Potential of Rhizobium and PGPR to Enhance Growth and Fodder Yield of Berseem (Trifolium alexandrinum L.) in the Presence and Absence of Tryptamine
Potential of Rhizobium and PGPR to Enhance Growth and Fodder Yield of Berseem (Trifolium alexandrinum L.) in the Presence and Absence of Tryptamine
Fraza Ijaz1*, Umair Riaz2, Shazia Iqbal3, Qamar uz Zaman4, Muhammad Furqan Ijaz5, Hina Javed1, Muhammad Amjad Qureshi1, Zuhra Mazhar3, Ahmad Hassan Khan6, Hassan Mehmood7 and Ijaz Ahmad8
1Soil Bacteriology Section, Agriculture Biotechnology Research Institute, AARI Faisalabad, Pakistan; 2Soil and Water Testing Laboratory for Research, Bahawalpur-63100, Pakistan; 3Institute of Soil and Environmental Sciences, University of Agriculture, Faisalabad, Pakistan; 4Department of Environmental Sciences, The University of Lahore, Lahore, Pakistan; 5Soil and Water Testing Laboratory for Research, Chiniot, Pakistan; 6Fodder Research Sub-station, Ayub Agricultural Research Institute, Faisalabad, Pakistan; 7Department of Soil Sciences, University College of Agriculture and Environmental Sciences, Islamia University of Bahawalpur, Bahawalpur, Pakistan; 8Ecotoxicology Research Program, National Agricultural Research Centre, Islamabad, Pakistan.
Abstract | Rhizobium is well recognized because of their symbiotic association especially with leguminous forages and form special structure, i.e., nodules, with 90% of Fabaceae family and displayed all possible means for improving the fertility of soils. Rhizobium also forms a symbiotic association with non-legumes because of their great colonizing capability and helps in improving crop growth. Physiological precursors are also used in association with rhizobium because of their higher solubility in water, continuous providence of hormones as well as low cost. This study was conducted to evaluate the role of Rhizobium, with and without Tryptamine on growth and yield parameters of Berseem. Total of eight treatments was planned to conduct an experiment. Results revealed that the nodule mass (0.523 g plant-1), highest plant height (103.45 cm), number of seeds per head, thousand seeds weight were recorded with Co-inoculation + Tryptamine @ 10-5 M (T8). Nutritious Parameters crude protein (30.23%), neutral detergent fiber (33.45%) and acid detergent fiber (26.56%) gave significant results as compared to control (T1). Results indicated that the combined application of Rhizobium species and Tryptamine performed better by improving growth and yield and quality parameters. It is concluded that precursor-inoculum combination is an effective approach and should be tested in different ecologies.
Received | February 27, 2019; Accepted | April 05, 2019; Published | May 06, 2019
*Correspondence | Fraza Ijaz, Soil Bacteriology Section, Agriculture Biotechnology Research Institute, AARI Faisalabad, Pakistan; Email: frazaijaz@gmail.com
Citation | Ijaz, F., U. Riaz, S. Iqbal, Q. Zaman, M.F. Ijaz, H. Javed, M.A. Qureshi, Z. Mazhar, A.H. Khan, H. Mehmood and I. Ahmad. 2019. Potential of rhizobium and PGPR to enhance growth and fodder yield of berseem (Trifolium alexandrinum L.) in the presence and absence of tryptamine.Pakistan Journal of Agricultural Research, 32(2): 398-406.
DOI | http://dx.doi.org/10.17582/journal.pjar/2019/32.2.398.406
Keywords | Berseem, Tryptamine, Rhizobium, Plant growth promoting rhizobacteria, Precursor-inoculum
Introduction
Plant growth promoting rhizobacteria (PGPR) form an association with Rhizobia and make colonization through roots. PGPR improve N2 fixation and nodulation process in leguminous crops (Paungfoo-Lonhienne et al., 2019). Rhizobium and PGPR co-inoculation have both positive and negative impact on plant growth. It caused a decrease in growth due to the competition for attachment sites on root surfaces and by the production of antibiotics (Mirza et al., 2007). Rhizobium and PGPR co-inoculation enhances growth by the reduction in direct spur of rhizobial growth/survival in the soil and ethylene level (Shaharoona et al., 2006), by the increase in phosphate solubilization and number of Rhizobium colonization sites, pathogen containment due to antibiotics production and hormone-induced amplification of the root system that results in enhanced nutrient uptake (Chebotar et al., 2001; Barea et al., 2005; Mishra et al., 2009). Bacterial and fungal-inoculation increased the stomatal conductance, water use efficiency (WUE) and photosynthetic rate (Vivas et al., 2003) and the relative water content of plants (Hamdia et al., 2004). Plants inoculated with Rhizobium and PGPR possessing ACC deaminase activity bears longer roots that help the plants to uptake more water from soil under stressed conditions (Hamdia et al., 2004).
