal thickness (MT) µm |
1.3750 ± 0.24 c |
2.1500 ± 0.18 a |
1.5750 ± 0.05 bc |
1.8500 ± 0.05 ab |
1.6273 ± 0.51 bc |
| Jejunum | |||||
| Villus Tall (VT) µm |
1.1000 ± 0.20 abc |
1.4429 ± 0.48 a |
0.7833 ± 0.13 bc |
1.1600 ± 0.25 ab |
0.7667 ± 0.29 c |
| Villus Width (VW) µm |
0.1333 ± 0.05 c |
0.2500 ± 0.10 ab |
0.1714 ± 0.07 bc |
0.3400 ± 0.13 a |
0.2429 ± 0.08 abc |
| Crypt Depth (CD) µm |
0.2000 ± 0.10 b |
0.2778 ± 0.11 b |
0.2250 ± 0.09 b |
0.4714 ± 0.09 a |
0.2286 ± 0.12 b |
| Mucosal thickness (MT) µm |
1.4286 ± 0.44 ab |
1.5800 ± 0.66 ab |
1.5667 ± 0.24 ab |
1.8333 ± 0.11 a |
1.1000 ± 0.41 b |
| Ileum | |||||
| Villus Tall (VT) µm |
0.8333 ± 0.08 bc |
1.0800 ± 0.36 b |
0.9833 ± 0.13 bc |
1.6667 ± 0.21 a |
0.7714 ± 0.28 c |
| Villus Width (VW) µm |
0.1000 ± 0.00 c |
0.5000 ± 0.17 b |
0.1167 ± 0.04 c |
0.7400 ± 0.09 a |
0.1125 ± 0.03 c |
| Crypt Depth (CD) µm |
0.2000 ± 0.08 c |
0.3800 ± 0.08 b |
0.2000 ± 0.09 c |
0.5800 ± 0.22 a |
0.2286 ± 0.07 c |
| Mucosal thickness (MT) µm |
1.1200 ± 0.16 c |
1.9500 ± 0.19 ab |
1.5000 ± 0.25 bc |
1.8200 ± 0.29 ab |
2.0778 ± 0.68 a |
abc Means within the same row carrying different superscripts were significantly different at (P ≤ 0.05).
NSPI: Native soy protein isolate . MSPI: Methylated soy protein isolate
Statistical Analysis
The data was analyzed by using SPSS 24.0 and expressed as the mean ± standard deviation (SD). The variation was assessed by one-way (ANOVA) and the differences between experimental groups were calculated by Duncan’s multiple-range test (Duncan, 1955). Statistical significance of the results calculated at (P ≤ 0.05).
RESULTS
Growth Performance Parameters
Growth performance of broilers fed the experimental diets was shown in Table (3). During the starter period, the BW, BWG, FI and FCR increased (P ≤ 0.05) significantly by experimental diets through group 2 to 5 as compared to group fed on control diet. During the grower period, experimental diets had non-significant (P ≥ 0.05) effect on BW& BWG while FI & FCR significantly increased (P ≤ 0.05) in broilers fed on diets supplemented with 2NSPI:2MSPI and 1 NSPI: 1MSPI. The results of the finisher period and overall performance revealed that supplementation of diet with 2NSPI:0MSPI and 1NSPI:1MSPI significantly (P ≤ 0.05) increased the BW and BWG and significantly (P ≤ 0.05) decreased FCR but had non-significant effect (P ≥ 0.05) on feed intake.
Carcass Traits
Statistical analysis of data of carcass traits was presented in Table (4). Carcass dressing % was non-significantly (P ≥ 0.05) affected, while Intestine % was significantly (P ≤ 0.05) increased for T2 through T5 as compared to the T1. There was non-significant (P ≥ 0.05) difference in the heart and spleen percentages among experimental groups versus the control group. Liver, thymus and bursa percentages significantly (P ≤ 0.05) increased in 2NSPI:0MSPI and 2NSPI:2MSPI compared to control group. Gizzard % was significantly (P ≤ 0.05) increased 2NSPI:2MSPI, while insignificant (P≥0.05) increased on other experimental groups compared to group fed on control diet.
Biochemical Parameters Of Blood
Concerning to the biochemical data represented at Table (5), comparing to the control group, broilers fed on NSPI and / or MSPI showed a significant (P ≤ 0.05) increase at the serum levels of total proteins and total globulins as a result of increasing γ – globulins concentration and also showed marked elevation at the growth hormone level with a significant (P ≤ 0.05) decrease at A/G ratio. These changes were clearer at 0NSPI:2MSPI supplemented group than 2NSPI:0MSPI for 35 days. Double exposure to both MSPI and NSPI revealed more significant results than the single exposure and the concentration 2% was more obvious and preferable than 1%. Meanwhile, serum albumin, α1 – globulin, α2 - globulin and β – globulin were non-significantly changed at all groups.
