Reproductive Biology of Snow Trout Schizothorax plagiostomus
Reproductive Biology of Snow Trout Schizothorax plagiostomus
Arif Jan*, Abdul Rab, Haroon, Rooh Ullah, Tauheed Ullah and Ikram Ullah
Department of Zoology, Shaheed Benazir Bhutto University, Sheringal, Dir Upper
ABSTRACT
Different aspects of reproductive biology of S. plagiostomus were studied in this work from February 2014 to January 2015. Fishes were collected from Sheringal valley right from Kumrat thal valley to Chukyatan Dir Upper. Fecundity and Gonado-somatic index was studied using gravimetric to infer about breeding season and reproductive potential. The mean value of absolute fecundity recorded was 14670.39 while mean value of relative fecundity was noted as 34.78. The mean value recorded for conditioned factor was 0.912 g/cm3. Relationship of fecundity with body length, body weight, ovary weight and the interrelationship of body length and body weight was established statistically using Linear Regression. The values of determination coefficient (R2) at P <0.001 for correlation between fecundity and body length, body weight and ovary length were recorded to be 0.8246, 0.8065 and 0.9120. The same value for association between body length and body weight was 0.9749. All the values are highly significant showing strong correlation. The GSI values ranged from 2 to 13.5. Highest value was observed in the month of March while lowest value is recorded in the month of April and October. Females to males ratio was found to be 1.04 (P>0.05). The study provides basic information about the reproductive potential and behavior of S. plagiostomus which will be handy towards its culture.
Article Information
Received 06 January 2017
Revised 15 July 2018
Accepted 27 March 2019
Available online 30 July 2019
Authors’ Contributions
AJ performed the research and wrote the article. Haroon and RU helped in sample collection. TU and IU assisted in lab work. AR helped in proofreading the paper.
Key words
Sheringal, Kumrat Thal valley, Gonado Somatic index (GSi), Chukyatan.
DOI: http://dx.doi.org/10.17582/journal.pjz/2019.51.5.sc9
* Corresponding author: arifjan@sbbu.edu.pk
0030-9923/2019/0005-1991 $ 9.00/0
Copyright 2019 Zoological Society of Pakistan
The cold water fisheries of Pakistan are limited to higher latitudes of the northern half of the country. In the northern areas of Pakistan three mountain systems extend from west to east, they are Himalayas and its two sub-branches Karakoram and Hindu Kush. Indus river of Pakistan, which runs through the northern mountains itself originate in Tibet (China) receives a number of tributaries i.e. Swat, Jhelum, Neelum, Kunhar and Gilgit. Schizothoracines (genera Schizothorax and Schizopyge) are the major fishes of cold water streams and rivers, with the dominant species being Schizothorax plagiostomus (Akhtar, 1991). The cold water fisheries of Pakistan mainly is based on schizothoracines. So far no attempt has been made to culture schizothoracines in Pakistan (Akhtar, 1992). The fish is reported to have maximum dimensions of 2.3 kg in weight and 50 cm in length (Sharma, 1994). Fishes of sub-family Schizothoracinae are generally long-lived and have low fecundity with late maturity. This makes them vulnerable to overfishing (Chen and Cao, 2000). Kullander et al. (1999) found that the ova of S. plagiostomus get ripe in December but they spent later in April–June, mainly in May. The average fecundity found was 12744. The present study is proposed primarily to find out gonado-somatic index and Fecundity for Schizothorax plagiostomus at study site and to estimate the spawning season, spawning types, range, and monthly variation in gonado-somatic index based on observation of monthly proportion of macroscopic gonadal maturity stages to estimate the sex ratio and size. The study will also focus to find out the relation of size and distribution of oocyte round the year. The relationship of fecundity and relative fecundity to the standard length, total length, total weight and age will also be determined. Effect of ecological parameters on gonadal development has also been kept under examination in this study.
Materials and methods
Fish (Schizothorax plagiostomus) were collected from 10 different points at River Panjkora, right from Kumrat Valley to Chukyatan. Collection were made fortnightly from each and every part of the River Panjkora from February 2014 to January 2015. Our collection contained all the age groups, entire range of body lengths, and total distribution area. Cast nets, drag nets, stake nets, bag nets and some other types of traps were used to capture fishes depending on the topography, depth and velocity of water at different collection points.