Berseem (Trifolium alexandrinum L.) is one of the most important leguminous forages in the Middle-East and the Mediterranean region. It is important winter forage because of its nutritional value and contains more than 20% and 70% crude protein and dry matter digestibility respectively (Pal et al., 2004). In Pakistan, it is also cultivated as rabi (winter) fodder crop in irrigated areas. Berseem is usually grown in early autumn months, so before and during the months having a low temperature, it is used as feed for livestock. It is very productive after winter when temperatures rise. It grows better on alkaline and high moisture soils but slightly less drought-resistant (Hannaway et al., 2004; Joshi, 2018). It can be categorized by their productivity and branching behavior, into four cultivar groups. Miscawi and Kahdrawiare are the berseem cultivars having higher productive potential with branching types of growth attributes (Hannaway et al., 2004). It is an erect, sparsely hairy, 30 to 80 cm in height and annual forage legume (Hackney et al., 2007). Flowers are yellowish-white and form elliptical, dense clustered heads that are 2 cm in diameter. The fruit is a pod with one white to purplish-red seed, shallow taproot system, hollow stems, base branching, alternate leaves, with 2-3 cm broad × 4-5 cm long leaflets (Smoliak et al., 2006). The seeds are abundant under favorable conditions due to its comparable feed value, and it is compared to alfalfa; however, unlike alfalfa, it never showed bloat symptoms (Suttie, 1999; Hannaway et al., 2004). Berseem clover is also used as a green manure crop (Hannaway et al., 2004). Berseem can also be made into silage with oats or be fed chaffed and mixed with chopped straw (Suttie, 1999; Hannaway et al., 2004).
Tryptamine, a monoamine alkaloid with one indole ring, is similar in structure to the amino acid tryptophan. Tryptamine is found in many plants in small amounts. It influences plant microbiome and growth by acting as a feedstock for the metabolic pathways. It is found as a possible intermediate in the indole-3-acetic acid biosynthetic pathway. Total four tryptophan-dependent pathways occurred, 1): IPA Pathway (indole-3-pyrovate), 2): Tryptamine Pathway, 3): IAN Pathway (Indole-3-Acetonitrile), 4): IAM Pathway (Indole-3-Acetamide). The decarboxylation of tryptophan occurred, and then Tryptamine produced which convert it into indole-3-acetaldehyde with deamination. It has been demonstrated that Trp-dependent auxin biosynthesis is essential for embryogenesis, seedling growth, flower development, vascular pattern formation, and other developmental processes (Cheng et al., 2007; Stepanova et al., 2008; Tao et al., 2008).
However, considering the above-mentioned information, there is a need to conduct systematic work in Punjab province (Pakistan) on the factors limiting growth and fodder yield of Berseem with co-inoculation of PGPR and Rhizobia along with the foliar application of Tryptamine to enhance growth and fodder yield of berseem in the presence and absence of Tryptamine.
Materials and Methods
Study site
A field experiment was carried out to test the precursor Tryptamine with co-inoculation of Rhizobium and PGPR for growth and yield improvement of berseem fodder at Fodder Research Institute, Sargodha / Fodder Sub-station, Faisalabad.
Treatment plan
Experiment was comprised to eight treatment T1: control; T2: Rhizobium inoculation; T3: PGPR inoculation; T4: Co-inoculation; T5: control + Tryptamine @ 10-5 M (foliar spray); T6: Rhizobium +Tryptamine @ 10-5 M (foliar spray); T7: PGPR + Tryptamine @ 10-5 M (foliar spray) and T8: Co-inoculation + Tryptamine @ 10-5 M (foliar spray). Randomized complete block (RCB) design was followed while experimenting with an individual plot size of 15 m2 (5 m × 3 m). The seed of berseem cultivar (SB-11) was used as test cultivar. The crop was sown on 3 November 2017 at the seed rate of 8 Kg acre-1 and 45g plot-1. Efficient isolates of Rhizobium and PGPR species obtained from the Soil Bacteriology Section, Ayub Agriculture Research Institute (ARRI) Faisalabad used for inoculum preparation while the Tryptamine was obtained from the Merck Millipore, Germany. It was mixed with 10% sugar solution with 1 ml of gum acacia (2%) sticker solution and applied to the seed before sowing by using seed coating method. Seeds were enough shaken for proper mixing and coating of inoculants. After little air drying in the shade, seeds were sown in the field. Tryptamine was applied in the form of foliar sprays after each cutting. Total three cutting was done on January 4, 2018, February 14, 2018, and March 20, 2018. All other agronomic practices kept constant for irrigation and fertilizer application.
