Introduction
Hyperlipidemia occurs by a rise in total cholesterol (TC), triacylglycerol (TAG), low-density lipoprotein cholesterol (LDL-C) and reduced high-density lipoprotein cholesterol (HDL-C) (Rahaman et al., 2013). High cholesterol diet results in fatty liver, atherosclerosis, and heart failure (Machado and Diehl., 2016; Wójcik-Cichy et al., 2018). Oxidative stress can modulate immunity via controlling expression of cytokines (IL6) that regulate weight and metabolism (Walaa et al., 2019; Fernández-Gayol et al., 2019). Atherosclerotic plaque is formed when there is an engulfment of LDL-C by macrophages Producing foam cells and proinflammatory cytokines (Hopkins, 2013). Sub-endothelial oxidative stress contributes to the oxidization of LDL-C formation and the development of fatty streak (Brites et al., 2017).
Herbs have gained a much important medical role in recent years (Karam et al., 2017). Linum usitatissimum (flaxseed or linseed) contains α-linolenic acid (α-ALA), secoisolariciresinol diglucoside (SDG) lignan, and dietary fiber (Hall et al., 2006). Flaxseed has antioxidant, anti-inflammatory, anti-atherosclerotic, and cardio-protective properties (Zanwar et al., 2011; Naik et al., 2018). Daily supplementation of ethanolic extract of flaxseed (400mg/kg for three weeks exhibited hypoglycemic and antioxidant properties in diabetic rats (Ghule et al., 2012). With reduced LDL-C and increased HDL-C level, administration of ground flaxseed (15g/100 gm diet for 20-week) displayed a cardioprotective effect in AD-fed diabetic hamsters (Haliga et al., 2015). Flaxseed oil downregulated hepatic expression of a gene involved in lipid metabolism, TNF, IL-6, and ICAM in apo-E deficient mice (Han et al., 2015). Since there are limited studies on immunomodulatory and cardio-protective aspects of flaxseed seeds ethanolic extractThis work was conducted to determine the anti-inflammatory, anti-angiogenic, and anti-atherogenic impact of ethanolic flaxseed seeds extract in atherogenic dietfed rats. These characteristics were assessed through cardiac, and inflammatory markers, and expression of HMGCR and VEGF. Phytochemical identification of flaxseed seeds ethanolic extract was also performed to detect total phenolic, flavonoids, vitamin C contents.
Materials and Methods
Animals
Forty Wistar male rats (120-130gm) were obtained from the National Research Center Laboratory Animal House, Dokki, Giza, Egypt. Animals were kept in the animal house at the Faculty of Veterinary Medicine, Suez Canal University. The study was carried out in accordance with the «Guide for the Care and Use of Laboratory Animals». Atherogenic diet (AD) comprised of basal diet (355 gm/kg), cholesterol (10gm/kg), lard (310gm/kg), corn oil (10gm/kg), DL-Methionine(3gm/kg), casein (250 gm/kg), vitamin and mineral mix 60gm/kg), Yeast powder (1gm/kg), and sodium chloride mix (1 gm/kg) (Srinivasan et al., 2005).
Flaxseed seeds Ethanolic extract preparation
Flaxseed seeds were obtained from the Department of Horticulture, Agricultural Research Center, Dokki, Giza, Egypt, and confirmed at a facility in the Pharmacognosy Department, Faculty of Pharmacy, Cairo University, Egypt. Flaxseed seeds (500 g) were washed with distilled water, dried in hot air oven (40–60 oC), grounded, soaked twice in 96% ethanol for 24 hr, filtered, dried the filtrate, and stored at 4°C until further use (Samson et al., 2012).
