Stimulus Specific Response on Mechanical Properties of Isolated Visceral Muscle Tissues of a Reptile, Uromastix hardwickii
Stimulus Specific Response on Mechanical Properties of Isolated Visceral Muscle Tissues of a Reptile, Uromastix hardwickii
Arifa Savanur1,*, Tallat Naz1,2, Tayyaba Hamid1,3, Syed Abid Ali4, Mian Jahangir1,3 and Muhammad Abdul Azeem4
1Neuromuscular Research Unit, Department of Physiology, University of Karachi, Karachi 75270
2Department of Physiology, Sindh Medical College, Jinnah Sindh Medical University, Karachi
3Center of Excellence in Marine Biology, University of Karachi, Karachi
4H.E.J. Research Institute of Chemistry, International Center for Chemical and Biological Sciences, University of Karachi, Karachi 75270
4Department of Physiology, United Medical and Dental College, Ibrahim Hyedri Korangi, Karachi
ABSTRACT
Mechanical properties of a muscle have traditionally been described on the basis of the characteristics of parallel elastic component (PEC), series elastic component (SEC) and contractile element (CE). Reptiles in general and Uromastix in particular have been less investigated regarding the mechanical responses and the characteristics of elastic elements of their visceral muscle tissues. Therefore, a comparative study was conducted to determine the mechanical properties of esophageal and intestine tissue strips of Uromastix hardwickii. Tissues were subjected to electrical stimulation in order to observe the graded mechanical response, and the quick isotonic release method was used for the measurement of stiffness in the series elastic component. The higher stiffness in intestinal tissues as compared to esophageal tissue of Uromastix is probably associated with its lesser compliance due to a lesser quantity of SEC that makes the intestine stiffer, as its muscle cells are less embedded in a collagen matrix. A significant difference between both types of muscle strips was observed in the rate of force re-development and change in their length. Thus, the present study provokes the need for quantitative histological and histo-chemical studies on the non-contractile proteins of Uromastix smooth muscle.
Article Information
Received 19 February 2019
Revised 11 April 2019
Accepted 25 May 2019
Available online 21 May 2020
Authors’ Contribution
AS designed the study and helped in experiments. TN did the experiments and wrote the article. TN, TH and SAA analyzed the data. AS, TH and SAA edited the manuscript. MJ helps in interpretation. MAA conceived the idea and reviewed the final manuscript.
Key words
Stiffness, Series elastic components, visceral muscles, Uromastix.
DOI: https://dx.doi.org/10.17582/journal.pjz/20190219020207
* Corresponding author: arifa.savanur@gmail.com
0030-9923/2020/0005-1941 $ 9.00/0
Copyright 2020 Zoological Society of Pakistan
Introduction
All isolated muscle has some compliant elements in series with the contractile elements. The mechanical properties and location of the elastic component of smooth muscles has been the subject of many investigations (Stephens and Kromer, 1971; Herlihy and Murphy, 1974; Herrera et al., 2005; Speich, 2006, 2007, 2009), and there is a general consensus that this component is less stiff than the corresponding component of skeletal muscle. In other words, the compliance of activated whole smooth muscle is greater than that of activated whole skeletal muscle (Herlihy and Murphy, 1974; Mulvany and Halpern, 1976).
According to the cross bridge theory, cross bridges and their attachments have elastic properties that are masked by the presence of compliant passive structures in series with the contractile apparatus. The reduction of force in an activated fiber resulting from a rapid decrease in length is a linear function of the length decrease. This function of the muscle fiber is thought to be the characteristic of the series elastic component (SEC). Compliance of the parallel elastic component (PEC) is also dependent on the anatomic arrangement and fraction of collagen in the tissue (Glavind, 1993). Collagen constitutes about 50% of smooth muscle, as seen in biopsies (Glavind, 1993; Mulvany, 1984).