Chemical Composition of Breast Muscle
Chemical composition of breast meat was shown in Table (6). The results revealed that the single or co-dietary supplementation with NSPI and/ or MSPI had no significant effect (P≥ 0.05) on Moisture, Ash and ether extract percent. Crude protein percent was significantly increased in broilers that fed on diets supplemented with 2NSPI:2MSPI and 1NSPI:1MSPI and non-significantly affected (P≤ 0.05) in other experimental groups as compared to control group.
Intestinal Histology
The results of intestine histology was shown in Table (7) revealed that duodenal villus tall, duodenal villus width, duodenal crypt depth and duodenum mucosal thickness were significantly (P≤ 0.05) increased in broiler chickens fed on diets supplemented with 2% NSPI and 1%NSPI : 1%MSPI. Co-dietary supplementation with 1% NSPI &1% MSPI and single dietary supplementation with 2 % NSPI significantly increased jejunal villous width and jejunal crypt depth and insignificantly increase jejunal villous tall and jejunal mucosal thickness. Ileal villous width, ileal crypt depth and ileal mucosal thickness were significantly (P≤ 0.05) increased in broiler chickens fed on diets supplemented with 2% NSPI and 1%NSPI: 1%MSPI, while, ileal villous tall was significantly increased (P≤ 0.05) in broiler chickens fed on diets supplemented with 1%NSPI: 1%MSPI and insignificantly increased in broiler chickens fed on diet supplemented with 2%NSPI. Villous tall, length, crypt depth and mucosal thickness of different parts of the small intestine were not significantly different (P≥ 0.05) in groups fed diets supplemented with 2MSPI and 2NSPI:2MSPI compared with the control group (Plate 1). Cross and longitudinal sections of the intestine of chicken fed 2NSPI and 1NSPI:1MSPI showed broad and thickened villous tips in most intestinal villi and an increase in the absorptive surface (Plate 2).
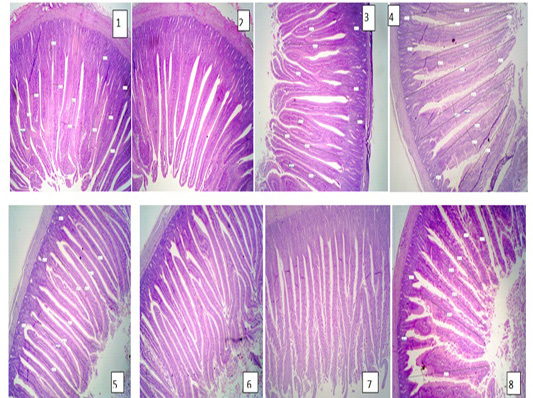
Plate 1: Figure 1,2,3 (Control group); Figure 4,5,6 (2MSPI); Figure 7,8 (2NSPI: 2MSPI): Cross and longitudinal sections of the intestine of the chicken showing normal tall and thin intestinal villi. H&E (40 X).
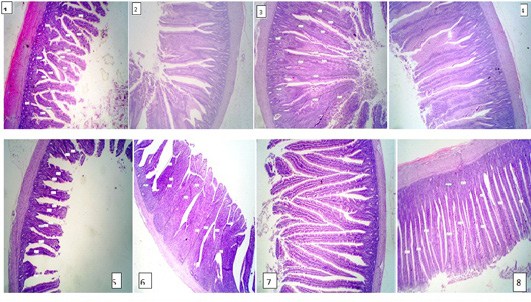
Plate 2: Figure 1,2,3,4 (2NSPI) and figure 5,6,7,8 (1NSPI:1MSPI ); cross and longitudinal sections of the intestine of the chicken showing broad and thickened villous tips in most intestinal villi and an increase in the absorptive surface. H&E (40 X).
DISCUSSION
In poultry nutrition, great attention is directed to protein products and their ingredients as they assist in the body tissue building, increasing growth and immunity (Beskiet al., 2015). The results of the present study showed that 2NSPI:0MSPI and 1NSPI:1MSPI supplemented diets had significantly improved the growth performance of the broiler chickens than other groups. This improvement in the growth performance may be attributed to the significant increase in the level of growth hormone (GH) concentration as it mostly altered by the composition of diet (Merimee et al., 1976), and the greater villous length, villous width, mucosal thickness and crypt depth in different parts of the small intestine, indicating an increase in the absorptive surface which resulted in better nutrients utilization. Ingestion of protein rich diet enhances the circulating GH secretion such as single meal ingestion of amino acids and dietary protein or intravenous injection of amino acids in large amounts (Knopf et al., 1965; Knopf et al., 1966; Collier et al., 2005). Vasconcelos et al. (2017) reported that inclusion of 6 % and 9 % soy protein concentrate resulted in greater villous tall and crypt depth in different parts of the small intestine, indicating an increase in the absorptive surface which resulted in better nutrients utilization. Cortés (2012) reported a decrease in the anti-nutritional factors of soybean as a result of mono competent protease supplementation to the diet of broilers, increase villous length and crypt depth, with increased the absorptive surface and villous: crypt ratio at 14 days of age. Beski and Iji, (2015) reported positive effect of processed soy protein on body weight, feed utilization and jejunum histomorphology at 24 d of age when included to corn or wheat based diets by (25, 50 or 100 g/kg diet).