Nutritious parameters
For each cut, the nutritive value of the forage was assessed with measures including crude protein (CP), neutral detergent fiber (NDF) and acid detergent fiber (ADF). NDF and ADF analyzed sequentially (Van Soest et al., 1991) using the filter bag method. Nitrogen was analyzed using the distillation procedure. Crude protein (%) was calculated by the following equation.
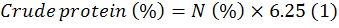
Statistical analysis
The experiment was led down in a randomized complete block design with three replicates. Statistical analysis of the data was done following the methods of analysis of variance (ANOVA) (Steel et al., 1997) by using the Statistix 8.1. The Least Significance Difference (LSD) values between the treatments were calculated at 5% probability levels.
Results and Discussion
Fresh fodder yield
Fresh fodder yield of berseem plants affected by different treatments is depicted in (Figure 1). The fresh fodder yield was highest (42.6 ± 0.67 t ha-1) at T8 {Co-inoculation + Tryptamine @ 10-5 M (foliar spray)} which was 46% more than control treatment (T1). While the minimum increase was found with T5 (Control + Tryptamine @ 10-5 M) treatment (PGPR Inoculation) but it was also 10% more than control (Figure 1). The increasing trend for fresh fodder yield was as T8> T6> T7 > T4 > T2> T3 > T5 > T1.
Dry matter yield
The dry matter yield of berseem plants is significantly affected by different treatments. Results showed that Rhizobium application and exogenous application of Tryptamine at different doses significantly enhanced dry matter yield. The highest dry matter yield was obtained from T8 {Co-inoculation + Tryptamine @ 10-5 M (foliar spray)} which was 39% more than control (T1) where no Rhizobium inoculation and Tryptamine were applied (Figure 2). The increasing trend for dry matter yield was as T8> T4> T6 > T7> T2> T5 > T3> T1.
Nodules per plant
Nodule formation is typically a characteristic of Rhizobium, the microbe responsible for nodule formation in legumes. In this study, nodules were formed on the berseem crop. Maximum nodules (24 nodules plant-1) were formed on plants which were treated with Co-inoculation + Tryptamine @ 10-5 M (T8). Whereas, the minimum nodules were formed, where no treatment was applied (T1) (Table 2). The trend for increasing nodules per plants was as T8> T7 = T6 = T4 > T2> T3 = T5> T1.
Nodule mass
The nodules mass was also affected by applied treatments. The highest nodule mass (0.523 ± 0.005 g plant-1) was recorded with T8 (Co-inoculation + Tryptamine @ 10-5 M) while minimum (0.023 ± 0.005 g plant-1) with T5 (control) (Table 2). The increasing trend for nodules mass was as T8> T4> T7 > T3 > T6> T2 > T1 > T5.
Plant height
The highest plant height (103.45 cm) was recorded with Co-inoculation + Tryptamine @ 10-5 M (T8). However, the lowest was found with control treatment where no inoculation has been performed, and no Tryptamine was applied (Figure 3).
Number of seeds per head
Data regarding the number of seed per head showed in Table 2. The maximum seeds per head showed by Co-inoculation + Tryptamine @ 10-5 M (T8). The decreasing trend was as T8> T7> T6 > T4 > T2> T3 > T5 > T1.
Thousand seeds weight
The last harvest was kept for seed production, and after maturity, the biomass of each plot was kept in the drying yard under the sun for one week, and threshing was operated to separate seed from the dried foliage. The seed from each plot was calculated following the same method employed in the calculation of green fodder yield ha-1. T8 obtained the highest thousand seed weight (Co-inoculation + Tryptamine @ 10-5 M) followed by T7 (Table 2).
Nutritious parameters
Crude protein: The results regarding crude protein contents (%) showed in Table 2. The maximum was crude protein contents (%) was determined in T8 (Co-inoculation + Tryptamine @ 10-5 M) which was significantly higher (P<0.001) than that of all other forage cuts. However, the minimum (97.88%) crude protein value was obtained by T1 (control).