Smooth muscles isolated from various viscera have a very long functional range, therefore these studies are disputed (Ford and Gilbert, 2007). Even the geometric organization of the contractile units can be altered when the muscle cells adapt to different lengths (Herrera et al., 2005). The generation of force and shortening are strongly influenced by the passive tissue elements when smooth muscle undergoes certain length changes (Meiss and Pidaparti, 2004, 2005). Previous studies regarding stiffness measurements (Meiss, 1993; Peiperet al., 1986; Yamakawaet al., 1990) indicated that the effects were confined to changes in the cross bridge, but according to Meiss and Pidaparti (2005), the curve defining the shortening-dependent stiffness in tracheal smooth muscle is highly sensitive to changes in radial connective tissue parameters, and not in the active tissue element. Speich et al. (2006, 2007) proposed a mechanical model to justify adjustable passive stiffness in smooth muscle. Their model is based on the addition of a novel cross-linking element to a hybrid Kelvin/Voigt visco-elastic model (Fung, 1993; Murphy, 1980). They focus on the existence of a variable parallel elastic component (VPEC) in addition to SEC and PEC. The VPEC element represents intracellular structures responsible for the adjustable component of passive stiffness. Most recently, Speich, (2009) and Almasari et al. (2010) reported that by increasing the number of KCl- induced contractions or the duration of a contraction, the amount of adjustable passive stiffness also increases. Further studies on pulmonary arterial smooth muscle by Syyong et al. (2008) revealed that force recovery is the characteristic of length adaptation that is due to passive stress relaxation. According to the authors, this will be needed in future studies to understand abnormalities. The compliance of SEC is higher in smooth muscle because the absolute length of the SEC in smooth muscle is much longer than that in striated muscle (Dorbin and Canfield, 1973, 1977) and also due to the small size of smooth muscle cells which are embedded in a collagen matrix rather than connected to tendons, like in case of skeletal muscles (Gabella, 1977). Studies of SEC properties in dog common carotid artery showed that skeletal and cardiac muscle exhibit similar SEC extensions when subjected to the isometric quick release method, but smooth muscle exhibits a somewhat greater SEC extension, and the greater compliance is due to differences in the morphological basis, i.e., less viscoelastic contractile component than that of skeletal and cardiac muscle (Dorbin and Canfield, 1973, 1977). Using amphibian smooth muscle, Harris and Warshaw (1991) reported that the stiffness of a single isolated smooth muscle cell decreased as the length decreases. Thus, each type of muscle is tuned to a specific application for their molecular and mechanical process. The simplest approach for characterizing the contractile system of a muscle is the measurement of its mechanical properties. Smooth muscle mechanics have been less studied on the spiny tail reptile Uromastix hardwickii. However, its skeletal muscle mechanics have been extensively studied by Azeem and Shaikh (2006), Azeem et al. (2006) and Farooq et al. (2007). Different visceral muscle tissues of Uromastix and other reptiles have not been compared among themselves with respect to stiffness of their SEC. The present study is, therefore, an attempt to determine and compare the series elastic component’s stiffness in esophageal and intestinal smooth muscles of Uromastix hardwickii.
Materials and Methods
Isolation of intestinal and esophageal tissue
Adult Uromastix hardwickii of both sex, weighing 180-200 g, were used for the experiments. Standard and ethical procedure was adopted for the handling of animals for dissection and isolation of smooth muscle tissue strips (Savanur et al., 2014). Isolated muscle tissues were separated from the surrounding connective tissues and blood vessels. The whole of the intestine and esophagus was washed by flushing with reptilian buffer solution (NaCl: 100mM, KCl: 2mM, CaCl2.2H2O: 1.8mM, Na2HPO4.2H2O: 5.8mM, KH2PO4: 1.2mM; pH=7.4) through the lumen twice or thrice by using a small pipette or syringe (without needle). Then it was immediately kept in bulk in 400-500ml of oxygenated (95% O2 and 5% CO2) reptilian buffer solution at room temperature.
Fixation and recording from muscle strip
For recording purpose, approximately 1-3 cm long segments were cut from intestine or esophagus. A single strip was then immediately transferred in the external muscle chamber that was filled with Reptilian buffer solution. One end of the strip was fixed into the pin that was glued in the muscle chamber, and the other end was attached by an entomology pin, shaped as a hook and a nylon thread that was associated with the hook, passing through a pulley and tied with the leaf of an isotonic transducer. A calibrated isotonic force transducer (Harvard Apparatus, UK) was used to measure the mechanical activity of muscles for transmission through the interface transducer unit of an Oscillograph channel amplifier. A pair of stimulating electrodes was placed beneath the muscle strip to stimulate the tissue.
