Population Density and Aggressiveness of Macrophomina Phaseolina Isolates from Sindh, Pakistan
Population Density and Aggressiveness of Macrophomina Phaseolina Isolates from Sindh, Pakistan
Khadim Hussain Wagan1*, Muhammad Ibrahim Khaskheli2, Jamal-U-Ddin Hajano1 and Abdul Ghani Lanjar2
1Department of Plant Pathology, Sindh Agriculture University, Tandojam, 70060-Pakistan; 2Department of Plant Protection, Sindh Agriculture University, Tandojam, 70060-Pakistan.
Abstract | Macrophomina phaseolina causing charcoal rot of sunflower is major fungal pathogen which can survive for months to years in soil and also over season in the seed. Therefore, population density and their aggressiveness to cause the disease is playing critical role for management of this pathogen. For such purpose colony forming units (cfu) per gram of soil and seed were determined and capability of previously characterized M. phaseolina isolates to cause the disease was tested on HO-1 variety. Additionally, most virulent isolate was characterized through sequence and phylogenetic analysis. There was significant variation (Df= 54, F=7.4, P=0.0000) among cfu per gram of M. phaseolina in soil samples collected during field survey at different districts of Sindh during 2015 and 2016 growing year. In soil sample maximum 41.371 cfu per gram of M. phaseolina with range of 27.00-48.40 were counted from Badin during 2016 followed by 40.2 with range of 27.00-45.20 during 2015 growing season. The plant tissues were carrying more M. phaseolina cfu as compared to soil samples. However, cfu count of M. phaseolina was significantly varying among districts, locations and year of samplings (Df= 54, F= 4, P= 0.0000). Similarly, as soil samples collected from Badin, plant tissue samples also gave maximum cfu per gram of M. phaseolina from Badin and then reduced gradually in Thatta, Tando M. Khan, Hyderabad, Shaheed Benazirabad, Mirpurkhas, Sanghar, Dadu, Sukkur and Khairpur. All the isolates could cause infection. Maximum plant mortality (46.67%) was recorded in plants inoculated with MPS16 isolate followed by MPS17 (43.33%) and MPS12 (43.0%). Similarly, length of necrotic lesions was also significantly higher in MPS16 (6.67 cm) followed by MPS17 (6.33 cm), MPS12 (6.30 cm), MPS15 (6.17 cm) and MPS 13 (6.07 cm). The predicted isolate (MPS16) was aligned with different species and observed that MPS16 belongs to M. phaseolina isolate. The sequence showed high identity with sequences with Macrophomina sp. from the NCBI database (100%); In contrast, alignments among the same genera and species demonstrated 91–98% identity. We then predicated that isolated encoded culture (MPS16) predetermine with Macrophomina sp.
Received | November 20, 2018; Accepted |March 10, 2019; Published | April 04, 2019
*Correspondence | Khadim Hussain Wagan, Department of Plant Pathology, Sindh Agriculture University, Tandojam, 70060-Pakistan; Email: khwagan@hotmail.com
Citation | Wagan, K.H., M.I. Khaskheli, J.D. Hajano and A.G. Lanjar. 2019. Population density and aggressiveness of macrophomina phaseolina isolates from Sindh, Pakistan. Sarhad Journal of Agriculture, 35(2): 400-407.