Neutral detergent fiber and acid detergent fiber: The results regarding neutral detergent fiber (NDF) showed in Table 2. The highest (33.45%) NDF was found with T8 (Co-inoculation + Tryptamine @ 10-5 M) and lowest (23.21%) with T1 (control). The results regarding Acid Detergent Fiber (ADF) showed in Table 2. The highest (26.56%) NDF was found with T8 (Co-inoculation + Tryptamine @ 10-5 M) and lowest (17.34%) with T1 (control).
Agriculture industry and community-related to fodder sector, particularly in the twenty-first century faces a lot of challenges, associated with loss of soil fertility status, the increment in the use of synthetic fertilizers, high dose of pesticides, drought, salinity, fluctuating climatic conditions, and growing attack on crops by pathogens and pest. The worldwide necessity to boost agricultural production from rapidly shrinking land resources had placed substantial strain on the fragile agro-ecosystem. Agricultural sustainability, conservational security, and increased crop production can be attained by engaging eco-friendly approaches like the use of bio inoculants (Bhardwaj et al., 2014).
Different Rhizobium spp. isolated from different sources is reported to make an association with non-legumes and act as PGPR (Adnan et al., 2016). Rhizobium species as PGPR possess various mechanisms which are responsible for stimulating plant growth such as production of phytohormone, siderophores, cyanide, killing harmful pathogens by lytic enzymes, antibiotics, enhancing micro and macro-nutrients mobilization like phosphate solubilization, quorum-sensing signal interference,
Table 1: Effect of Treatments on Different Characteristics of Berseem Crop.
| Treatment | Nodules plant-1 | Nodule mass (g plant-1) | Number of Leaves Plant-1 | Number of Branches Plant-1 |
|
T1: Control |
11±0.98e |
0.088±0.001d |
69.50±5.12f |
13.33±1.2f |
|
T2: Rhizobium Inoculation |
16±1.21c |
0.105±0.002c |
83.45±7.12c |
20.03±2.12c |
|
T3: PGPR Inoculation |
15±1.02d |
0.125±0.014bc |
80.23±7.36d |
17.56±1.74d |
|
T4: Co-Inoculation |
19±1.40b |
0.140±0.001b |
86.45±8.12b |
21.01±2.22b |
|
T5:Control + Tryptamine @ 10-5 M |
15±0.99cd |
0.023±0.002e |
76.45±7.26e |
13.98±1.11e |
|
T6: Rhizobium + Tryptamine @ 10-5 M |
19±1.51b |
0.124±0.004bc |
85.67±8.23b |
21.45±1.89b |
|
T7: PGPR +Tryptamine @ 10-5 M |
19±1.24b |
0.136±0.001b |
86.45±8.24b |
22.56±1.99b |
|
T8: Co-Inoculation + Tryptamine @ 10-5 M |
24±1.93a |
0.523±0.001a |
90.67±9.45a |
24.34±2.12a |
| LSD value | 0.653 | 0.0180 | 1.56 | 0.98 |
Means ±SE sharing the same letter(s) in a column do not differ significantly at p<0.05 according to LSD Test.
Table 2: Effect of Treatments on Yield and Nutritious Parameters of Berseem Crop.
| Treatment | No of seeds per head | 1000-seeds weight (g) | Crude Protein (%) | Neutral detergent fiber (%) | Acid Detergent fiber (%) |
|
T1: Control |
10.34±0.89f |
1.20±0.85f |
15.45±1.14f |
23.21±2.13e |
17.34±1.15d |
|
T2: Rhizobium Inoculation |
22.67±1.24c |
2.11±0.97c |
22.34±1.77c |
27.40±1.32c |
20.56±2.36c |
|
T3: PGPR Inoculation |
19.78±1.89d |
1.92±0.64d |
19.45±1.68d |
25.01±2.45d |
18.67±1.78d |
|
T4: Co-Inoculation |
26.32±2.45b |
2.30±1.11b |
26.01±2.14b |
28.67±2.68b |
22.90±2.38b |
|
T5: Control + Tryptamine @ 10-5 M |
13.67±1.04e |
1.54±078e |
17.56±1.67e |
24.78±1.99d |
17.68±1.68d |
|
T6: Rhizobium + Tryptamine @ 10-5 M |
27.41±2.14b |
2.30±1.68b |
26.45±2.43b |
29.41±2.56b |
23.01±2.12b |
|
T7: PGPR +Tryptamine @ 10-5 M |
28.45±2.12b |
2.31±2.14b |
27.34±1.65b |
30.45±3.01b |
23.45±2.45b |
|
T8: Co-Inoculation + Tryptamine @ 10-5 M |
32.34±2.89a |
2.42±2.33a |
30.23±2.69a |
33.45±3.09a |
26.56±2.56a |
| LSD value | 1.23 | 0.05 | 0.91 | 0.29 | 1.01 |
Means ±SE sharing the same letter(s) in a column do not differ significantly at p<0.05 according to LSD Test.