Determination of SEC stiffness in esophagus and intestine
The muscle strip was mounted horizontally in the muscle chamber. It was then extended by adjusting the position of the force transducer with the help of a macro-manipulator to provide stretch on the muscle strip until a desired passive tension was recorded on the Oscillograph. At this passive tension, the length of the muscle strip was taken as initial length measured by using the measuring scale. The strip was then allowed to incubate for 20 min in the muscle bath at room temperature. Later for stimulation of intestinal and esophageal strips, tetanic currents of 240 Hz for 30 sec were used to produce a mechanical (graded) response, while the paper speed of the Oscillograph was adjusted at 0.5 mm/sec or 1 mm/sec. In order to measure the SEC stiffness, the muscle strip was quickly released from stretch by a sudden anticlockwise movement of the macro-manipulator knob and kept at this reduced level of stretch for 30 sec, to complete the record taken on the Oscillograph before and after release of stretch of the smooth muscle strip. All such records of graded mechanical response obtained on stimulation followed by quick release were used for the analysis and calculation of various mechanical parameters including the SEC stiffness.
Graded response
A gradual change in initial base line after exposure to electrical stimulation represents a graded mechanical response. The magnitude of the graded response was measured in mm by drawing a vertical line “P1” from base line to the peak of response as shown in Figure 1. The magnitude of the graded response was then calculated in grams by using the transducer calibration according to the following formula (1):

Where, y is the calibration obtained from deflection of the Oscillograph pen on applying a 5gm weight on the leaf spring of the isotonic transducer and x is the total amplitude of the mechanical response represented as P1 in Figure 1.
The graded response was then converted into kg/cm2 by using the formula (2):
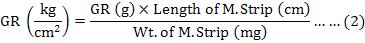
SEC stiffness (ΔP/ΔL)
The stiffness of the SEC was estimated by using the formula (3) as described by Hellstrand (1984):
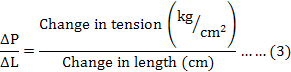
Where, ΔP = P1-P2 (change in tension after quick release measured from the record as shown in Figure 1), ΔL = L1-L2 (change in length after quick release measured by using a ruler), P1 is the tension at initial length (L1), P2 is the tension after quick release at the new length (L2), L1 is the initial length of muscle strip and L2 is the length of the strip after quick release.
Rate of rise of contraction or force redevelopment (ΔP/Δt)
The rate of force redevelopment during the graded mechanical response was calculated by using the formula (4) as described by Klemt and Peiper (1978):

Where, Δt is the time required by the muscle strip to change tension from P1 to P2.
Rate of length change
The rate of change in muscle length was calculated by using the formula (5) as described by Per Hellstrand (1984):
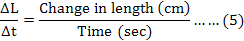
Where, Δt is the time required by the muscle strip to change length from L1 to L2.
Results and discussion
To understand the architecture, mechanics and underlying molecular mechanism of smooth muscle, the contractility following quick release imposed during active contraction has been demonstrated by a number of studies (Gunst, 1986; Gunst et al., 1993; Meiss, 1993). The data obtained on quick release are illustrated in Figure 1 and were used to compute the stiffness of the series elastic component, graded mechanical response, rate of force redevelopment and rate of length change. All of these parameters were then compared in intestinal and esophageal smooth muscles of Uromastix.
Stiffness of series elastic components (SEC)
The stiffness of the SEC was estimated by the change in tension/change in length (ΔP/ΔL) as described by Hellstrand (1984) and Stephens and Seow (1989).