Experimental protocol
Rats were divided into four groups, (n=10 each). Group I (Control) fed on basal pelleted diet and water. Starting from 7th week of the experiment, rats were supplemented with 1ml distilled water per-os daily till the end of the experiment (12th week). Group II (AD) fed the atherogenic diet (AD) for three months. Starting from 7th week of the experiment, rats were supplemented with 1ml distilled water per-os daily till the end of the experiment. Group III(AD+EEFS) fed AD for three months. Starting from the 7th week, rats were supplemented with ethanolic extract of flaxseed seeds (EEFS) 400mg/kg/day (Ghule et al., 2012). Group IV (EEFS) fed on a standard rat pelleted diet for three months. Starting from 7th week, rats were supplemented with EEFS 400mg/kg/day till the end of the experiment.
Collection of blood, liver and heart samples
At the end of the experiment, rats were fasted overnight and euthanized. Blood samples were collected and centrifuged for serum separation. Heart and liver samples were dissected. Liver tissues were kept at -80°C for as an assessment of the rate of expression of HMGCR, and VEGF. Heart tissue was used for histological analysis.
Analysis of serum lipid profile, cardiac enzyme activity, and inflammatory markers
Commercially available kits were used to determine TC (Cat. No.1105, Vitro Science, Egypt), TAG (Cat. No. 702040050, Vitro Science, Egypt), HDL-C (Cat. No. 282000005, Vitro Science, Egypt). Lactate dehydrogenase (LDH) LDH ELISA Kit, Cat. No. MBS043166, MyBioSource, USA, creatinine kinase- MB (CK-MB) CK-MB Isoenzyme ELISA Kit, Cat. No: MBS008782, MyBioSource, USA, aspartate aminotransferase (AST), Cat. No. TR70121, Thermo Fisher Scientific, USA, serum high-sensitivity C-Reactive Protein (hs-CRP), hs-CRP ELISA kit, Cat. No: MBS2500438 MyBioSource, USA, Interleukin-6 (IL-6) IL-6 ELISA kit, Cat. No: MBS355410 MyBioSource, USA, and Interleukin-10 (IL-10) IL-10 ELISA kit, Cat No. MBS175998, MyBioSource, USA as per manufacturer’s instructions. Previously published protocols and procedures were used to determine the concentration of LDL- C, (Davidson and Rosenson, 2009), VLDL- C (Zhao et al., 2017), and Castelli Risk Index (CRI-I and CRI-II) cardiovascular risk (Olamoyegun et al., 2016).
Molecular investigations
The extraction of total RNAs of the liver was done (RNeasy Mini Kit, Qiagen) following manufacturer guidelines. Transformation of RNA to cDNA was carried out (RevertAid Reverse Transcriptase (cDNA synthesis kit, ThermoFisher, Catalog number: EP0441). QuantiTect SYBR green PCR kit (3 x 1.7 ml 2x QuantiTect SYBR Green PCR Master Mix, 2 x 2 ml RNase-Free Water, Cat. No./ID: 204143 ) was applied to quantify 3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMGCR) (Morral et al., 2007), and vascular endothelial growth factor (VEGF) expression (Peng et al., 2014). Previously published primers and probes were synthesized (Metabion, Germany) (Table 1). Thermal conditions included 94°C for 15 min, followed by 40 cycles of each of 94°C for 15s and 60°C for the 30s. Endogenous gene (β-actin) was used for normalization, and expression quantification was determined (Bahr et al., 2019).
Quantification of phenolic, flavonoid compounds and antioxidant activity
Total phenolics levels in EEFS were evaluated using the Folin-Ciocalteau assay and described as gallic acid equivalents (GAE/g) extract (Dandge et al., 2011). Total flavonoid level was detected and described as quercetin equivalents (mg QE /g)) according to the previously described method (Kumar and Roy, 2018). DPPH (2,2-diphenyl-2-picryl-hydrazyl free radical) assay was used to determine the antioxidant activity in EEFS (Norshazila et al., 2010).
Chromatographic identification of EEFS total phenolic, flavonoids and vitamin C content
For the detection of phenolic compounds, standards such as gallic, p-coumaric, caffeic, syringic, cinnamic, chlorogenic, and ferulic acid were used.Referring to flavonoids, standard such as kaempferol, catechins, quercetin, rutin, hesperetin, rosmarinic acid, quercitrin, and apigenin were applied. Vitamin C standard was used for its measurement. The contents of the above said components were determined through HPLC (Agilent Technologies, USA) following the previously published protocol (Croci et al., 2009).