organic compounds, inducing systemic resistance, nitrogen fixation, biofilm formation, releasing ACC deaminase and symbiotic relation between plant and microbes (Compant et al., 2005; Jimenez Gomez et al., 2016). Rhizobium, are recognized for making the symbiotic association with legumes and are accountable to fix nitrogen, capable of producing hormones may be considered the most credible mean for plant growth promotion (Mehboob et al., 2008). Rhizobium inoculation significantly affected the growth and development of berseem. Effect of all isolates was variable, and it was more manifest when tryptamine was applied exogenously (Zahir et al., 2004). In our study, Rhizobium inoculation with and without PGPR and Tryptamine increased the growth and yield attributes of the berseem as compared to control treatment (Table 1, 2; Figures 1, 2 and 3). These results are in agreement with Zahir et al. (2004) who stated that increase in growth and yield attributes of the fodder crop was might be due to more production of auxin and many researchers have also observed potential of Rhizobium sp to biosynthesize auxin either in the presence or absence of L-TRP (Hussain et al., 2013; Zahir et al., 2010a, b).
A significant effect in the nodules per plant, nodule mass, number of leaves, number of branches, number of seeds per pod and 1000-seeds weight was noticed in the current study (Table 1, 2). Increase in the fresh fodder weight, dry matter yield, and plant height, while the separate application of PGPR also increased the fresh fodder weight, dry matter yield, and plant height but less than the separate application of Rhizobium inoculation. Improvement in growth and yield at the sole application of tryptamine might have occurred due to the IAA biosynthesis, hormonal imbalance and lower level of ethylene. Sole inoculation of Rhizobium species improved maize growth and yield that might have occurred due to the release of organic acids and siderophores, increased plant hormone production, better root colonization, better nutrient uptake and improved systemic resistance (Hmissi et al., 2011; Mehboob et al., 2011; Akhtar et al., 2013). Increased auxin biosynthesis is associated with better root colonization and better root system architecture that improved the growth and yield of the berseem crop (Zahir et al., 2004, 2005, 2010). These results are confirmed by another finding where Auxin biosynthesis potential as IAA equivalents improved root (Khalid et al., 2001).
The plant growth promoting rhizobacteria (PGPR), are characterized by the following inherent distinctiveness’s: (i) they must be proficient to colonize the root surface (ii) they must survive, multiply and compete with other microbiota, at least for the time needed to express their plant growth promotion/protection activities and (iii) they must promote plant growth (Kloepper, 1994). Our study indicated that co-inoculation along with Tryptamine @ 10-5 M provided the best results (Figure 1, 2 and 3) about fodder fresh weigh, dry matter yield and plant height. The combined application of Rhizobium + PGPR + Tryptamine @ 10-5 M promoted the yield of fodder. This might have occurred due to the improved biosynthesis of auxins in the rhizosphere (Zahir et al., 2005, 2010).
Similarly, Kang et al. (2007) observed an enhanced enzyme activity (T5H) coupled with a dose-dependent increase in serotonin in the rice seedling tissues, grown in the presence of tryptamine. T5H is a terminal enzyme responsible for serotonin synthesis using tryptamine as a substrate. The T5H enzyme occurred predominantly as a soluble protein and exhibited broad substrate specificity toward aromatic amino acids and their derived amine compounds such as tryptamine and octopamine. However, dopamine derived from phenylalanine did not affect T5H enzyme catalysis, suggesting that T5H harbors a certain level of substrate specificity among aromatic amino acid derivatives (Jang et al., 2004). The conversion of tryptophan into indole-3-acetic aldehyde (IAA) may involve an alternative pathway in which Tryptamine is formed as in pseudomonads and azospirilla.
A significant effect in the neutral and acid detergent fiber and crude protein was also observed by the application of Rhizobium and RGPR inoculations in a singlet and combined form using the tryptamine of berseem crop (Table 2). High plant crude protein content in maize might be due to better root growth and root system because of improved growth hormone in the rhizosphere (Hussain et al., 2009). Soil N and available P improved because of Precursor-inoculum interaction. The reason behind this improvement might be the solubilization of phosphates by organic acids production, enhanced root system for nutrients acquisition, increased microbial activities due to the precursor and thus increased berseem crop quality attributes (Fatima et al., 2006; Mehboob et al., 2011).