The gravid samples of fishes were dissected with a longitudinal incision; ovaries were taken out and kept immersed in Gilson’s fluid for hardening and easy counting (Friedland et al., 2005). For each female fish, two to three sub samples each of about 1 to 2 g ovarian material were taken from the different parts of the ovaries and were weighed to the nearest of 0.001 g with a digital electronic balance before preservation in 10% phosphate-buffered formalin was used as preservative. Fecundity was estimated according to Murua et al. (2003) as follows:
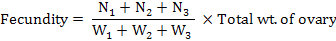
Where, N1 to N3 are the number of eggs in each sub-sample and W1 to W3 are the weights of these sub-samples, respectively.
First correlation and then Linear Regression analysis was used to interpret the relationships among Standard length, total weight, age, environmental conditions, gonado somatic index and fecundity using the following formula (Duponchelle et al., 2000). SigmaPlot version 10 built 10.0.0.54 was used to estimate relationships among various parameters recorded during the study. Chi-square was used to check the sex ratio of the collected fishes.
Log F = 3.3828 log TL ─ 1.1034
Log F = 0.944BW + 1.6707
Log F = 0.9561Log of OW + 2.5869
W= aLb
Where, F is absolute fecundity, TL is total length in cm, BW is body weight, OW is ovary weight, W, is the weight of fish (g), L is the length of fish (cm), a is the rate of change of weight with length (intercept) and b is weight at unit length (slope).
Results and discussion
The mean value recorded for the weight of ovary was 44.83 g (Supplementary Table I). Supplementary Table I also shows the absolute and relative fecundities. The mean value of absolute fecundity recorded was 14670.39 while mean value of relative fecundity was noted as 34.78. The mean value recorded for conditioned factor was 0.912 g/cm3.
Figure 1 shows relationship of log of fecundity with body length, ovary weight and body weight. A strong relationship was observed in all the three parameters. The correlation of coefficient for log of fecundity with body length, ovary weight and body weight were found to be 0.8246, 0.8065 and 0.912, respectively at p<0.001
Similar results were reported by Bahuguna and Khatri (2009) and Jan et al. (2014). Strong correlation of the fecundity with the length and weight of fish and with the weight of ovary has also been noticed by Bagenal (1957), Lehman (1953) and May (1967).
Figure 2 shows significant relationship between length and weight of the female gravid fishes dissected for fecundity estimation. Supplementary Table I contains the data recorded for length and weights of the selected specimens. When log of body length was plotted against log of body weight, the plot showed a strong positive correlation. The value of coefficient of determination was found to be 0.9749. The relationship between the two variables can be expressed by the following equation:
Log of BW = 3.4967 Log of BL - 2.8069
The linear regression model for the said two variables is: The L-W relationship was estimated by using the equation for finding the value of conditioned factor to check the general well-being of the fishes at study site.
The mean value of conditioned factor was found to be 0.912 depicting almost isometric growth of the collected samples. Similar results were reported by Goel et al (2011) for Schizothorax richardsonii and by Sundar (1985) for Schizothorax curvifrons.
Fecundity is thought to be an important feature in step towards the evolution of a new species Nikolskii (1965). Fecundity of snow trout is comparatively less than other plain counterparts as well as other coldwater fishes (Agarwal et al., 1988; Agarwal, 1996; Thapliyal et al., 2002). However this may be due to the struggle for existence in adverse coldwater environment, but it has also caused low production of the group.
A very basic finding of the current study was that fecundity i.e. the number of eggs increased linearly with increase in weight. This findings is supported by various previous workers Jhingran (1968), Pathani (1981), Sunder (1992), Islam and Hossain (1990), Hussain et al. (2003), Mohan (2005), Offem et al. (2008), Bahuguna and Khatri (2009), Gaur and Pathani (1996) and Lawson (2011). All the correlations such as fecundity and body weight, body length and ovary weight were found highly significant (p< 0.005). Das and Singh (1969), Hussain et al. (2003), Bahuguna and Khatri (2009) and Alam and Pathak (2010) found similar relationship between fecundity and ovary weight, body weight and body length.
We have found that gastrosomatic index of S. plagiostomus is inversely related to their maturity levels. Similar findings have been found by Kausar et al. (2012). The gonadosomatic index was found high in the breeding season while lowest value was observed visually for gastrosomatic index in this part of the year. Similar conclusions have been recorded by Kausar et al. (2012), Jhingran (1978) and Bhatnagar (1972) for Labeo fimbriatus, Desai (1973) for Tor tor and Thakur (1978) for Clarius batrachus. Malhotra (1967) also recorded identical observations for Schizothorax niger.