The results summarized in Table I demonstrate that the stiffness of the SEC is significantly higher (P<0.005) in intestinal muscle as compared to esophageal muscle of Uromastix (Fig. 3B). A previous study of Gabella (1977) on taenia coli of guinea pig has demonstrated that smooth muscle cells are smaller in size and embedded in a collagen matrix rather than connected to tendons like in skeletal muscles, therefore their SEC compliance is higher and stiffness is less than that of skeletal muscle. The compliance of the parallel elastic component is also dependent on the anatomic arrangement and fraction of collagen in the tissue (Glavind, 1993). Studies by Dorbin and Canfield (1973, 1977) revealed that the absolute length of the series elastic elements of smooth muscles are longer than in striated muscle, therefore the SEC in smooth muscles is more compliant than that in skeletal muscles. Cox (1984) revealed that the differences in the stiffness of the SEC are dependent on SEC properties, on muscle length, on anatomical variability of muscles in a given animal, and on species differences. In addition to above studies, Meiss and Pidaparti (2004, 2005) tested the radial constraint hypothesis on tracheal smooth muscle of mongrel dogs and reported that stiffness varies with the intensity of muscle contraction. Accordingly, when muscle begins to shorten against a small after load, its internal axial forces generated by the cross bridge array are proportional to the small axial force, while the connective tissue forces present in the radial direction are negligible, and stiffness at this point is very low. On the other hand, when muscle shortened fully (at constant volume), the external force has not changed, but the measured axial stiffness and the tissue diameter increased significantly, and the radial connective tissue is now strained, providing an increased (internal) load on the cross bridge array. The adaptive response to length change (Syyong et al., 2008) due to the adjustable passive stiffness of smooth muscle (Meiss and Pidaparti, 2005; Almasari et al., 2010; Speich et al., 2006, 2007, 2009) has also been studied to understand the active and passive component in the length dependent stiffness.
Thus, in view of the above mentioned reports, it is suggested that the intestinal and esophagus smooth muscle of Uromastix also possess passive stiffness during contraction, and this stiffness changes as the length of the muscle changes, and the lesser SEC stiffness in esophageal muscle than in intestinal muscle may be due to the reason that their smooth muscle cells are smaller in size, highly embedded in a collagen matrix, their series elastic elements are also larger in size, and thus this makes esophageal muscle more compliant and less stiff than intestinal muscle.
Table I.- A comparison of the average values of the magnitude of graded response, stiffness of series elastic components (ΔP/ΔL), rate of rise of contraction (ΔP/Δt) and rate of length change (ΔL/Δt) measured from the records of mechanical response of esophageal and intestinal smooth muscles of Uromastix hardwickii.
|
Mechanical parameters |
Visceral muscles |
P |
|
|
Esophageal strips |
Intestinal strips |
||
|
Magnitude of response (kg/cm2) |
0.0079 ± 0.00226 (10) |
*0.0091 ± 0.00226 (10) |
P > 0.05 |
|
Stiffness of S.E.C ΔP/ΔL (kg/cm3) |
0.0782 ± 0.0164 (10) |
0.1487 ± 0.0150 (10) |
P < 0.005 |
|
Rate of force redevelopment ΔP/Δt (kg/cm2/sec) |
0.0005 ± 0.00009 (10) |
0.0008 ± 0.0001 (10) |
P < 0.025 |
|
Rate of length change ΔL/Δt (cm/sec) |
0.0081 ± 0.0010 (10) |
0.0056 ± 0.0005 (10) |
P < 0.025 |
All the values presented are Mean±SE. Values in parenthesis represent number of observations (n). P, represent the level of significance. Value with asterisk represents negatively graded response.