The improvement in the growth performance also may be attributed to that processed soy products have lower amount of oligosaccharides and antigenic substances and the procedures concerned in NSPI and MSPI processing may result in improved energy and amino acids availability (Batal and Parsons, 2002, 2003), increased digestibility of amino acids and crude protein than that of SBM (Sohn et al., 1994; Grala et al., 1998) resulting in better nutritive value than SBM leading to improvement of chick performance (Peisker, 2001). Van der Eijk (2015) reported increased body weight and feed efficiency as a result of inclusion of processed soy protein to broiler starter diet. Jankowski et al. (2009) reported higher final body weight of turkeys consuming SBM-NSPI and NSPI diets, while, incorporation of MSPI as a replacement for SBM did not affect the final body weight but improved feed utilization efficiency. Van der Eijk (2015) reported higher 8-week body weight of turkeys by partial or complete replacement of SBM with NSPI, while inclusion of MSPI in replacement of SBM improved feed utilization significantly. In another study, inclusion of NSPI 10% in piglet weaning diet resulted a significant improvement in performance of piglet (Siugzdaite et al., 2008), and partial substitution of SBM with extruded NSPI in a high-SBM diet 40% resulted in a significant improvement in the growth performance (Lenehan et al., 2007). In contrast, Vasconcelos et al. (2017) found no effect of NSPI inclusion on BW, BWG and Feed efficiency in broiler at any studied period (1-7, 1-21 and 1-40 days of age). Elbing et al. (2015), Batal and Parsons (2003) reported no significant effect of soy protein isolate on the broiler performance. A decreased average daily gain and FCR was reported in the research of Xianglun et al. (2017). Heat oxidized soy protein isolate decreased body weight of broiler (Wu et al., 2014; Chen et al., 2015).
The results of the present study revealed no significant effect of NSPI or MSPI on carcass dressing %, while Intestine % was significantly increased by NSPI and / or MSPI supplementation. In contrast, Vasconcelos et al. (2017) and Beski et al. 2015 found that the relative weight of the small intestine of the birds which received processed soy product was decreased slightly than those of control birds and there was an improvement in the carcass yield at 35 days. Also heat oxidized soy protein isolate not affect the relative weight of gizzard; however relative anterior intestine weight significantly decreased (Chen et al., 2015).
In the present study, NSPI and MSPI supplemented diets significantly improved the immune status of the birds indicated by an increase in the blood circulating γ- globulins levels and increased size of the thymus and bursa fabrics. The results may be attributed to the function of soy proteins as immunological substances and its ability to enhance the linear increase in lymphocyte numbers (Nikoskelainen et al., 2007) which responsible for the manufacturing of immunoglobulin (Ortega and Mellors, 1957). Soy proteins are compounds that meet the need of all essential amino acid to maintain normal growth (Wu et al., 2014). Plasma proteins play essential roles in colloid osmotic pressure maintenance, assuring the glucose level, transportation of selective minerals and hormones as well as enzymes and immune system building in the living organisms (Filipović et al., 2007). Gamma globulins (immunoglobulins) are synthesized in the reticulo- endothelial system (RES) cells and responsible for the immunological reactions (Jolles et al., 2005).
The results of the meat composition were consistent with the result of (Krawczyk et al., 2015) who reported that dietary supplementation with yellow lupine at 8%, 16% and 24% had no significant effect on moisture %, protein %, ether extract % and Ash % of turkey breast muscle. Laudadio & Tufarelli (2010) reported that the inclusion of micronized – dehulled peas at (400 g/kg) had no significant effect on moisture %, protein % and ash % but significant decrease fat % of broiler breast muscle.
CONCLUSIONS
From the obtained results we can conclude that single supplementation with 2% NSPI or co-dietary supplementation with 1NSPI:1MSPI had positive influence on growth performance and gut health. Both single and co-dietary supplementation with NSPI and MSPI had potential effects on immune status of broiler chickens.
Acknowledgements
The authors acknowledge the Department of Nutrition and Clinical Nutrition, Faculty of Veterinary Medicine, Zagazig University (Egypt) for their cooperation.
Conflict of interest
The authors declare that they have no competing interests.
Authors Contribution
Anaam E. Omar: Animal work, sample collection, and manuscript preparation.
Shimaa A. Amer: Designing the experiment, animal work, sample collection, manuscript preparation, statistical analysis, and publishing the article “corresponding author”.
Wafaa A. M. Mohamed: Sample collection, serum biochemical parameters analysis, and manuscript preparation.
Ali Osman and Mahmoud Sitohy: Preparation of native soy protein isolate and methylated soy protein isolate and manuscript preparation.
ReferenceS