A total of 701 fishes were collected out of which 358 were females and 343 males. Overall sex ratio was found to be 1.04. Supplementary Table II shows male to female ratio of fish caught from different sites of the river. Almost similar findings were reported by Olurin and Savage (2011). Fagade et al. (1984) attributed this natural phenomenon as a powerful mechanism for population regulation. Moreover, females are found to invest more energy for reproductive potential than males of the respective species. Fish can store energy in the liver and other tissues to meet the requirements of spawning (Liao and Chang, 2011).
A number of factors like difference in longevity of opposite sexes, different growth rate of both sexes and sampling techniques adapted influence sex ratio (Wu and Wu, 1992; Liao and Chang, 2011). In our study, proportions of females in the overall population and in the population of larger adults were greater than those of males, which reflect faster growth and a greater longevity of females than males. Similar conclusion was found in Schizothorax o’connori by Ma et al. (2012).
Our findings indicated that late maturation, low fecundity and aggregation-spawning were typical characteristics of S. plagiostomus, and these characteristics render the population particularly vulnerable to overexploitation. Regulations and check and balance on harvesting, can prevent overexploitation of these fishes to a large extent. Morphometric similarity of S. plagiostomus with other species from shizothorancine family is also one reason for it’s over exploitation.
There is supplementary material associated with this article. Access the material online at: http://dx.doi.org/10.17582/journal.pjz/2019.51.5.sc9
Statement of conflict of interest
The authors declare no conflict of interest.
References
Agarwal, N.K, 1996. Fish reproduction. A.P.H. Publishing Corporation, New Delhi, pp. 157.
Agarwal, N.K., Singh, W. and Singh, H.R., 1988. J. Indian Fish. Assoc., 18: 537-548.
Akhtar, N., 1991. The northern areas (Pakistan). Fisheries profile, feasible sites for trout culture and an overall sector development perspective. Report for Project PAK/91/008, Rome, FAO, pp. 29.
Akhtar, N., 1992. Pakistan’s cold water fisheries and trout farming sector study: Trends, opportunities and challenges. Report for FAO/UNDP Projects PAK/88/048 and PAK/91/008, Rome, FAO, pp. 75.
Alam, M. and Pathak, J.K., 2010. Int. J. Pharma. Biosci., 1: 1-6.
Bagenal, T.B., 1957. J. Mar. biol. Assoc., U.K., 36: 339-375. https://doi.org/10.1017/S0025315400016854
Bahuguna, S.N. and Khatri, S., 2009. Our Nature, 7: 116-121. https://doi.org/10.3126/on.v7i1.2558
Bhatnagar, G.K., 1972. J. Inland Fish. Soc. India, 4: 26-37.
Chen, Y.F. and Cao, W.X., 2000. In: Fauna sinica osteicthtyes cypriniformes III (ed. P.Q. Yue). Science Press, Beijing, pp. 273-388.
Das, S.M. and Singh, H., 1969. Kashmir Sci., 4: 77-88.
Desai, V.R., 1973. Proc. Indian natl. Sci. Acad., 19: 228-248.
Duponchelle, F., Cecchi, P., Corbin, D., Nuñez, J. and Legendre, M., 2000. Environ. Biol. Fish., 57: 155-170. https://doi.org/10.1023/A:1007575624937
Fagade, S.O., Adebisi, A.A. and Atanda, A.N., 1984. Arch. Hydrobiol., 100: 493-500.
Friedland, K.D., Ama-Abasi, D., Manning, M., Clarke, L., Kligys, G. and Chambers, R.C., 2005. J. Sea Res., 54: 307-316. https://doi.org/10.1016/j.seares.2005.06.002
Gaur, S.K. and Pathani, S.S. 1996. Indian J. Fish., 43: 381-384.
Gayanilo, F.C. and Pauly, D., 1997. FAO ICL ARM stock assessment tools (FISAT): References Manual, FAO Computerized Information Series (Fisheries), FAO, pp. 262.
Goel, C., Ashoktaru, B., Veena, P., Shahnawaz, A. and Rohit, K., 2011. World J. Fish. Mar. Sci., 3: 485-488.
