Synthesis and Application of Chitosan-Starch Based Nanocomposite in Wastewater Treatment for the Removal of Anionic Commercial Dyes
Synthesis and Application of Chitosan-Starch Based Nanocomposite in Wastewater Treatment for the Removal of Anionic Commercial Dyes
Amtul Jamil Sami1*, Madeeha Khalid1, Sara Iqbal1, Maira Afzal1 and A.R. Shakoori2
1Institute of Biochemistry and Biotechnology, University of the Punjab, Quaid-i-Azam Campus, Lahore 54590 Pakistan
2School of Biological Sciences, University of the Punjab, Quaid-i-Azam Campus, Lahore 54590 Pakistan
ABSTRACT
Chitosan is a cationic biopolymer that can be used for sustainable waste water treatment as a coagulant-flocculent due to its ability to coagulate various ionic substances such as metal ions as well as dyes. In the current study novel polymer composites were prepared using natural polymers chitosan and starch in molds. Structure of composites was analyzed using scanning electron microscopy. The physical parameters such as weight and swelling degree of composite were studied. Chitosan starch based composites were used in treatment of waste water to remove a toxic anionic dye Congo red. Congo red solution (0.015 mM) was treated with polymer composite at neutral pH overnight with 150 rpm orbital shaking at room temperature. The same procedure was performed for commercial dyes. The dye was removed due to the interaction with the polysaccharide starch and flocculation phenomenon. Chitosan starch based polymer composites can be used as an efficient and cost effective method to treat waste water polluted with carcinogenic dyes by coagulation-flocculation. Moreover this method is beneficial over other methods that employ toxic polymers or mineral coagulants.
Article Information
Received 24 June 2016
Revised 05 August 2016
Accepted 15 August 2016
Available online 19 September 2016
Authors’ Contributions
AJS performed pilot experiments; ARS and AJS designed the experiments which were carried out by MK, SI and MA. AJS, MK and ARS analysed the results and drafted discussion. All the authors read and approved the manuscript.
Key words
Chitosan, Starch, Waste water treatment, Nanocomposite, Flocculation
DOI: http://dx.doi.org/10.17582/journal.pjz/2017.49.1.21.26
* Corresponding author: yingbinwang@126.com
0030-9923/2017/0001-0021 $ 9.00/0
Copyright 2017 Zoological Society of Pakistan
INTRODUCTION
Green materials or biopolymers are under investigation for various applications. One very important biopolymer of special importance is chitosan (Mohanty et al., 2002). Chitosan is a deacetylation product of chitin, an abundant naturally occurring polysaccharide. It is abundantly present and extracted from shells of insects and marine animals such as crab, shrimps etc. (Renault et al., 2009a, 2009b; Mukherjee et al., 2004). Chitosan along with other biopolymers such as cellulose, starch, polysaccharides etc. can be used for making green material nano-composites and membranes for sustainable water treatment (Sharma et al., 2013). Chitosan based composites are being widely studied for water treatment and purification processes. Chitosan is less crystalline than chitin due to the specific antiparallel chains of chitosan and their cross linking. Chitosan has a higher degree of hydration and thus its swelling capacity and dye adsorption capability is higher. This mechanism provides a basis for formation of composite materials for water treatment (Carlstrom, 1957; Peesan et al., 2003). Starch also assists the function of chitosan as it aids in the adsorption of the dye and various macromolecules on the surface of composites.
Textile and leather industries utilize various types of dyes in their manufacturing procedure in bulk quantities which results in production of tons of liters of industrial waste water containing residual dyes. These dye compounds have brilliant colors and are toxic to the environment so in order to protect the environment from this industrial waste and to reuse and recycle the waste water it is of utmost importance to devise cost effective ways to remove dyes from waste industrial water (Lee et al., 2006, Reddy et al., 2005). In this respect nano-composites and membrane technologies are widely explored and used to remove toxic dyes from water (Petrinic et al., 2007, Qin et al., 2007; Riera-Torres et al., 2010, Vishnu and Joseph, 2007; Zahrim et al., 2011).