DOI | http://dx.doi.org/10.17582/journal.sja/2019/35.2.400.407
Keywords | Macrophomina phaseolina, Colony forming units, Aggressiveness, Isolates and characterization
Introduction
Macrophomina phaseolina, a dominant fungus of sunflower causing charcoal rot is mainly found in soil, but also reported as seed borne; therefore, it overwinter season in the soil, plant debris and in the infected seeds. Usually charcoal rot infection starts when sunflower roots come into contact with small sclerotia of M. phaseolina (Suriachandraselvan and Seetharaman, 2000; Krizmanic et al., 2004). The fungus becomes more active and reproductive in summer season than winter season (Dwivedi et al., 1990). Premature death of leaf and wilting symptoms are induced through infection of M. phaseolina under favourable conditions for successful infection (Gupta and Chauhan, 2005; Khan, 2007). The disease becomes more economically important and encouraged by dry condition and hot season (Schwartz and Gent, 2016). After harvesting of crop, microsclerotia in the plant residue returned to the soil. There is contradictive information regarding time period during which microsclerotia of the fungus can be infective in naturally infested soil. Short et al. (1980) mention survival period of 24 months in plant debris or under the soil surface for M. phaseolina. In another study Reis et al. (2014) reported 35 months survival period of the fungus and its ability to cause the infection. The fungus can survive for 39 months in infested seeds and 37 months in plant debris as microsclerotia and mycelium depending source of survival has also been reported (Suriachandraselvan and Seetharaman, 2000; Krizmanic et al., 2004; Gupta et al., 2012). Gupta et al. (2012) mentioned survival of the microsclerotia up to 15 years under favourable conditions when it is associated with suitable host but its disease-causing potential is not addressed. Lodha et al. (2002) reported that sclerotia of M. phaseolina were unable to be completely eradicated from infected residues of cluster bean at 48-62 0C thermal range. In addition, application of fungicides to check the pathogen sometimes becomes ineffective due to variability among populations of the same pathogen showing different properties (Kushwaha et al., 2017).
Isolates of this fungus are divergent in various morphological and other aspects like pigmentation of mycelium, distribution of microsclerotia, formation of pycnidia and chlorate phenotypes (Almomani et al., 2013). Iqbal and Mukhtar (2014) reported 65 isolates of M. phaseolina from Punjab and Khyber Pakhtunkhwa (KPK) provinces of Pakistan, which are varying in morphological characteristics and pathogenic nature to infect mungbeen crop. Ashraf et al. (2015) conducted such study in Punjab province to differentiate morphologically and virulent potential of 24 isolates of this fungus from maize crop, which were significantly changed in the geographic regions. Moreover, morphological and virulent variation among isolates of M. phaseolina collected from mash is examined by Riaz et al. (2007). In another study, thirty three M. phaseolina isolates were recovered from different sunflower fields of Egypt and all isolates were pathogenic to both tested sunflower cultivars H11-008 and H11-009, in the pathogenicity test (Aboshosha et al., 2007). Mahdizadeh et al. (2011) recovered fifty two M. phaseolina isolates from 24 host plant species during a survey of 14 Iranian provinces. Saleh et al. (2010) evaluated the degree of populations of M. phaseolina by comparing 143 isolates from different crops. However, all these studies were conducted in other geographical locations and the reported population and isolates of M. phaseolina were quite different than current study. Thus, may not represent the actual position of Sindh Province that has drastically affected by this serious disease in recent days.
Moreover, such variation is a regular phenomenon among pathogens including fungi, hence there was a need to assess aggressiveness and proper characterization of M. phaseolina isolates from sunflower for better understanding and effective management to avoid any epidemic of the disease in near future in this region. In the current study, the virulence and aggressiveness of the pathogen was greatly varies from one locality to another.
Materials and Methods
Plant sample assay
Five diseased plants from each farmer’s field were collected at random from 10 different major sunflower growing districts of Sindh Province and brought into the laboratory. Plant samples were properly washed and dried. Plant pieces without secondary roots were ground in blender, later powder was properly mixed. Weight of 0.2 g powder was mixed in 100 ml of sterilized PDA medium (Mihail and Alcorn, 1982) and poured in five petri plates. All petri plates were incubated in darkness at 30 0>C. After 10 days, number of colony forming units (cfu) was counted under stereoscopic microscope to decide the microsclerotia per gram of root tissues.