Hunter, J.R. and Macewicz, B.J., 2003. In: Modern approaches to assess maturity and fecundity of warm and cold water fish and squids (eds. O.S. Kjesbu, J.R. Hunter and P.R. Witthames). John Grieg Grafisk AS, Norge, Norway, pp. 57-68.
Hunter, J.R., Macewicz, B.J., Lo, N.C.H. and Kimbrell, C.A., 1992. Fish. Bull. U.S., 90: 101-128.
Hussain, L., Alam, M.A. Islam, M.S. and Bapary, M.A., 2003. Pakistan J. biol. Sci., 6: 231-233.
Islam, M.S. and Hossain, M.A., 1990. Univ. J. Zool. Rajshahi Univ., 9: 69-74.
Jan, M., Jan, U. and Shah, G.M., 2014. J. Threat. Taxa, 6: 5375-5379.
Jhingran, V.G. and Sehgal, K.L., 1978. Coldwater fisheries of India. Inland Fisheries Society of India, Barrackpore, India, pp. 239.
Kausar, N., Shah, M. and Jan, U., 2012. Int. J. Sci. Nature, 3: 928-930.
Khan, N., 2012. Afri. J. Pl. Sci., 6: 21-31.
Kraus, G., Tomkiewicz, J. and Köster, F.W., 2002. Canadian J. Fish. aquat. Sci., 59: 1908-1920. https://doi.org/10.1139/f02-159
Kullander, S.O., Fang, F., Delling, B. and Ahlander, E., 1999. Fishes of the Kashmir Valley. Department of Vertebrate Zoology, Swedish Museum of Natural History.
Lawson, E.O., 2011. J. Fish. aquat. Sci., 6: 264-271.
Lehman, B.A., 1953. Fecundity of Hudson River Shad. U.S. Fish Wildl. Serv. Res. Rep., 33: 1-8.
Liao, Y.Y. and Chang, Y.H., 2011. Zool. Stud., 50: 296-308.
Ma, B.S., Xie, C.X., Huo, B., Yang, X.F. and Chen, S.S., 2012. Zool. Stud., 51: 1066-1076.
Malhotra,Y.R., 1967. Indian J. Fish., 14: 313-317. https://doi.org/10.1093/nq/14-8-313a
May, A.W., 1967. J. Fish. Res. Bd. Canada, 24: 1531-1551. https://doi.org/10.1139/f67-127
Mohan, M., 2005. Indian J. Fish., 52: 451-457. https://doi.org/10.1093/notesj/gji410
Murua, H., Kraus, G., Saborido-Rey, F., Witthames, P.R., Thorsen, A. and Junquera, S., 2003. J. Northw. Atlantic Fish. Sci., 33: 33-54. https://doi.org/10.2960/J.v33.a3
Nikolskii, G., 1965. Theory of fish population dynamics as the biological background for rational exploitation and management of fishery resources. Nauka, Moscow.
Offem, B.O., Samsons, Y.A. and Omoniyi, I.T., 2008. J. Anim. Pl. Sci., 18: 130-138.
Olurin K.B. and Savage, O.D., 2011. Int. J. Fish. Aquacul., 3: 146-150.
Pathani, S.S., 1981. Proc. Indian Acad. Sci. (Anim. Sci.), 90: 253-260. https://doi.org/10.1007/BF03185999
Sharma, B.P., 1994. FAO Fish. Rep., 405(Suppl): 90-94.
Sivashanthini, K., Charles, G.A. and Shutharshan, S., 2008. J. Fish. aquat. Sci., 3: 320-327. https://doi.org/10.3923/jfas.2008.320.327
Sundar, S., 1985. Geobios New Rep., 4: 16-19.
Sunder, S., 1992. In: Recent researches in coldwater fisheries (ed. K.L. Sehgal). FAO, Rome, pp. 157-171.
Thakur, N.K., 1978. Int. Rev. Gesamten Hydrobiol., 63: 421-431. https://doi.org/10.1002/iroh.19780630310
Thapliyal, B.L., Agarwal, N.K. and Rawat, U.S., 2002. J. Inland Fish. Soc. India, 33: 77-80.
Wu, Y.F. and Wu, C.Z. (eds.), 1992. The fishes of the Qinghai-Xizang Plateau. Sichuan Publishing House of Science and Technology, Chengdu, pp. 599.
To share on other social networks, click on any share button. What are these?