Statistical analysis
The results were analyzed through (SPSS, version 20). Data were described as mean± SD. One-way Analysis made a comparison of Variance (ANOVA) followed by Duncan Multiple Range Test. The significance was considered at P<0.05.
Results
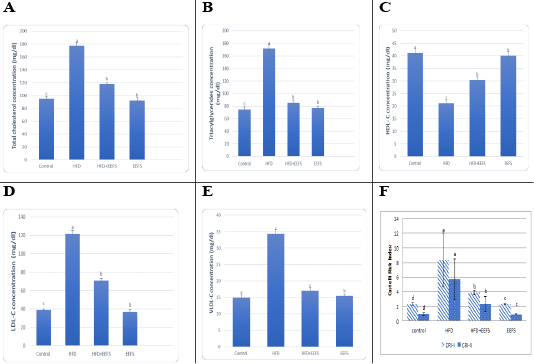
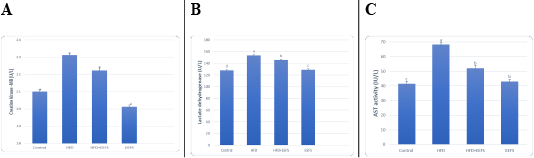
Effect of flaxseed seeds ethanolic extract on lipid profile, cardiac, inflammatory, and angiogenic markers in AD- treated groups
AD induced hyperlipidemia displayed an increased TC, TAG and LDL-C, VLDL-C values, along with an upregulation in the rate of expression HMGCR. The HDL-C concentration as decreased on the other hand. An elevated CRI-I, CRI-II, LDH, and AST activity indicated the risk of cardiovascular disease. Inflammatory and oxidative burden as indicated by higher serum pro-inflammatory IL 6, hs-CRP level, and a regressed level of the antioxidant IL-10. An over-expression of VEGF was observed than control. EEFS treatment after AD-feeding resulted in hypolipidemic, anti-inflammatory, antioxidant, antiangiogenic, and antiatherogenic effects. No significant difference was observed between EEFS and control rats indicating its safe usage (Figures 1, 2, 3 and 4).
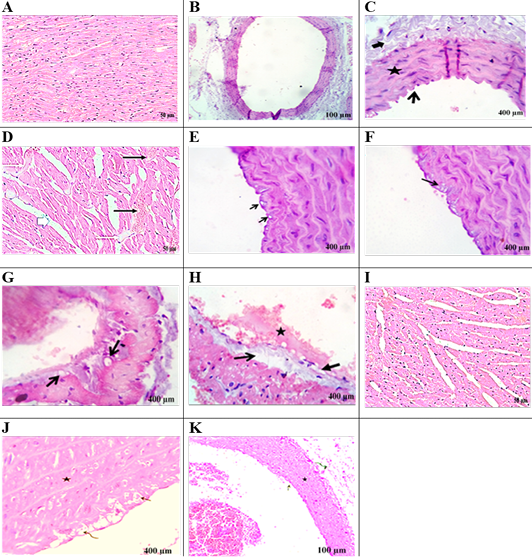
Histological picture of heart and aorta
Figure 5A showed regular arranged cardiac myofibers with single, ovoid, and centrally located nuclei myocytes in the heart of the control group. Figure 5B, C exhibited normal intimal, medial,and adventitial structures of the aorta. Heart section in AD-group (Figure 5D) showed severe myocardial degenerative changes with disarrayed of cardiac myofibers. Hyaline degeneration, interstitial edema along with cardiac hypertrophy, severe congestion, and dilatation of blood vessels was also observed. Aorta section of the AD-group displayed intimal-vacuolation of the endothelial cells (Figure 5E). Atheromatous plaques (round cells and macrophages were formed from fat vacuoles under the endothelium (Figure 5F). Focal endothelial destruction of the endocardium with subendothelial deposition of fat vacuole was observed (Figure 5G, 5H). Heart section in the AD+EEFS group displayed tissue restoration with mild hyaline degeneration, interstitial edema, and congested blood vessels (Figure 5I). Aorta presented normal tunica intima, tunica media, and tunica adventitia (Figure 5J). The presence of a few degenerative and necrotic changes in the endothelial and subendothelial layers was also evident (Figure 5K).