Soil sample assay
To estimate microsclerotia population in each farmer’s field, soil samples from 0-15 cm soil depth were collected from each field and pooled in to composite sample. Sampling was done in “X” pattern with the length of approximately 50 m in each direction. All composite soil samples were kept at room temperature for 24 hours and then passed through 2 mm sieve. The microsclerotia population of M. Phaseolina present in composite soil sample was estimated by using the methods described by Mihail and Alcorn (1982). Colony counts were done after 10 days of incubation at 30oC with the help of stereoscopic microscope to determine colony forming units (CFU) per gram of soil. The data on colony forming units (CFU) was observed after 4, and 7 days of inoculation. The data for CFU count in soil dilution plate technique is presented here is from 10-1 dilution and calculated by:
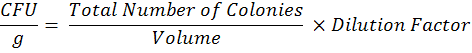
Molecular characterization of virulent isolate
Five samples from each aggressive isolate of M. phaseolina were selected for DNA extraction. Fungal genomic DNA was purified using Gentra Puregene Yeast Kit following the manufacturer’s instructions. Quality of DNA was determined on 1.5% gel using UVP Benchtop transilluminator (302 nm). Conserved regions of fungal genomic DNA were amplified using ITS1-5´-TCCGTAGGTGAACCTGCGG-3´ and ITS4-5´-TCCTCCGCTTATTGATATGC-3´ primers and methodology for running PCR reaction was as described Mirhendi et al. (2007). Approximately, 5µl of amplified products were visualized on 2% gel. The PCR products were sent to the company for sequencing of DNA of each sample. The nucleotide sequence of each sample was blasted by using nucleotide internet database of National Center for Biotechnology Information Database (NCBI). The Phylogenetic tree was constructed through Neighbor-Joining method associated taxa clustered (bootstrap 1000 replicates). The Nucleotide sequence obtained from the National Center for Biotechnology Information Database (NCBI). The obtained sequences were analyzed with Clustalx 1.83 and the Phylogenetic tree was constructed through MEGA (version 3.1). The circled isolate denotes the member cultured in present study.
Degree of virulence of isolates and pathogenicity
The degree of virulence of M. phaseolina isolates collected from different farmer’s fields of sunflower of Sindh and previously characterized in our study (Wagan et al., 2018) was tested in complete randomized design with three replications. The potting mixture containing clay loam soil, sand and farm yard manure at 1:1:1 ratio prepared and autoclaved for sterilization. Earthen pots of 9-inch diameter were filled with the soil containing inoculum of M. phaseolina isolates 2 g per kg soil. Soil infestation was performed one day before sowing of seeds. Apparently, looking healthy seeds of HO-1 variety were dipped in 0.01% mercuric chloride for 30 seconds then washed two times in distilled water to eliminate the pathogens on surface of seed. 10 seeds were sown per pot and the pots without fungus were served as control. Water was given to the plants as needed. MP isolates were re-isolated from developed lesions for confirmation of pathogenicity test. After 45 days of sowing data on mortality percentage, root colonization percentage from inoculated and un-inoculated plants was recorded.
Results and Discussion
Macrophomina phaseolina population density
The population density of M. phaseolina was highest in the fields of sunflower in which disease severity was highest and showed great proportion. There was significant variation (Df= 54, F=7.4, P=0.0000) among colony forming units (cfu) of M. phaseolina in soil samples collected during field survey at different districts of Sindh during both years of study. Significantly maximum 41.371 cuf per gram of M. phaseolina with range of 27.00-48.40 were counted from Badin during 2016 followed by 40.2 with range of 27.00-45.20 during 2015 growing season (Table 1). Minimum 14.8 (ranging 7.00-19.60) and 14.6 (ranging 6.00-18.60) cfu per gram of M. phaseolina were counted at Khairpur during 2016 and 2015, respectively (Table 1).
Overall M. phaseolina cfu were higher during 2016 ranging from 41.371 to 14.8 as compared to 2015 ranging 40.2 to 14.6 (Table 1, Figure 1). M. phaseolina population was well established in Badin, Thatta, Tando M. Khan, Hyderabad and it was reduced to Khairpur and Sukhar Districts. This finding links with cropping history, that crop is grown in district Badin and Thatta at larger areas and is continuously grown due to shortage of irrigation; thus, fungus seems to establish itself in these areas.
The presence of M. phaseolina was further confirmed by analyzing the plant tissues of infected plants. The plant tissues were carrying more M. phaseolina cfu as compared to soil samples. However, cfu count was significantly varying among districts, locations and year of sampling (Df= 54, F= 4, P= 0.0000). Similarly, as soil samples collected from Badin, plant tissue samples also gave maximum cfu from Badin and then reduced gradually in Thatta, Tando M. Khan, Hyderabad, Shaheed Benazirabad, Mirpurkhas, Sanghar, Dadu, Sukkur and Khairpur (Table 2).