Graded response
In our present study, esophageal and intestinal smooth muscles of Uromastix were exposed to electrical stimulation (240 Hz); different graded responses have been observed as shown in Figure 2A and B. A positive graded response was observed in the esophagus of Uromastix. In contrast, the intestine of Uromastix showed quite unique behavior and produced a negative graded response on electrical stimulation is shown in Figure 3A. These results suggest that the positive graded response that was produced in the esophagus of Uromastix might be a contractile response that was evoked by electrical stimulation. Earlier studies revealed that electrical stimulation is responsible to release acetylcholine from intrinsic nerves of rat ileum, and this would be responsible to evoke contractions (Kurjak et al., 1999; Blandina et al., 1984; Jankovic et al., 2004). The negative graded response that was produced in the intestine of Uromastix might indicate relaxation, as it was also reported in literature that the electrical stimulation produces an inhibitory response in different smooth muscles of different animals. For example, studies on smooth muscles like mouse colon (Shuttleworth et al., 1997), mouse ileum (Ward et al., 1994), guinea pig taenia caeci (Bridgwater et al., 1995), guinea pig ileum (Crist et al., 1992; Bywater and Taylor, 1986), human colon (Keef et al., 1993) and human jejunum (Stark et al., 1993) demonstrated that the electric stimulation evoked inhibitory junction potentials (IJPs) which showed slow and fast hyper polarization. Furthermore, IJPs result in relaxation of gastrointestinal smooth muscles, and these IJPs are mediated by inhibitory non-adrenergic non-cholinergic (NANC) neurotransmitters (Bywater and Taylor, 1986). These evidences strengthen our hypothesis that the negative graded response produced in Uromastix intestine might be due to hyper polarization that was mediated by NANC transmitters and results in relaxation. A comparative study between esophagus and intestine of Uromastix presented in this work in terms of graded response is shown in Figure 3A. The insignificantly higher graded response in positive direction produced in esophagus as compared to intestine of Uromastix may probably be related with some variability in thin to thick filament ratio. According to Murphy et al. (1974), the larger thin to thick filament ratio provides the basis for larger force development by providing a larger number of potential cross bridges at any given muscle length.
Rate of force redevelopment and length change
When muscle strips were quickly released from the desired initial length during contraction, a fall in tension was observed at the new length. The time required for developing the new tension, and the time required to reach the new length was also determined in the present study. Accordingly, it was observed that the intestine of Uromastix showed a significantly higher (P<0.025) rate of force development but lesser (P<0.025) rate of length change as compared to the esophagus (Table I; Fig. 3C, D). As mentioned earlier, the intestinal muscles of Uromastix produce higher tension and also have greater SEC stiffness than esophagus muscles, which is in good agreement with Hill’s hypothesis (1970) that the greater tension makes the SEC stiffer. Moreover, Warshaw and Fay (1984) reported that the rate of tension recovery is a consequence of actively cycling cross bridges. Their argument was based on the entire loss of the ability to recover tension when cells were contracted in the rigor state. Therefore, it is suggested in the present study that the greater stiffness of the SEC in intestine was responsible for the slow development of tension, as the SEC is stiffer in them. It is assumed that intestinal tissue takes more time to overcome the resistance imposed on the contractile components by the non-contractile elements. In contrast, the fact that intestine takes less time to change its length as compared to esophagus of Uromastix, indicates that the speed of shortening is higher in intestine in spite of the fact that it contains a higher stiffness. Furthermore, there are indications of some morphological differences in esophageal and intestinal muscles, studies of which are in progress and will be described elsewhere.
Conclusions
The present study is the first of its kind on intestinal and esophageal smooth muscle of Uromastix hardwickii focusing on the behavior of the elastic component. It is concluded that intestinal tissues have a stiffer SEC, which is responsible for the slow development of force because it takes more time to overcome the resistance imposed on the contractile component by the non-contractile component. Additional studies are required to understand the complex behavior of smooth muscle related to the differences in histological & morphological aspects that influence the mechanical behavior serving the different physiological roles of different tissues in this animal.
Acknowledgements
This work was supported by a grant from the Dean of Science, University of Karachi, Pakistan.
Statement of conflict of interest
There is no conflict of interest.
References
Arifa, S., Azeem, M.A. and Erum, A., 2004. Intestinal contractions under the influence of Ethanol used for simple and succussed drug dilution. Int. J. Biol. Biotech., 1: 625-630.
Arifa, S., Azeem, M.A., Saify, Z.S. and Ahmed, S.I., 1997. Comparative effect of simple and triturated dilution of acetylcholine and adrenaline on intestinal contraction parameters. Proceed. 2nd Biennial conf. Pharmacol. Therapy, pp. 93-99.
Azeem, M.A. and Shaikh, H.A., 2006. Effect of season on length-tension relation of gastrocnemius muscle of Uromastix hardwickii. Pak. J. Physiol., 2: 17-21.