Conclusions and Recommendations
A field investigation was accompanied to assess the interaction of Rhizobium and PGPR to improve development, growth and fodder harvest of Berseem in the existence and lack of tryptamine. The results displayed that the collective plan of co-inoculation (PGPR + Rhizobium) + Tryptamine @ 10-5 M significantly improved the physical, nutritious, growth parameters as compared to all treatment. Precursor-inoculum interaction is an effective strategy to improve the berseem fresh and dry fodder yield. Comprehensive studies in different ecologies regarding the physiological and biochemical aspects are required to confirm this approach.
Acknowledgment
Authors are thankful to Ayub Agricultural Research Station, Faisalabad for providing space for experiments and reviewers for improvement of the manuscript.
Author’s Contribution
Fraza Ijaz conducted the research, Umair Riaz, Shazia Iqbal, and Muhammad Furqan Ijaz prepared the draft while Hina Javed and Muhammad Amjad Qureshi guided step by step for the research study, Zuhra Mazhar prepared the references, Ahmad Hassan Khan provided the research material, Ijaz Ahmad, Qamar Uz Zaman and Hassan Mehmood technically improved the manuscript and graphs.
References
Adnan, M., Z. Shah, N. Saleem, A. Basir, H. Ullah, M. Ibrahim, J.A. Shah, A. Khan and S.R.A. Shah. 2016. Isolation and evaluation of summer legumes rhizobia as PGPR. Pure Appl. Biol. 5(1): 127. https://doi.org/10.19045/bspab.2016.50017
Akhtar, N., I. Arshad, M.A. Shakir, M.A. Qureshi, J. Sehrish and L. Ali. 2013. Co-inoculation with rhizobium and bacillussp to improve the phosphorus availability and yield of wheat (Triticumaestivum L.). J. Anim. Plant Sci. 23(1): 190-197.
Badria, F.A. 2002. Melatonin, serotonin, and tryptamine in some Egyptian food and medicinal plants. J. Med. Food. 5(3): 153-157. https://doi.org/10.1089/10966200260398189
Barea, J.M., M.J. Pozo, R. Azcon and C. Azcon-Aguilar. 2005. Microbial co-operation in the rhizosphere. J. Exp. Bot. 56(417): 1761-1778. https://doi.org/10.1093/jxb/eri197
Bhardwaj, D., M.W. Ansari, R.K. Sahooand N. Tuteja. 2014. Biofertilizers function as a key player in sustainable agriculture by improving soil fertility, plant tolerance and crop productivity. Microb. Cell Fact. 13(1): 66. https://doi.org/10.1186/1475-2859-13-66
Chebotar, V.K., C.A. Asis and S. Akao. 2001. Production of growth-promoting substances and high colonization ability of rhizobacteria enhance the nitrogen fixation of soybean when coinoculated with Bradyrhizobiumjaponicum. Biol. Fert. Soil. 34(6): 427-432.
Cheng, Y., X. Dai and Y. Zhao. 2007. Auxin synthesized by the YUCCA flavin monooxygenases is essential for embryogenesis and leaf formation in Arabidopsis. Plant Cell. 19(8): 2430-2439. https://doi.org/10.1105/tpc.107.053009
Compant, S., B. Reiter, A. Sessitsch, J. Nowak, C. Clément and E.A. Barka. 2005. Endophytic colonization of Vitisvinifera L. by plant growth-promoting bacterium Burkholderia sp. strain PsJN. Appl. Environ. Microb. 71(4): 1685-1693. https://doi.org/10.1128/AEM.71.4.1685-1693.2005
Fatima, Z., M. Zia and M.F. Chaudhary. 2006. Effect of Rhizobium strains and phosphorus on growth of soybean (Glycine max) and survival of Rhizobium and P solubilizing bacteria. Pak. J. Bot. 38: 459-464.
García, J.L., A. Probanza, B. Ramos, J. Barriuso and F.G. Mañero. 2004. Effects of inoculation with plant growth promoting rhizobacteria (PGPRs) and Sinorhizobiumfredii on biological nitrogen fixation, nodulation and growth of Glycine max cv. Osumi. Plant Soil. 267(1-2): 143-153. https://doi.org/10.1007/s11104-005-4885-5
Hackney, B., B. Dear and G. Crocker. 2007. Berseem clover.