Metal coagulants as well as polymers have a tendency to coagulate dyes and ionic impurities from water. Polymer containing composites and membranes coagulate and flocculate these impurities (Bratby, 2006). Coagulation is a mechanism in which any dissolved or soluble material destabilizes and start to aggregate in a solution, whereby flocculation refers to as the process in which these insoluble particle come close together and aggregate forming, even larger insoluble particles the agglomerates (Leiknes, 2009). The size of dye molecule, molecular weight and high anionic charge also assist in coagulation and flocculation of dye molecules (Yu et al., 2002).
The mechanism behind the dye removal by natural coagulants is complex and involves various interactions. Electrostatic interactions, charge neutralization and bridging are among the major interactions involved in dye adsorption and coagulation flocculation. Natural polymers are long chain molecules with free charge bearing ends. The dyes used in dying and textile industry are mostly anionic or neutral in charge and relatively smaller in structure. The polymers owing to their large structure offer a large number of binding sites to attach dye molecules thereby neutralizing the charge and precipitating the molecule. Those polymers that do not contain free charge bearing groups cause interparticle bridging thus sorbing small molecules in the spaces present in their architecture this bridging occurs because of π electrons interacting with OH groups of the polysaccharides. Thus natural polysaccharides are very effective in water treatment (Verma et al., 2010; Miller et al., 2008; Yoshida et al., 1964; Yin, 2010).
Metal and mineral coagulants tend to increase the metal and salt content of treated water; moreover, sludge may be produced as a byproduct of coagulation activity of these compounds. Polymers especially biopolymers are regarded to be advantageous over the mineral coagulants as biopolymers are eco-friendly and do not add any toxic or unwanted residual compounds in treated water (Bolto, 1995; Beltrán-Heredia and Sánchez Martín, 2008; Verma et al., 2011).
MATERIALS AND METHODS
Materials
Following chemicals of analytical grade were used: Chitosan extracted from shrimp cells purchased from Sigma-Aldrich, starch (Sigma-Aldrich) and 1% glutaraldehyde. Besides that waste water from local fabric dyers was used for analysis. Glycine sodium hydroxide buffer pH10, potassium phosphate buffer pH 4.0 and Tris-Cl buffer pH 7.4 were used in different experiments.
Preparation of chitosan-starch based nanocomposite
Starch solution (2%) was prepared in 2% acetic acid and 2% chitosan solution was prepared in acetic acid. Both the solutions were mixed in 3:7 ratio with constant stirring at room temperature. Glutaraldehyde (1%) as a cross linking agent and few drops of glycerol (as a plasticizer) were added under stirring. The solution was then casted in the moulds (Diameter: 2.5cm) for solidification. 2ml solution was added in each mould. The moulds were kept in oven at 45ᵒC overnight (Baran et al., 2004). The nano-material based composite were removed from the moulds the next morning and were stored in sealed bags.
Structural analysis and physical characteristics of nanomaterial
Structural analysis of the prepared chitosan starch nano composite was done by scanning electron microscopy using Hitachi S-3700 N. The outer structure and architecture was viewed and analyzed under different magnifications (500X, 1000X, 2000X, 3000X) of electron microscope.
The physical characteristics i.e. swelling capacity of nano material based composite was checked under acidic potassium phosphate buffer pH 4.0, physiological Tris-Cl buffer pH 7.4 alkaline pH glycine sodium hydroxide buffer pH 10. The chitosan starch composites were immersed in each of the three buffers separately. After specific period of time, composite was taken out of flask by spatula. The weight of each sample was taken. All the experiments were run in triplicates.
The swelling in distilled water and different buffers was calculated by formula
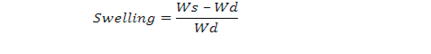
where Ws is weight of swelled sample and Wd is weight of dry sample (Gulrez et al., 2011).
Removal of Congo red dye by flocculation
0.15mM solution of Congo red dye was prepared in 0.1M Tris-Cl buffer pH 7.4. The starch chitosan nano composites were placed in each solution, each with a control. The solutions were kept on mechanical shaker for 12 h at 150rpm at room temperature and then observed periodically. After 12 h the solutions were filtered using Whatman’s filter paper No. 1 the filtrate and precipitates were analyzed.