Data set as (Mean ± S.D). Means with different superscript letters within the same raw are significantly different at P<0.05. AD: atherogenic diet; EEFS: ethanol extract of flaxseed seeds.
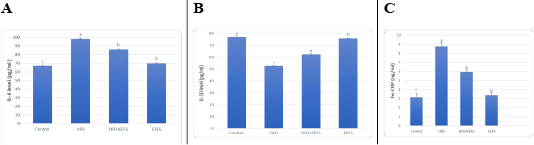
Figure 3: The effect of flaxseed seeds ethanolic extract on inflammatory markers in AD- treated groups.
Table 1: HMGCR and VEGF rat primers sequences.
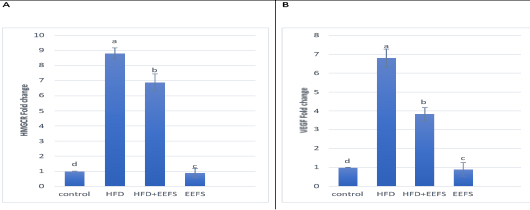
Figure 4: The effect of flaxseed seeds ethanolic extract on liver mRNA expression of HMGCR and VEGF in AD- treated groups.
Data noted as (Mean ± S.D). Means with different superscript letters within the same raw are significantly different at P<0.05. AD: high fat diet, EEFS: ethanol extract of flaxseed seeds. (A) HMGCR: 3-hydroxy-3-methylglutaryl-coenzyme A reductase (B) VEGF: vascular endothelial growth factor.
Quantification of phenolic, flavonoids, and antioxidant capacity of EEFS
The total phenolic content was found to be 16.15 ±0.59mg GAE /g, while the concentration of total flavonoids was 2.5±0.58mg QE mg/g, and had antioxidant activity as 132.49±0.56 (Table 2).
Table 2: Measurement of total phenolic, flavonoids, vitamin C content and antioxidant capacity of EEFS.
|
Total flavonoids (mg QE /g) |
Total phenolic |
|
| 132.49±0.56 | 2.5±0.58 | 16.15 ±0.59 |
Chromatographic screening for detection of total phenolic, flavonoids and vitamin C levels
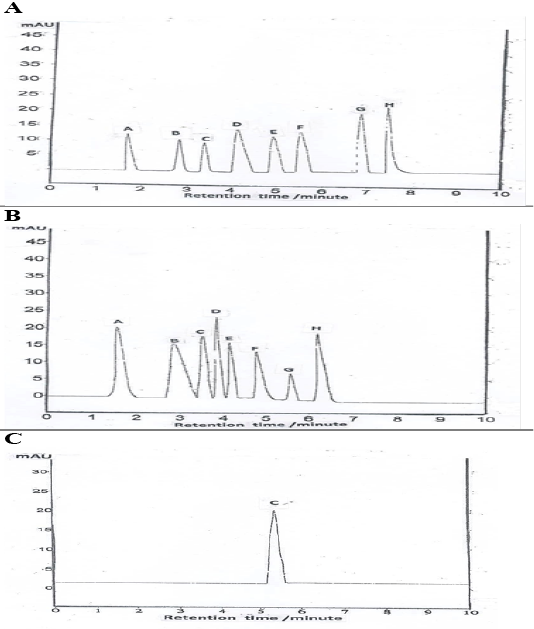
HPLC chromatogram revealed the presence of syringic acid as the major phenols followed by salicylic, cinnamic, p-coumaric, gallic, ferulic, caffeic, and chlorogenic acid (Table 3, Figure 6A). Quantification of total flavonoids compounds revealed the presence of rosmarinic acid at the greatest value, followed by catechins, quercetin, rutin, quercitrin, kaempferol, hesperetin, and apigenin (Table 3, Figure 6B). Vitamin C level is shown in (Table 3, Figure 6C).