Table 1: Population of Macrophomina phaseolina in soil samples collected from different farmer’s fields of sunflower.
| District | Year | |||||
| 2015 | 2016 | |||||
| Range of cfu g-1of soil | cfu Mean | Range of cfu g-1of soil | cfu Mean | |||
| Badin | 27.20-45.20 | 40.2 | b | 28.00-48.40 | 41.371 | a |
| Thatta | 25.60-43.80 | 38.229 | d | 26.20-45.00 | 38.743 | c |
| Tando M.Khan | 24.00-42.20 | 35.457 | f | 25.40-43.00 | 36.171 | e |
| Hyderabad | 22.80-40.20 | 33.171 | h | 24.20-41.80 | 34.6 | g |
| Mirpurkhas | 16.20-30.80 | 25.229 | k | 18.00-32.00 | 26.229 | j |
| S. Benazirabad | 18.00-34.80 | 26.571 | j | 20.00-35.20 | 27.4 | i |
| Sanghar | 15.00-27.20 | 22.286 | m | 16.20-28.80 | 23.2 | l |
| Dadu | 11.40-24.00 | 19.686 | o | 12.60-25.00 | 20.943 | n |
| Khairpur | 6.00-18.60 | 14.6 | r | 7.00-19.60 | 14.8 | r |
| Sukkur | 6.20-19.80 | 15.229 | q | 8.00-21.00 | 15.914 | p |
| LSD (P < 0.05) | 0.3851 | |||||
| SE | 0.1960 | |||||
cfu = Colony Forming Unit; Note: The alphabetical letters showing the homogenous grouping in column are not significant with each other.
Table 2: Population of Macrophomina phaseolina in infected plant samples collected from different farmer’s fields of sunflower.
| District | Year | |||||
| 2015 | 2016 | |||||
| Range of cfu g-1in plant | cfu Mean | Range of cfu g-1in plant | cfu Mean | |||
| Badin | 168.00-208.60 | 194.31 | b | 172.20-215.00 | 198.8 | a |
| Thatta | 160.00-201.40 | 184.23 | d | 167.00-206.40 | 187.2 | c |
| Tando M.Khan | 152.80-195.40 | 179.4 | f | 160.00-200.80 | 182.37 | e |
| Hyderabad | 150.00-193.60 | 172.34 | h | 160.00-198.20 | 178.49 | g |
| Mirpurkhas | 138.40-180.00 | 160.31 | l | 140.80-185.20 | 164.94 | k |
| S. Benazirabad | 141.20-188.00 | 166.6 | j | 145.40-192.80 | 170.74 | i |
| Sanghar | 132.00-175.80 | 156.91 | n | 136.00-180.40 | 159.69 | m |
| Dadu | 127.00-166.60 | 151.8 | p | 130.20-171.40 | 155 | o |
| Khairpur | 111.40-140.00 | 129.66 | t | 115.20-146.80 | 132.17 | r |
| Sukkur | 115.80-146.00 | 131.14 | s | 119.00-150.00 | 134.69 | q |
| LSD (P<0.05) | 0.4118 | |||||
| SE | ±0.2096 | |||||
cfu = Colony Forming Unit; Note: The alphabetical letters showing the homogenous grouping in column are not significant with each other.
The overall M. phaseolina cfu in plant samples were more during 2016 growing year than 2015, ranging from 198.8 to 134.69. M. phaseolina population in plant samples was well established in Badin, Thatta, Tando M. Khan, Hyderabad and it was reduced to Khairpur and Sukhar districts (Table 2).
Molecular characterization of MPS16 isolate
The virulent isolate MPS16 associated with the different species of fungi was characterized on morphological criteria and phylogenetic analysis of internal transcribed spacer (ITS) regions. In present study, we determined domain consisting of proteins sequences obtained from various sources aligned by using redundant entries. A combined rooted neighbor-joining (NJ) tree (1000 replicates) was generated through MEGA 4.5 by following the default parameters; poisson correction, pairwise deletion and bootstrap. Conserved motifs protein sequences of sorted member were identified using a motif based sequence analysis tool. The BLASTn search for the resulting motifs in NCBI and MS-Homology databases was carried out to determine their significance. In order to understand the putative role of fungal isolates, we analyzed the deduced amino acid sequence by BLASTn and searched in NCBI database indicated as inquiry aligned with isolated strains. Comparison of the nucleotide sequence and phylogenetic analysis of isolates was also performed which revealed that sequences belong to different groups (species) (Figure 2). The isolated fungi were classified into different species, the most common of which aligned the isolates encoded culture-1 using ITS region. The predicted isolate (MPS16) aligned with different species and observed that MPS16 belongs to M. phaseolina isolate. The sequence showed high identity with sequences with Macrophomina sp. from the NCBI database (100%); In contrast, alignments among the same genera and species demonstrated 91–98% identity. We then predicated that isolated encoded culture (MPS16) predetermine with Macrophomina sp.