Wastewater treatment for the removal of commercial dyes
Wastewater samples were obtained from local fabric dyer shops in 3 different colors namely red, see green and yellow. Sample solution (20 ml) was taken in a conical flask. The starch chitosan nano composite were placed in textile waste water containing dye. For each sample control was prepared in a similar fashion without the nano composite. The solutions were kept on mechanical shaker for 12 h at 150 rpm at room temperature for the removal of dye. After 12 h the solutions were filtered using Whatman’s filter paper No. 1. The filtrate and precipitates were analyzed.
RESULTS AND DISCUSSION
Chitosan starch nanocomposite
The nanocomposite was obtained by mixing starch and chitosan in specified ratio along with glutaraldehyde. Chitosan and starch produced a nano composite (Fig.1B, C). The scanning electron micrographs of composites indicated spherical molecules of nano-scale size dispersed evenly (Fig. 1B). The amount of glutaraldehyde affects the architecture of the composite whereas glycerol acts as a plasticizer and provides stability to the composite (Chung et al., 2010). Considerable concentrations of cross linker are required to form cross link. In the presence of low concentrations of cross linker the cross linking is not complete and thus a composite type material is formed which is visible in Figure 1B (Elson, 1980; Rangel-Mendez et al., 2010).
Physical properties of chitosan-starch nano-composite
The chitosan-starch based nano composite were prepared and dried as described earlier. Figure 2 shows weight increase of starch chitosan nanocomposite at different pH. The weights of dried samples were determined and the mean weight was recorded to be 57.2 mg.
Figure 3 shows the swelling of composite at different pH values. The nanocomposites and membranes formed of chitosan have a considerable swelling capacity because of the architecture. Chitosan has exposed amino groups at the ends that are charged. These charged species can attract ionic compounds as well as water. The hydration capacity of chitosan depends upon this structure, the amount of chitosan and the degree of polymerization or cross linking between the polymers. The swelling capacity was highest at acidic pH as at low pH the NH2 groups are more stable and their ability to bind with water is highest (Szygula et al., 2009). The swelling ratio of nanocomposite is low as there is no crosslinking between the two polymers. Scanning electron micrographs of the composites indicates that the architecture is made up of nano molecules of starch and chitosan which provides evidence for low swelling ratio.
Removal of Congo red dye by flocculation
Flocculation studies were conducted by immersing the nanocomposite completely in 20 ml of standard solution of Congo red dye in 100 ml conical flask. The mixture was placed on orbital shaking and the flocculation was observed at various intervals of time results are shown in Figure 4.
Figure 4 indicates the removal of a standard anionic dye congo red dye. Congo red flocculation is clearly visible in all the samples. Considerable swelling of composite was also observed in the samples. Adsorption of composite as well as coagulation flocculation resulted in about 90% dye removal as a result of the activity of the starch chitosan nanocomposite (Chassary et al., 2004; Chiou and Li, 2003; Guibal, 2004). The aminopolysacchride present in the chitosan interact very effectively with various molecules. The presence of amines assists in the coagulation and flocculation of the various mineral as well as dye materials. The NH2 groups have a strong tendency to attract anionic dye particle at low or acidic pH (Chassary et al., 2004; Chiou and Li, 2003; Guibal, 2004; Jabli et al., 2011; Guibal and Roussy, 2006; Yoshida et al., 1993). At acidic pH the degree of protonation of amino group increases thereby increasing electrostatic interaction with anionic molecules. At neutral pH the binding of cationic particles is promoted via same electrostatic mechanism. Thus at every pH the coagulation flocculation activity of chitosan is considerable. Along with electrostatic mechanisms other interactions also assist in the coagulation property of chitosan. Starch also aids in the function of chitosan as it also contains amylose chains that are helpful in attracting anionic dye molecules and other minerals (Szygula et al., 2009; Miller et al., 2008). Both of these natural coagulants interact with dyes with 2 possible mechanisms i) Charge neutralization and ii) Bridging (Verma et al., 2008).