Hamdia, M.A.E.S., M.A.K. Shaddad and M.M. Doaa. 2004. Mechanisms of salt tolerance and interactive effects of Azospirillumbrasilense inoculation on maize cultivars grown under salt stress conditions. Plant Growth Regul. 44(2): 165-174. https://doi.org/10.1023/B:GROW.0000049414.03099.9b
Hannaway, D.B. and C. Larson. 2004. Berseem clover (Trifoliumalexandrinum L.). Oregon State Univ. Species Sel. Inf. Sys.
Hmissi, I., S. Gargouri and B. Sifi. 2011. Attempt of wheat protection against Fusarium culmorum using Rhizobium isolates. Tunis. J. Plant Prot. 6: 75-86.
Hussain, M.I., H.N. Asghar, M. Arshad and M. Shahbaz. 2013. Screening of multi-traits rhizobacteria to improve maize growth under axenic conditions. J. Anim. Plant Sci. 23(2): 514-520.
Husssain, M.B., I. Mehboob, Z.A. Zahir, M. Naveed and H.N. Asghar. 2009. Potential of Rhizobium spp. for improving growth and yield of rice (Oryza sativa L.). Soil Environ. 28(1): 49-55.
Jang, S.M., A. Ishihara and K. Back. 2004. Production of coumaroylserotonin and feruloylserotonin in transgenic rice expressing pepper hydroxycinnamoyl-coenzyme A: Serotonin N-(hydroxycinnamoyl) transferase. Plant Physiol. 135: 346–356. https://doi.org/10.1104/pp.103.038372
Jiménez-Gómez, A., E. Menéndez, J.D. Flores-Félix, P. García-Fraile, P.F. Mateos and R. Rivas. 2016. Effective colonization of spinach root surface by Rhizobium. In Biol. Nitrogen Fixation and Beneficial Plant-Microbe Interaction (pp. 109-122). Springer, Cham. https://doi.org/10.1007/978-3-319-32528-6_10
Joshi, M. 2018. Textbook of field crops. PHI Learning Pvt. Ltd.
Kang, S., K. Kang, K. Lee and K. Back. 2007. Characterization of tryptamine 5-hydroxylase and serotonin synthesis in rice plants. Plant Cell Rep. 26(11): 2009-2015. https://doi.org/10.1007/s00299-007-0405-9
Khalid, A., M. Arshad, Z.A. Zahir and M. Khalid. 2001. Relative efficiency of rhizobacteria for auxin biosynthesis. J. Biol. Sci. 8(1): 750-754.
Khuong, T., Y. Zheng, C. Chao and C. Lovatt. 2010. Foliar-applied trytophan, a precursor of IAA biosynthesis, increases fruit set and fruit size of citrus. In Proceedings of the 37th Annual Meeting of the Plant Growth Regulation Society of America, Portland, Oregon, USA, 8-12 August 2010 (pp. 97-101). Plant Growth Regul. Soc. Am.
Kloepper, J.W. 1994. Plant growth-promoting rhizobacteria (other systems). Azospirillum/plant Assoc. 137-166.
Mehboob, I., Z.A. Zahir, A. Mahboob, S.M. Shahzad, A. Jawad and M. Arshad. 2008. Preliminary screening of rhizobium isolates for improving growth of maize seedlings under axenic conditions. Soil Environ. 27: 64-71.
Mehboob, I., Z.A. Zahir, M. Arshad, A. Tanveer and F. Azam. 2011. Growth promoting activities of different rhizobium spp., in wheat. Pak. J. Bot. 43(3): 1643-1650.
Mehboob, I., Z.A. Zahir, M. Arshad, A. Tanveer and F. Azam. 2011. Growth promoting activities of different rhizobium spp., in wheat. Pak. J. Bot. 43(3): 1643-1650.
Mirza, B.S., M.S. Mirza, A. Bano and K.A. Malik. 2007. Co-inoculation of chickpea with Rhizobium isolates from roots and nodules and phytohormone-producing Enterobacter strains. Aust. J. Exp. Agric. 47: 1008-1015. https://doi.org/10.1071/EA06151
Mishra, P.K., S. Mishra, G. Selvakumar, S. Kundu and H. Shankar Gupta. 2009. Enhanced soybean (Glycine max L.) plant growth and nodulation by Bradyrhizobiumjaponicum-SB1 in presence of Bacillus thuringiensis-KR1. Acta Agric. Scand. Sec. B. 59(2): 189-196.