Note: The Phylogenetic tree was constructed through Neighbor-Joining method associated taxa clustered (bootstrap 1000 replicates). The Nucleotide sequence obtained from the National Center for Biotechnology Information Database (NCBI). The obtained sequences were analyzed with Clustalx 1.83, and the Phylogenetic tree was constructed through MEGA (version 3.1). The circled isolates denote the member culture in present study.
Degree of virulence of isolates and pathogenicity
Aggressiveness of all 32 isolates was tested using HO-1 cultivar. All the isolates could cause infection (Table 3). Mortality % was recorded highly significant among all the isolates (Df= 31, F= 1224.76, P= 0.0000). However, maximum plant mortality (46.67%) was recorded in plants inoculated with MPS16 isolate followed by MPS17 (43.33%) and MPS12 (43.0%).
Similarly, length of necrotic lesions was also significantly higher in MPS16 (6.67 cm) followed by MPS17 (6.33 cm), MPS12 (6.30 cm), MPS15 (6.17 cm) and MPS 13 (6.07 cm) (Table 3). There was highly significant variation among the necrotic lesions produced by isolates (Df= 31, F= 6.13231, P= 0.0000).
Table 3: Pathogenicity and of degree of virulence of Macrophomina phaseolina isolates obtained from different districts of Sindh.
| Isolate | Pathogenicity test | Mortality (%) | Necrotic lesions(cm) | ||
| MPS1 | + | 36.67 | d | 5.60 | d |
| MPS2 | + | 26.67 | g | 4.67 | fg |
| MPS3 | + | 30.00 | f | 5.13 | e |
| MPS4 | + | 23.33 | h | 4.33 | h |
| MPS5 | + | 16.67 | i | 3.87 | i |
| MPS6 | + | 10.00 | k | 3.27 | kl |
| MPS7 | + | 6.67 | l | 2.90 | m |
| MPS8 | + | 13.33 | j | 3.33 | k |
| MPS9 | + | 26.67 | g | 4.47 | gh |
| MPS10 | + | 30.00 | f | 5.00 | e |
| MPS11 | + | 26.67 | g | 4.87 | ef |
| MPS12 | + | 43.00 | b | 6.30 | b |
| MPS13 | + | 40.00 | c | 6.07 | bc |
| MPS14 | + | 36.67 | d | 5.77 | cd |
| MPS15 | + | 40.00 | c | 6.17 | b |
| MPS16 | + | 46.67 | a | 6.67 | a |
| MPS17 | + | 43.33 | b | 6.33 | b |
| MPS18 | + | 36.67 | d | 5.63 | d |
| MPS19 | + | 33.33 | e | 5.53 | d |
| MPS20 | + | 30.00 | f | 5.07 | e |
| MPS21 | + | 13.33 | j | 3.33 | k |
| MPS22 | + | 16.67 | i | 3.67 | ij |
| MPS23 | + | 13.33 | j | 3.50 | jk |
| MPS24 | + | 10.00 | k | 3.00 | lm |
| MPS25 | + | 6.67 | l | 2.80 | m |
| MPS26 | + | 10.00 | k | 2.97 | lm |
| MPS27 | + | 6.67 | l | 2.83 | m |
| MPS28 | + | 6.67 | l | 2.87 | m |
| MPS29 | + | 3.33 | m | 2.40 | n |
| MPS30 | + | 3.33 | m | 2.17 | no |
| MPS31 | + | 3.33 | m | 2.07 | o |
| MPS32 | + | 3.33 | m | 2.13 | no |
| SE | 0.5729 | 0.1544 | |||
|
LSD (P 0.05) |
1.1452 | 0.3085 | |||
Note: The alphabetical letters are showing the same homogenous groups in column are not significant with each other.