Wastewater treatment
The method optimized for dye removal of standard anionic dye congo red was used for three commercial waste water samples taken from fabric dyers from the local market. All the experiments were done under the conditions described above. After incubation of dye samples with chitosan starch nanocomposite the solutions were filtered to estimate the amount of dye precipitation.
In Figure 5 conical flasks indicate controls and test samples (in duplicates) showing commercial dye sample with nanocomposite after 12 h (A i, B i, C i). Precipitates of flocculated dye sample obtained on filter paper are also clearly visible for all the commercial samples (A ii, B ii, C ii). Within 12 hours ~90% flocculation was observed in the all the samples. The filtrates obtained after filtration of samples were analyzed and found to contain minimal amount of dye (Fig. 5 A iii, B iii, C iii, in each case test tubes from left to right indicate control without nanocomposite and test samples in duplicates, respectively). The nanocomposites were removed from the flocculated samples and observed carefully. Due to dye adsorption the color of the composites was changed. The surface of the nanocomposite was also found to be smooth after adsorption of the dye (Fig. 5 A iii, B iii, C iii samples of nanocomposite are indicated below the test tubes).
The mechanism of commercial dye flocculation is similar to that discussed for congo red. High concentration of NaCl is used in dyeing process therefore the commercial dye samples contained considerable amount of salt. As the molar ratio of salt increases the precipitation of dye also increases (Tan et al., 2000) thus facilitating the coagulation of charged molecules.
Conclusion
In the current investigation, experiments were performed for the removal of commercial dyes obtained from local market fabric dyers, using chitosan starch based nanocomposites. The electrostatic and hydrophobic interactions between starch and chitosan provide better dye removal effects as compared to the use of chitosan alone, reported by other researchers. It is an effective method for waste water treatment especially in countries like Pakistan where there is mass release of wastewater containing toxic dyes in sewerage water. The work presented is the first report of waste water treatment using green composite based on chitosan and starch and could be useful in sustainable waste water treatment.
Conflict of interest statement
We declare that we have no conflict of interest.
References
Baran, E.T., Mano, J.F. and Reis, R.L., 2004. Starch–chitosan hydrogels prepared by reductive alkylation cross-linking. J. Mat. Sci.: Mat. Med., 15:759-765. http://dx.doi.org/10.1023/B:JMSM.0000032815.86972.5e
Beltrán-Heredia, J. and Sánchez Martín, J., 2008. Azo dye removal by Moringa oleifera seed extract coagulation. Colorat. Technol., 124:310-317. http://dx.doi.org/10.1111/j.1478-4408.2008.00158.x
Bolto, B.A., 1995. Soluble polymers in water purification. Progr. Polym. Sci., 20:987-1041. http://dx.doi.org/10.1016/0079-6700(95)00010-D
Bratby, J., 2006. Coagulation and flocculation in water and wastewater treatment. IWA publishing.
Carlström, D., 1957. The crystal structure of α-chitin (poly-N-acetyl-D-glucosamine). J. Biophys. Biochem. Cytol., 3:669-683. http://dx.doi.org/10.1083/jcb.3.5.669
Chassary, P., Vincent, T. and Guibal, E., 2004. Metal anion sorption on chitosan and derivative materials: a strategy for polymer modification and optimum use. React. Funct. Polym., 60:137-149. http://dx.doi.org/10.1016/j.reactfunctpolym.2004.02.018
Chiou, M.S. and Li, H.Y., 2003. Adsorption behavior of reactive dye in aqueous solution on chemical cross linked chitosan beads. Chemosphere, 50:1095-1105. http://dx.doi.org/10.1016/S0045-6535(02)00636-7
Chung, Y.L., Ansari, S., Estevez, L., Hayrapetyan, S., Giannelis, E.P. and Lai, H.M., 2010. Preparation and properties of biodegradable starch–clay nanocomposites. Carbohyd. Polym., 79: 391-396.