Control group: (A) heart revealed regularly arranged cardiac myofibers with single, ovoid and centrally located nuclei myocytes. (B, C) aorta viewing normal intimal (open arrow), medial (star) and adventitial structures (closed arrow) of aorta. AD- group: heartsection in (D) showing sever myocardial degenerative changes with disarrayed of cardiac myofibers. Additionally, hyaline degeneration (white thin arrows), interstitial edema alongside cardiac hypertrophy (white thick arrows), sever congestion, and dilatation of blood vessels (black arrows). AD- group: aorta section showing: (E) Intimal-vacuolation of the endothelial cells (arrows).(F) Atheromatous plaques under the endothelium (arrow) and formed from fat vacuoles, round cells and macrophages.(G) Subendocardial deposition of fat vacuoles (arrows).(H) Focal endothelial destruction of the endocardium (closed arrow) with subendothelial deposition of fat vacuole (open arrow) with early thrombus formation (star). AD+EEFS group heart section in (I) displaying tissue restoration with mild hyaline degeneration, interstitial edema and congested blood vessels are detected. AD+EEFS group aorta section revealing: (J) normal tunica intima (open arrow), tunica media (star) and tunica adventitia (curved arrow). (K) show degenerative changes mostly fatty change beside necrotic changes in the endothelial and subendothelial layers with apparently normal tunica media (star).
Table 3: Chromatographic screening of total flavonoids, phenolic, and vitamin C levels (μg/ml) of EEGS.
|
Chloro- genic acid |
Caffeic acid | Ferulic acid | Gallic acid | P-Coumaric acid | Cinnamic acid | Salicylic acid | Syringic acid | Total Phenolic |
| 6.96 | 8.41 | 11.38 | 12.54 | 14.57 | 19.14 | 20.61 | 21.52 | |
| Apigenin | Hesperetin | Kaempferol | Quercitrin | Rutin | Quercetin | Catechins | Rosmarinic acid | Total Flavonoid |
| 0.67 | 1.38 | 1.53 | 1.58 | 1.7 | 1.91 | 2.09 | 2.3 | |
| 0.214 | Vitamin C | |||||||
(A): Data performs total phenolic compound (A-gallic acid, B-caffeic acid, C chlorogenic acid, D-cinnamic acid, E- ferulic acid, F- p- coumaric acid, G- Salicylic acid, H- Syringic acid); (B): Data performs total flavonoids compound (A-Catechins, B- Quercitrin, C-rutin, D-rosmarinic, E- kaempferol, F-hesperetin, G-apigenin, H-quercetin); (C): Data performs vitamin C content.
Discussion
The study confirmed AD- induced hyperlipidemia by an increase in TAG, LDL-C, VLDL-C, and TC, and a decrease in HDL-C content. This agrees with previous observations (Jiang et al., 2018; Feng et al., 2019). Naik et al. (2018) reported a relationship between hypercholesterolemia to elevated cholesterol uptake, an overexpression of HMGCR, and increased free fatty acids esterification. A minimized HDL-C level may contribute regression in the LCAT activity that favors cholesterol uptake from tissues to hepatic HDL (Kunnen and Van Eck, 2012).
Hyperlipidemia-induced atherosclerosis elevated CRI-I, CRI-II did progress cardiac damage indicated by the raised activity of LDH and AST. These findings agree with Subramania et al. (2017). Dyslipidemia and impaired cholesterol efflux are pivotal factors for cardiovascular disease.Furthermore, an accumulation of macrophage and cholesterol contents results in mitochondrial dysfunction and cell apoptosis (Yao and Tabas, 2001; Han et al., 2018). In the current work, AD-induced inflammatory burden was evident by rise in the levels of pro-inflammatory IL-6, hs CRP, and a decrease in IL-10. This corroborates with the finding reported previously (Siasos et al., 2011; Kondo et al., 2018; Fernández-Gayol et al., 2019). Following atherogenic diet feeding, hs-CRP raised significantly, which is known to controls the inflammatory process of atherogenesis (Kleemann and Kooistra, 2005). These findings mean that AD-induced hyperlipidemia is accompanied by an inflammatory response and cardiac damage. Histopathologically, this can be evident by hyaline degeneration, interstitial edema, cardiac hypertrophy, and the presence of atheromatous plaques under the endothelium of aorta.