The better understanding with regards to variability within pathogen population for traits, survival and fitness in different agro-ecological regions, definitely may support in design improved management strategies (Kaur et al., 2012). In our study, thirty two M. phaseolina isolates were obtained from infected plant samples of sunflower collected from different ten districts viz., Badin, Thatta, Hyderabad, Tando Muhammad Khan, Nawabshah, Mirpurkhas, Sanghar, Dadu, Sukkur and Khairpur. Significant variation in characteristics was noticed among collected isolates. In current study, the M. phaseolina population density was found highest in the fields of sunflower. Higher intensity of charcoal rot of sunflower caused increase in the mortality rate of the plants. Such results are in consistency with the previously findings. The disease severity has been influenced by the population of infective sclerotia in the soil (Khan, 2007). The disease becomes more economically important and encouraged by dry condition and hot season (Schwartz and Gent, 2016).
Pathogenicity test conducted using HO-1 cultivar showed that all isolates were pathogenic in nature. They could infect the sunflower plants and caused mortality after inoculation. MPS16 was found highly pathogenic in nature. Furthermore, there was significant variation in genetic diversity in MPS16 with other isolates and was found most destructive based on pathogenicity test. Previously, M. phaseolina showed three types of colony growth (dense, feathery and restricted). Chlorate resistant isolates produced dense growth on medium containing potassium chlorate, whereas, chlorate sensitive isolates produced feathery and restricted growth on medium containing potassium chlorate (Atiq et al., 2001). Linhai et al. (2011) characterized 35 isolates of M. phaseolina isolates from sesame on the base of colour produced by density of aerial mycelia, sclerotia quantity, sclerotium size and growth speed of colony. Similarly, Saleh et al. (2010) evaluated the degree of populations of M. phaseolina by comparing 143 isolates from maize, sorghum, soybean fields and from eight plant species of tall grass prairie. Growth phenotypes of isolates were calculated on medium containing chlorate. They observed dense phenotype of the medium obtained from sorghum; whereas, either feathery or restricted phenotypes from other hosts. Likewise, Similarly, Mahdizadeh et al. (2011) recovered fifty two M. phaseolina isolates from 24 host plant species during a survey of 14 Iranian provinces. The colony characteristics of each isolate were noted on the basis of chlorate phenotype, relative growth rate at 30 0 C and 37 0 C, average size of microsclerotia and time to microsclerotia formation. They found that feathery colony phenotype was the most common (63.7%) on the chlorate selective medium. While, isolates did not clearly differentiate to the specific group according to the host or geographical origins, however, generally the isolates from the same host or the same geographic origin tend to group nearly. M. phaseolina isolates were identified on the basis of morphology characteristic and their thermophilic nature. The pycnidia were sub spherical to flask shaped, dark brown, papillate at ostioles and 120-440 µm in diameter. Conidia which were produced from phialides on the inner surface of the pycnidial wall were ellipsoid to obovoid, aseptate, hyaline, smooth and 18-34 × 6-10 µm in size. Sclerotia were sub spherical hard, smooth, 50-140 µm in diameter (Sato et al., 1999). Furthermore, Jana et al. (2003) discussed genetic variation of 43 isolates of M. phaseolina collected from geographically distinct regions of India over a range of hosts. Karunanithi et al. (1999) described cultural and pathogenic variation of isolates of M. phaseolina into three groups. Isolates were significantly varied in ability to produce sclerotia. Our results are in continuity with the above findings that all collected isolates were producing various colour, density and pattern of mycelial growth and were able to cause the infection to host plant. The highly pathogenic isolate was used for further studies.
Conclusions and Recommendations
Based on current findings it is concluded that Macrophomina phaseolina causing charcoal rot of sunflower is major fungal pathogen which can survive in soil and plant debris. There was significant variation among cfu per gram of M. phaseolina in soil and plant samples. Significantly maximum cfu per gram of M. phaseolina from infected plant and infested soil was recorded at Badin and Thatta; however, the lower values for these parameters were recorded at Sukhar, Dadu and Khairpur during both growing seasons of 2016 and 2015. MPS16 isolate collected from fields of Badin showed higher aggressiveness in term of plant mortality and length of necrotic lesions. The phylogenetic analysis of virulent isolates MPS16 based on internal transcribed spacer (ITS) regions showed high identity with the sequences of Macrophomina sp., from the NCBI database (100%).