Elson, C.M., Davies, D.H. and Hayes, E.R., 1980. Removal of arsenic from contaminated drinking water by a chitosan/chitin mixture. Water Res., 14: 1307-1311.
Furlan, F.R., da Silva, L.G.D.M., Morgado, A.F. and de Souza, A.A.U., 2010. Removal of reactive dyes from aqueous solutions using combined coagulation/flocculation and adsorption on activated carbon. Resour. Conserv. Recycl., 54:283-290. http://dx.doi.org/10.1016/j.resconrec.2009.09.001
Guibal, E., 2004. Interactions of metal ions with chitosan-based sorbents: A review. Separat. Purif. Technol., 38:43-74. http://dx.doi.org/10.1016/j.seppur.2003.10.004
Guibal, E. and Roussy, J., 2007. Coagulation and flocculation of dye-containing solutions using a biopolymer (Chitosan). React. Funct. Polym., 67:33-42. http://dx.doi.org/10.1016/j.reactfunctpolym.2006.08.008
Gulrez, S.K.H., Al-Assaf, S. and Phillips, G.O., 2011. Hydrogels: Methods of preparation, characterization and applications. In: Progress in molecular and environmental bioengineering from analysis and modeling to technology applications (ed. A. Carpi) INTECH Open Access Publisher, pp. 115-170. http://dx.doi.org/10.5772/24553
Jabli, M., Baouab, M.H.V., Roudesli, M.S. and Bartegi, A., 2011. Adsorption of acid dyes from aqueous solution on a chitosan-cotton composite material prepared by a new pad-dry process. J. Eng. Fibers Fabrics, 6: 1-12.
Lee, J.W., Choi, S.P., Thiruvenkatachari, R., Shim, W.G. and Moon, H., 2006. Evaluation of the performance of adsorption and coagulation processes for the maximum removal of reactive dyes. Dyes Pigm., 69:196-203. http://dx.doi.org/10.1016/j.dyepig.2005.03.008
Leiknes, T., 2009. The effect of coupling coagulation and flocculation with membrane filtration in water treatment: A review. J. environ. Sci., 21:8-12. http://dx.doi.org/10.1016/S1001-0742(09)60003-6
Miller, S. M., Fugate, E. J., Craver, V. O., Smith, J. A., & Zimmerman, J. B. (2008). Toward understanding the efficacy and mechanism of Opuntia spp. as a natural coagulant for potential application in water treatment. Environ. Sci. Technol., 42:4274-4279. http://dx.doi.org/10.1021/es7025054
Mohanty, A.K., Misra, M. and Drzal, L.T., 2002. Sustainable bio-composites from renewable resources: opportunities and challenges in the green materials world. J. Polym. Environ., 10:19-26. http://dx.doi.org/10.1023/A:1021013921916
Mukherjee, M., Swami, A., Ramteke, D.S., Moghe, C.A. and Sarin, R., 2004. Role of conventional and non-conventional coagulants with and without polyelectrolyte in treatment of refinery wastewater. Pollut. Res., 23:417-26.
Peesan, M., Rujiravanit, R. and Supaphol, P., 2003. Characterisation of beta-chitin / poly (vinyl alcohol) blend films. Polym. Test., 22:381-387. http://dx.doi.org/10.1016/S0142-9418(02)00118-6
Petrinić, I., Andersen, N.P.R., Šostar-Turk, S. and Le Marechal, A.M., 2007. The removal of reactive dye printing compounds using nanofiltration. Dyes Pigm., 74:512-518. http://dx.doi.org/10.1016/j.dyepig.2006.11.003
Qin, J.J., Oo, M.H. and Kekre, K.A., 2007. Nanofiltration for recovering wastewater from a specific dyeing facility. Separ. Purif. Technol., 56:199-203. http://dx.doi.org/10.1016/j.seppur.2007.02.002
Rangel-Mendez, J.R., Davila-Rodriguez, J.L. and Barrios, V.A.E., 2010. Chitin based biocomposites for removal of contaminants from water: A case study of fluoride adsorption. INTECH Open Access Publisher.