Azeem, M.A., Arifa, S., Erum, A. and Saify, Z.S., 1999. Cardiac and intestinal contraction under the influence of triturated drug dilutions. J. Pharmaceut. Sci., 12: 15-20.
Azeem, M.A., Saima, G. and Sadaf, A., 2006. Mechanical characteristics of rib cage elevator muscle of reptile Uromastix hardwickii. Pak. J. Physiol., 2: 38-44.
Almasari, A.M., Ratz, P.H., Bhatia, H., Klausner, A.P. and Speich, J.E., 2010. Rhythmic contractions generates adjustable passive stiffness in rabbit detrusor. J. appl. Physiol., 108: 544-553. https://doi.org/10.1152/japplphysiol.01079.2009
Blandina, P., Barattini, M., Fantozzi, R., Masini, E., Brunelleschi, S. and Mannaioni, P.F., 1984. Mediator release from isolated rat ileum in response of field stimulation. Agents Actions, 14: 405-409. https://doi.org/10.1007/BF01973838
Brigdewater, M., Cunnane, T.C. and Brading, A.F., 1995. Characteristic features of inhibitory junction potentials evoked by single stimuli in the guinea pig isolated taeniacaeci. J. Physiol., 485: 145-155. https://doi.org/10.1113/jphysiol.1995.sp020719
Bywater, R.A.R. and Taylor, G.S., 1986. Non-cholinergic excitatory and inhibitory junction potentials in the circular smooth muscle of the guinea pig ileum. J. Physiol., 374: 153-164. https://doi.org/10.1113/jphysiol.1986.sp016072
Crist, J.R., He, X.D. and Goyal, R.K., 1992. Both ATP and the peptide VIP are inhibitory neurotransmitters in guinea pig ileum circular muscle. J. Physiol., 447: 119-131. https://doi.org/10.1113/jphysiol.1992.sp018994
Cox, R.H., 1984. Mechanics of blood vessels: Conduit arteries. In: Smooth muscle contraction (ed. N.L. Stephens). Marcel Dekker, Inc., New York, pp. 405-425.
Daniel, J.C., 1983. Agamid. In: The book of Indian Reptiles. Bombay Natural History Society, Oxford University Press, India, pp. 42-50.
Dorbin, P.B. and Canfield, T.R., 1973. Series elastic and contractile elements in vascular smooth muscle. Circulation Res., 33: 454-464. https://doi.org/10.1161/01.RES.33.4.454
Dorbin, P.B. and Canfield, T.R., 1977. Identification of smooth elastic component in intact carotid artery. Am. J. Physiol., 232: H122-H130. https://doi.org/10.1152/ajpheart.1977.232.2.H122
Farooq, S.N., Azeem, M.A., Rakkah, N.I.A. and Mutafa, S.M.A., 2007. Effect of cast immobilization on contractile characteristics of skeletal muscles of Uromastix. Pak. J. Physiol., 3: 35-40.
Ford, L.E. and Gilbert, S.H., 2007.The significance of variable passive compliance in smooth muscle. J. appl. Physiol., 102: 1735-1736. https://doi.org/10.1152/japplphysiol.00130.2007
Fung, Y.C., 1993. Biomechanics. Springer-Verlag, New York, USA. https://doi.org/10.1007/978-1-4757-2257-4
Gabella, G., 1977. Arrangement of smooth muscle cells and intramuscular septa in the Taenia coli. Cell Tissue, 184: 195-212. https://doi.org/10.1007/BF00223068
Glavind, E.B., Forman, A., Svane, D., Andersson, K.E. and Tottrup, A., 1993. Mechanical properties of isolated smooth muscle from human rectum and internal anal sphincter. Am. J. Physiol., 265: G792-G798. https://doi.org/10.1152/ajpgi.1993.265.4.G792
Gunst, S.J., 1986. Effect of length history on canine tracheal smooth muscle. Am. J. Physiol. Cell Physiol., 250: C146-C154. https://doi.org/10.1152/ajpcell.1986.250.1.C146
Gunst, S.J., Wu, M.F. and Smith, D.D., 1993. Contraction history modulates isotonic shortening velocity in smooth muscle. Am. J. Physiol., 265: C467-C476. https://doi.org/10.1152/ajpcell.1993.265.2.C467
Herlihy, J.T. and Murphy, R.A., 1974. Force-velocity and series elastic characteristics of smooth muscle from hog carotid artery. Circulation Res., 34: 461-466. https://doi.org/10.1161/01.RES.34.4.461
Herrera, A.M., McParland, B.E., Bienkowska, A., Tait, R., Pare, P.D. and Seow, C.Y., 2005. ‘Sarcomere’ of smooth muscle: Functional characteristics and ultra-structural evidence. J. Cell Sci., 118: 2381-2392. https://doi.org/10.1242/jcs.02368
Harris, D.E. and Warshaw, D.M., 1991. Length vs. active force relationship in single isolated smooth muscle cell. Am. J. Physiol. Cell Physiol., 265: C1104-C1112. https://doi.org/10.1152/ajpcell.1991.260.5.C1104
Hellstrand, P., 1984. Mechanical transient and the force velocity relation in smooth muscle. In: Smooth muscle contraction (ed. N.L. Stephens). Marcel Dekker Inc., New York, USA, pp. 113-129.