Acknowledgements
The authors acknowledge the administrative staff of the Department of Plant Pathology, Sindh Agriculture University, Tandojam 70060-Pakistan for providing laboratory facilities.
Author’s Contribution
KHW, designed and conducted experiments and prepared manuscript; MIK, designed experiment and prepared manuscript; JH, analyzed data and prepared manuscript; AGL, prepared manuscript. All authors read and approved the final manuscript.
References
Aboshosha, S.S., S.I. Atta-Alla, A.E. El-korany and E. El-Argawy. 2007. Characterization of Macrophomina phaseolina isolates affecting sunflower growth in El-Behera Governorate, Egypt. Int. J. Agric. Biol. 9(6): 807-815.
Almomani, F., M. Alhawatema and K. Hameed. 2013. Detection, identification and morphological characteristic of Macrophomina phaseolina: the charcoal rot disease pathogens isolated from infected plants in Northern Jordan. Arch. Phytopathol. Plant Protect. 46(9): 1005–1014. https://doi.org/10.1080/03235408.2012.756174
Ashraf, W., S.T. Sahi, I. Haq and S. Ahmed. 2015. Morphological and pathogenic variability among Macrophomina phaseolina isolates associated with maize (Zea mays) in Punjab-Pakistan. Int. J. Agric. Biol. 17: 1037‒1042.
Atiq, M., A. Shabeer and I. Ahmed. 2001. Pathogenic and cultural variations in Macrophomina phaseolina, the cause of charcoal rot in sunflower. Sarhad J. Agric. 17(2): 253-255.
Dwivedi, S.K., R.S. Dwivedi and V.P. Tewari. 1990. Studies on pathogenic fungi inciting guava wilt in Varanasi. India. Phytopathol. 43(1): 116-117.
Gupta, G.K. and G.S. Chauhan. 2005. Symptoms, identification and management of soybean diseases. Technical bulletin 10. Nat. Res. Centre Soybean, Indore, India.
Gupta, G.K., S.K. Sharma and R. Ramteke. 2012. Biology, epidemiology and management of the pathogenic fungus Macrophomina phaseolina (Tassi) Goid with special reference to charcoal rot of soybean (Glycine max (L.) Merrill). J. Phytopathol. 160: 167-180. https://doi.org/10.1111/j.1439-0434.2012.01884.x
Iqbal, U. and T. Mukhtar. 2014. Morphological and pathogenic variability among Macrophomina phaseolina isolates associated with mungbean (Vigna radiata L.) Wilczek from Pakistan. Sci. World J. 1-9. https://doi.org/10.1155/2014/173869
Jana, T, T.R. Sharma, R.D. Prasad and D.K. Arora. 2003. Molecular characterization of Macrophomina phaseolina and Fusarium species by a single primer RAPD technique. Microbiol. Res. 158(3): 249–257. https://doi.org/10.1078/0944-5013-00198
Karunanithi, K., M. Muthusamy and K. Seetharaman. 1999. Cultural and pathogenic variability among the isolates of Macrophomina phaseolina causing root rot of Sesamum. Plant Dis. Res. 14(2): 113-117.
Kaur, S., G.S. Dhillon, S.K. Brar, G.E. Vallad, R. Chand and V.B. Chauhan. 2012. Emerging phytopathogen Macrophomina phaseolina: biology, economic importance and current diagnostic trends. Crit. Rev. Microbiol. 38(2): 136-151. https://doi.org/10.3109/1040841X.2011.640977
Khan, S.N. 2007. Macrophomina phaseolina as causal agent for charcoal rot of sunflower. Mycpah. 5(2): 111-118.
Krizmanic, M., I. Liovic, A. Mijic and M. Bilandzic. 2004. Sunflower breeding and seed production in the Agricultural Institute Osijek. Sjemenarstvo. 21 (5/6): 249-260.