Reddy, A.V.R., Trivedi, J.J., Devmurari, C.V., Mohan, D.J., Singh, P., Rao, A.P. and Ghosh, P.K., 2005. Fouling resistant membranes in desalination and water recovery. Desalination, 183:301-306. http://dx.doi.org/10.1016/j.desal.2005.04.027
Renault, F., Sancey, B., Badot, P.M. and Crini, G., 2009. Chitosan for coagulation / flocculation processes–an eco-friendly approach. Europ. Polym. J., 45:1337-1348. http://dx.doi.org/10.1016/j.eurpolymj.2008.12.027
Renault, F., Sancey, B., Charles, J., Morin-Crini, N., Badot, P.M., Winterton, P. and Crini, G., 2009. Chitosan flocculation of cardboard-mill secondary biological wastewater. Chem. Engineer. J., 155:775-783. http://dx.doi.org/10.1016/j.cej.2009.09.023
Riera-Torres, M., Gutiérrez-Bouzán, C. and Crespi, M., 2010. Combination of coagulation–flocculation and nanofiltration techniques for dye removal and water reuse in textile effluents. Desalination, 252:53-59. http://dx.doi.org/10.1016/j.desal.2009.11.002
Sharma, R., Dwivedi, S., Hristovski, K., Wu, Y. and Srinivasan, R., 2013. Green materials for sustainable water remediation and treatment. Volume 23. (eds. A. Mishra, J. H. Clark, G. A. Kraus, P. R. Seidl, A. Stankiewicz, & Y. Kou). Royal Society of Chemistry.
Szyguła, A., Guibal, E., Palacín, M.A., Ruiz, M. and Sastre, A.M., 2009. Removal of an anionic dye (Acid Blue 92) by coagulation–flocculation using chitosan. J. environ. Manage., 90:2979-2986. http://dx.doi.org/10.1016/j.jenvman.2009.04.002
Tan, B.H., Teng, T.T. and Omar, A.M., 2000. Removal of dyes and industrial dye wastes by magnesium chloride. Water Res., 34:597-601. http://dx.doi.org/10.1016/S0043-1354(99)00151-7
Verma, A.K., Dash, R.R. and Bhunia, P., 2012. A review on chemical coagulation/flocculation technologies for removal of colour from textile wastewaters. J. environ. Manage., 93:154-168. http://dx.doi.org/10.1016/j.jenvman.2011.09.012
Vishnu, G. and Joseph, K., 2007. Nanofiltration and ozonation for decolorisation and salt recovery from reactive dyebath. Colorat. Technol., 123:260-266. http://dx.doi.org/10.1111/j.1478-4408.2007.00093.x
Yin, C.Y., 2010. Emerging usage of plant-based coagulants for water and wastewater treatment. Process Biochem., 45:1437-1444. http://dx.doi.org/10.1016/j.procbio.2010.05.030
Yoshida, H., Okamoto, A., Yamasaki, H. and Kataoka, T., 1993. Breakthrough curve for adsorption of acid dye on crosslinked chitosan fiber. Stud. Surf. Sci. Cataly., 80:767-774. http://dx.doi.org/10.1016/S0167-2991(08)63587-9
Yoshida, Z.I., Osawa, E. and Oda, R., 1964. Intermolecular hydrogen bond involving a π-base as the proton acceptor. I. Detection by the refractive index method. J. Phys. Chem., 68:2895-2898. http://dx.doi.org/10.1021/j100792a025
Yu, Y., Zhuang, Y.Y., Li, Y. and Qiu, M.Q., 2002. Effect of dye structure on the interaction between organic flocculant PAN-DCD and dye. Indust. Engineer. Chem. Res., 41:1589-1596. http://dx.doi.org/10.1021/ie010745t
Zahrim, A.Y., Tizaoui, C. and Hilal, N., 2011. Coagulation with polymers for nanofiltration pre-treatment of highly concentrated dyes: A review. Desalination, 266:1-16. http://dx.doi.org/10.1016/j.desal.2010.08.012
To share on other social networks, click on any share button. What are these?