EEFS reversed AD-induced hyperlipidemia that may be due to their flavonoid and phenols content (Wang et al., 2016; Subramania et al., 2017). A study revealed that quercetin flavonoids minimize the expression of sterol regulatory element-binding protein-1c and fatty acid synthase in the NAFLD model (Li et al., 2013). A meta-analysis indicated that daily administration of 500 mg/day of vitamin C improved endothelial function in diabetic, atherosclerosis, and cardiac failure patient (Ashor et al., 2014). Indeed, due to its SDG, α- ALA, several studies ascertained the hypolipidemic prospect of flaxseed (Edel et al., 2015; Torkan et al., 2015). An ALA-rich diet reduces hepatic lipid deposition by stimulating β-oxidation and by inhibiting fatty acid synthesis (Murase et al., 2005). A study reported hydroxyl radical scavenging impact of SDG of flaxseed in vitro (Prasad, 2000). Another meta-analysis study elucidated that omega-3 treatment minimizes hs-CRP, IL-6, and TNF-α in a different population (Li et al., 2014). Itoh et al. (2010) suggested the antioxidant prospect of vanillic acid in cultured hepatic cells. An administration of quercetin (50 mg/kg) for eight weeks minimized the hepatic inflammation and fibrosis in CCl4-treated mice via depressing macrophages infiltration and downregulated expression of IL-1β, IL-6, and TNFα (Li et al., 2018). The study of Ham et al. (2016) elucidated that administration of 0.05% syringic acid for four months downregulated IL6, TNF-α, NF-κB in obese mice. Syringic acid suppressed the expression of VEGF protein and mRNA in zebrafish embryos (Karthi et al., 2014).The research of Rahbardar et al. (2018) revealed that rosmarinic acid displayed anti-inflammatory prospects in the neuropathic pain model in rats. Hence, EEFS induced hypolipidemic, immunomodulatory, and antioxidant properties via DPPH scavenging in vitro, and in vivo through the upregulation of IL-10.
AD-feeding for 12 weeks overexpressed VEGF in a pattern revealed previously (Forstermann and Sessa., 2012; Castiglione et al., 2018). VEGF played a crucial role in angiogenesis. The neovascularization within atherosclerotic plaques is accompanied by thrombotic cascades (Coulon et al., 2012; Camaré et al., 2017). Contrary to this, VEGF expression is suppressed by EEFS treatment in AD-treated rats. This corresponds to the observation of Surapaneni et al. (2015) who described the role of quercetin in minimizing VEGF expression in AD-fed rats. Fan et al. (2017) concluded the anti-inflammatory role of catechins in bowel disease. Francis et al. (2013) concluded that α-ALA control expression of oxidative and inflammatory mediators in vascular tissue minimizing atherosclerosis progression. The histopathological findings confirmed the anti-atherosclerotic and cardioprotective prospect of EEFS, where cardiac tissue restoration with mild hyaline degeneration, interstitial edema with normal tunica intima, tunica media, and tunica adventitia of aorta was observed. This indicates the anti-angiogenic activity of EEFS that may reverse AD-induced atherosclerosis cardiac damage.
Conclusion
Our research demonstrates the safe administration of EEFS, and its potential impact as a hypolipidemic, immunomodulatory, antiangiogenic, antiatherogenic, and cardioprotective herbal drug. These impacts are largely attributed to its phenolic, flavonoid, and vitamin C content, as well as antioxidant capacity.
Authors Contribution
All authors contributed equally.
Conflicts of interests
The authors declare no conflicts of interest.
References