Kushwaha, C., N. Rani and A.P. Bhagat. 2017. Nature, dissemination and epidemiological consequences in charcoal rot pathogen Macrophomina phaseolina. The Phytopathogen: Evol. Adaptation. 13: 978-1-315-36613-5.
Linhai, W., Z. Yanxin, Li Donghua, H. Junbin, W. Wenliang, L. Haixia and Z. Xiurong. 2011. Variations in the isolates of Macrophomina phaseolina from sesame in China based on amplified fragment length polymorphism (AFLP) and pathogenicity. Afr. J. Microbiol. Res. 5(31): 5584-5590.
Lodha, S., S.K. Sharma and R.K. Agarwal. 2002. Inactivation of Macrophomina phaseolina propagules during composting and effect of composts on dry rot severity and on seed yield of cluster bean. Eur. J. Plant Pathol. 108(3): 253-261. https://doi.org/10.1023/A:1015103315068
Mahdizadeh, V., N. Safaie and E.M. Goltapeh. 2011. Diversity of Macrophomina phaseolina based on morphological and genotypic characteristics in Iran. Plant Pathol. J. 27(2): 128-137. https://doi.org/10.5423/PPJ.2011.27.2.128
Mihail, J.D. and S.M. Alcorn. 1982. Quantitative recovery of Macrophomina phaseolina Sclerotia from Soil. Plant Dis. 66: 662-663. https://doi.org/10.1094/PD-66-662
Mirhendi, H., K. Diba, A. Rezaei, N. Jalalizand, L. Hosseinpur and H. Khodadadi. 2007. Colony-PCR is a rapid and sensitive method for DNA amplification in yeasts. Iran. J. Publ. Health. 36(1): 40-44.
Reis, E.M., C. Boaretto and A.L.D. Danelli. 2014. Macrophomina phaseolina: density and longevity of microsclerotia in soybean root tissues and free on the soil, and competitive saprophytic ability. Summa Phytopathol. 40: 128-133. https://doi.org/10.1590/0100-5405/1921
Riaz, A., S.H. Khan, S.M. Iqbal and M. Shoaib. 2007. Pathogenic variability among Macrophomina phaseolina (Tassi) Goid, isolates and identification of sources of resistance in mash against charcoal rot fungus, Macrophomina phaseolina. Phytopathol. 91 (2): 120–126.
Saleh, A.A., H.U. Ahmed, T.C. Todd, S.E. Travers, K.A. Zeller, J.F. Leslie and K.A. Garrett. 2010. Relatedness of Macrophomina phaseolina isolates from tall grass prairie, maize, soybean and sorghum. Mol. Ecol. 19: 79-91. https://doi.org/10.1111/j.1365-294X.2009.04433.x
Sato, T., K. Tomioka, T. Nakanishi and H. Koganezawa. 1999. Charcoal rot of yacon (Smallanthus sonchifolius (Poepp. et Endl.) H. Robinson), oca (Oxalis tubersa Molina) and pearl lupin (Tarwi, Lupinus mutabillis sweet) caused by Macrophomina phaseolina (Tassi) Goid. Bulletin of Shikoku. Nat. Agric. Exp. Station, 64: 1-8.
Schwartz, H.F. and D.H. Gent. 2016. High plains integrated pest management. https:// www.wiki. bugwood. Org/HPIPM: charcoal rot sunflower. 05.12.2016.
Short, G.E., T.D. Wyllie and P.R. Bristow. 1980. Survival of Macrophomina phaseolina in soil and residue of soybeans. Phytopathol. 70: 13-17. https://doi.org/10.1094/Phyto-70-13
Suriachandraselvan, M. and K. Seetharaman. 2000. Survival of Macrophomina phaseolina, the causal agent of charcoal rot of sunflower in soil, seed and plant debris. J. Mycol. Plant Pathol. 30(3): 402-405.
Wagan, K.H., M.I. Khaskheli, J.D. Hajano and A.G. Lanjar. 2018. Isolation and characterization of Macrophomina phaseolina isolates prevailing in Sindh, Pakistan. Pure Appl. Biol. 7(4): 1309-1315. https://doi.org/10.19045/bspab.2018.700152
To share on other social networks, click on any share button. What are these?