Hill, A.V., 1970. First and last experiments in muscle mechanics. Cambridge University Press, Cambridge, England, pp. 85-109.
Jankovic, S.M., Jankovic, S.V. and Milovanovic, D.R., 2004. Effect of the exogenous glutamate and the NMDA on electric field-stimulated contractions of isolated rat ileum. Pharmacol. Res., 50: 529-532. https://doi.org/10.1016/j.phrs.2004.02.011
Keef, K.D., Du, C., Ward, S. M., McGregor, B. and Sanders, K.M., 1993. Enteric inhibitory neural regulation of human colonic circular muscle: Role of nitric oxide. Gastroenterology, 105: 1009-1016. https://doi.org/10.1016/0016-5085(93)90943-7
Klemt, P. and Peiper, U., 1978. The dynamics of cross bridge movements in v vascular smooth muscle estimated from a single isometric contraction of the portal vein: The influence of temperature and calcium. Pflugers Arch., 378: 31-36. https://doi.org/10.1007/BF00581955
Kurjak, M., Sattler, D., Schusdziarra, V. and Allescher, H.D., 1999. Characterization of pre-junctional and post-junctional muscarinic receptors of the ascending reflex contraction in rat ileum. J. Pharmacol. exp. Ther., 290: 893-900.
Meiss, R.A. and Pidaparti, R.M., 2004. Mechanical state of airway smooth muscle at very short length. J. appl. Physiol., 96: 655-667. https://doi.org/10.1152/japplphysiol.00388.2003
Meiss, R.A. and Pidaparti, R.M., 2005. Active and passive component in the length dependent stiffness of tracheal smooth muscle during isotonic shortening. J. appl. Physiol., 98: 234-241. https://doi.org/10.1152/japplphysiol.00574.2004
Meiss, R.A., 1993. Persistent mechanical effects of decreasing length during isometric contraction of ovarian ligament smooth muscle. J. Muscle Res. Cell Motil., 14: 205-218. https://doi.org/10.1007/BF00115455
Mulvany M.J., 1984. The series elastic component of vascular smooth muscle. In: Smooth muscle contraction (ed. N.L. Stephens). Marcel Dekker Inc., New York, USA, pp. 151-159.