Pal, M., V. Karthikeyapandian, V. Jain, A.C. Srivastava, A. Raj and U.K. Sengupta. 2004. Biomass production and nutritional levels of berseem (Trifoliumalexandrium) grown under elevated CO2. Agric. Ecosyst. Environ. 101(1): 31-38. https://doi.org/10.1016/S0167-8809(03)00202-0
Panse, V.G. and P.T. Sukhatme. 1967. Statistical methods for agricultural workers. India. Counc. Agric. Res. New Delhi.
Paungfoo-Lonhienne, C., M. Redding, C. Pratt and W. Wang. 2019. Plant growth promoting rhizobacteria increase the efficiency of fertilisers while reducing nitrogen loss. J. Environ. Manage. 233: 337-341. https://doi.org/10.1016/j.jenvman.2018.12.052
Shaharoona, B., M. Arshad, Z.A. Zahir and A. Khalid. 2006. Performance of pseudomonas spp. containing ACC-deaminase for improving growth and yield of maize (Zea mays L.) in the presence of nitrogenous fertilizer. Soil Biol. Biochem. 38(9): 2971-2975. https://doi.org/10.1016/j.soilbio.2006.03.024
Smoliak, S., R.L. Ditterline, J.D. Scheetz, L.K. Holzworth, J.R. Sims, L.E. Wiesner, D.E. Baldridge and G.L. Tibke. 2006. Berseem Clover (Trifoliumalexandrinum). Montana State Univ. Anim. Range Sci. Ext. Serv. Forage Ext. program, Bozeman, USA
Steel, R.G., J. H. Torrie and D.A. Dickey. 1997. Principles and procedures of statistics: A biometrical approach. (3rd Ed.) McGraw-Hill book Co., NY. USA.
Stepanova, A.N., J. Robertson-Hoyt, J. Yun, L.M. Benavente, D.Y. Xie, K. Doležal, A. Schlereth, G. Jürgens and J.M. Alonso. 2008. TAA1-mediated auxin biosynthesis is essential for hormone crosstalk and plant development. Cell. 133(1): 177-191. https://doi.org/10.1016/j.cell.2008.01.047
Suttie, J.M. 1999. Berseem clover (Trifoliumalexandrinum). A searchable catalogue of grass and forage legumes, FAO.
Tao, Y., J.L. Ferrer, K. Ljung, F. Pojer, F. Hong, J.A. Long, L. Li, J.E. Moreno, M.E. Bowman, L.J. Ivans and Y. Cheng. 2008. Rapid synthesis of auxin via a new tryptophan-dependent pathway is required for shade avoidance in plants. Cell. 133(1): 164-176. https://doi.org/10.1016/j.cell.2008.01.049
Van Soest, P.J.V., J.B. Robertson and B.A. Lewis. 1991. Methods for dietary fiber, neutral detergent fiber and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 74: 3583-3597. https://doi.org/10.3168/jds.S0022-0302(91)78551-2
Vivas, A., A. Marulanda, J.M. Ruiz-Lozano, J.M. Barea and R. Azcón. 2003. Influence of a Bacillus sp. on physiological activities of two arbuscular mycorrhizal fungi and on plant responses to PEG-induced drought stress. Mycorrhiza. 13(5): 249-256. https://doi.org/10.1007/s00572-003-0223-z
Zahir, A.Z., H.M. Yasin, M. Naveed, M.A. Anjum and M. Khalid. 2010b. L-Tryptophan application enhances the effectiveness of Rhizobium inoculation for improving growth and yield of Mungbean (Vigna radiate L. Wilczek). Pak. J. Bot. 42(3): 1771-1780.
Zahir, Z.A., M.K. Shah, M. Naveed and M.J. Akhter. 2010a. Substrate-dependent auxin production by Rhizobium phaseoli improves the growth and yield of Vignaradiata L. under salt stress conditions. J. Microbiol. Biotechnol. 20: 1288– 1294. https://doi.org/10.4014/jmb.1002.02010
Zahir, Z.A., H.N. Asghar, M.J. Akhtar and M. Arshad. 2005. Precursor (L-tryptophan)-inoculum (Azotobacter) interactions for improving yields and nitrogen uptake of maize. J. Plant Nutr. 28: 805-817. https://doi.org/10.1081/PLN-200055543
Zahir, Z.A., M. Arshad and W.T. Frankenberger, Jr. 2004. Plant growth promoting rhizobacteria: application and perspectives in agriculture. Adv. Agron. 81: 96-168.
Zahir, Z.A., M. Arshad and W.T. Frankenberger. 2004. Plant growth promoting rhizobacteria: applications and perspectives in agriculture. Adv. Agron. 81: 98-169.
To share on other social networks, click on any share button. What are these?