Mulvany, M.J. and Halpern, W., 1976. Mechanical properties of vascular smooth muscle cells in situ. Nature, 260: 617-619. https://doi.org/10.1038/260617a0
Murphy, R.A., 1980. Mechanics of vascular smooth muscle. In: Handbook of physiology: The cardiovascular system, vascular smooth muscle. Am. Physiol. Soc. Sect. 2, Vol. II, Chap. 13 (ed. M.D. Bethesda). Wiley Online Library, pp. 325-351. https://doi.org/10.1002/cphy.cp020213
Murphy, R.A., Herlihy, J.T. and Megerman, J., 1974. Force generating capacity and contractile protein content of arterial smooth muscle. J. Gen. Physiol., 64: 691-705.https://doi.org/10.1085/jgp.64.6.691
Peiper, U., Vahl, C.F., Donker, E., Buchholz, D. and Schreiber, S., 1986. The temperature dependence of post-vibration tension recovery in intact and skinned rat tracheal smooth muscle. J. Muscle Res. Cell Motil., 7: 333-338. https://doi.org/10.1007/BF01753654
Savanur, A., Ali, S.A., Munir, I., Abbasi, A., Alam, M., Shaikh, H.A., 2014. Pharmacological and biochemical studies on the venom of a clinically important viper snake (Echis carinatus) of Pakistan. Toxicon, 80: 47-57. https://doi.org/10.1016/j.toxicon.2014.01.005
Savanur, A., Azeem, M.A. and Erum, A., 2000. Response of rabbit’s intestinal strips to simple and succussed drug dilutions. New Engl. J. Homeop., 9: 97-108.
Shuttleworth, C.W.R., Conlon, S.B. and Sanders, K.M., 1997. Regulation of citrulline recycling in nitric oxide-dependent neurotransmission in the murine proximal colon. Br. J. Pharmacol., 120: 707-713. https://doi.org/10.1038/sj.bjp.0700949
Speich, J.E., Almasari, A.M., Bhatia, H., Klausner, A.P. and Ratz, P.H., 2009. Adaptation of the length-tension relationship in rabbit detrusor. Am. J. Physiol. Renal Physiol., 297: F1119-F1128. https://doi.org/10.1152/ajprenal.00298.2009
Speich, J.E., Dosier, C., Borgsmiller, L., Quintero, K., Koo, H.P. and Ratz, P.H., 2007. Adjustable passive length-tension curve in rabbit detrusor. J. appl. Physiol., 102: 1746-1755. https://doi.org/10.1152/japplphysiol.00548.2006
Speich, J.E., Quintero, K., Dosier, C., Borgsmiller, L., Koo, H.P. and Ratz, P.H., 2006. A mechanical model for adjustable passive stiffness in rabbit detrusor. J. appl. Physiol., 101: 1189-1198. https://doi.org/10.1152/japplphysiol.00396.2006
Stark, M.E., Bauer, A.J., Sarr, M.G. and Szurszewski, J.H., 1993. Nitric oxide mediates inhibitory nerve input in human and canine jejunum. Gastroenterology, 104: 398-409. https://doi.org/10.1016/0016-5085(93)90407-4
Stephens, N.L. and Kromer, U., 1971. Series elastic component of tracheal smooth muscle. Am. J. Physiol., 220: 1890-1895. https://doi.org/10.1152/ajplegacy.1971.220.6.1890
Stephens, N.L. and Seow, C.Y., 1989. Changes of tracheal smooth muscle stiffness during an isotonic contraction. Am. J. Physiol., 256: C341-350. https://doi.org/10.1152/ajpcell.1989.256.2.C341
Syyong, H., Cheung, C., Solmon, D., Seow, C.Y. and Kuo, K.H., 2008. Adaptive response of pulmonary arterial smooth muscle to length change. J. appl. Physiol., 104: 1014-1020. https://doi.org/10.1152/japplphysiol.01203.2007
Ward, S.M., Burns, A.J., Torihashi, S. and Sanders, K.M., 1994. Mutation of the proto-oncogene c-kit blocks development of interstitial cells and electrical rythmicity in murine intestine. J. Physiol., 480: 91-97. https://doi.org/10.1113/jphysiol.1994.sp020343
Warshaw, D.M. and Fay, F.S., 1984. Tension transients in single isolated smooth muscle cells: Insight into the cross-bridge mechanism. In: Smooth muscle contraction (ed. N.L. Stephens). Marcel Dekker Inc., New York, USA, pp. 131-144.
Yamakawa, M., Harris, D.F., Fay F.S. and Warshaw, D.M., 1990. Mechanical transient of single toad stomach smooth muscle cells: Effect of lowering temperature and extra cellular calcium. J. gen. Physiol., 95: 697-715. https://doi.org/10.1085/jgp.95.4.697
To share on other social networks, click on any share button. What are these?










