Physicomorphic Response of Polyphagous Helicoverpa armigera Hübner (Lepidoptera: Noctuidae) Towards Different Host Plants
Physicomorphic Response of Polyphagous Helicoverpa armigera Hübner (Lepidoptera: Noctuidae) Towards Different Host Plants
Sajjad Ali1,*, M. Irfan Ullah2, Asif Sajjad1, Muhammad Zeeshan Majeed2, M. Aslam Farooqi1, M. Shahid Rizwan3, Qaiser Shakeel4, Sohail Akhter1, Muhammad Raheel4 and Muhammad Arshad2
1Department of Entomology, UCA&ES, The Islamia University of Bahawalpur, 63100, Bahawalpur
2Department of Entomology, University of Sargodha, 40100, Sargodha
3Cholistan Institute of Desert Studies, The Islamia University of Bahawalpur, 63100, Bahawalpur
4Department of Plant Pathology, UCA&ES, The Islamia University of Bahawalpur, 63100, Bahawalpur
ABSTRACT
How fitness of herbivore insects alters with different host plants in terms of their physicomorphic attributes has been the subject of great interest with point of their integrated pest management. Helicoverpa armigera (Noctuidae; Lepidoptera) -being highly polyphagous- is the pest of many crops and exhibits high fecundity and migrating efficiency. The present study aimed to evaluate its physicomorphic responses towards different host plants. The highest larval and pupal weights were observed when fed on gram, Cicer arietinum (L.). The maximum (97.23%) larval survivorship was recorded on gram while, the lowest (56.25%) on okra, Abelmoschus esculentus (L.). The consumption index (CI) was also observed to be the highest (1.49 %) in gram and the lowest (0.95 %) in tomato, Lycopersicon esculentum (L.) The sizes of fore and hind wings, head, femur and tibia were the maximum in the individuals fed on gram. Furthermore, physiological parameters of the adults were also significantly better when larvae were fed on gram. The relative growth rate (RGR) and relative consumption rate (RCR) values were the maximum (2.09 mg/mg/day and 10.14 mg/mg/day, respectively) in individuals fed on gram. Therefore, the efficiency of conversion of ingested food (ECI) was the highest (70.65%) in gram feeding as compared to other host plants. The areas of fore and hind wings (0.98 cm2 and 0.79 cm2) and hind tibia length (0.82 cm) were also greater in the adults fed on gram. Among natural host plants, gram was proved to be the highly nutritious food plant facilitating both the development and the survival effectiveness of this pest. Therefore, it is recommended to carefully include gram in a cropping scheme especially where H. armigera is a regular pest whilst future studies should focus on demographic and nutritional parameters of this pest as affected by different host plants.
Article Information
Received 13 April 2018
Revised 22 June 2019
Accepted 6 August 2019
Available online 18 May 2020
Authors’ Contribution
SA and MIU designed the study and wrote the article. SA and MA performed experimental work. AS, SA and MAF collected and analyzed the data. MSR, MZM, QS and MR helped in experimental work.
Key words
Helicoverpa armigera, Host plant utilization, Physicomorphic response, Feeding response, Herbivory, Polyphagous.
DOI: https://dx.doi.org/10.17582/journal.pjz/20180413130416
* Corresponding author: sajjad.ali@iub.edu.pk
0030-9923/2020/0005-1833 $ 9.00/0
Copyright 2020 Zoological Society of Pakistan
Introduction
Suitability of host plants among an array of available flora varies with insect species especially in lepidopteran insects in terms of female fitness (Du et al., 2016). Their larval development -morphology and physiology- is largely predictd by food source and environmental factors (Shanower et al., 1999). It is well established that insects exhibit stronger response towards genetic background of host plants than the external factors i.e. phenotypic response (Rajapakse and Walter, 2007). Although, polyphagous insects are adapted to a wide range of host plants, but their fitness may differ with plant species (Scriber, 2002). Phenotypic plasticity of polyphagous herbivores is a major factor in the diversification of their life and successful utilization of host plants (Görür, 2000).
Helicoverpa armigera (Lepidoptera: Noctuidae) is one of the major insect pests of many crops and causes considerable economic losses worldwide (Sharmad et al., 2005). Its preference to feed on harvestable parts of host plants, higher fecundity, increased mobility, migrating potential, polyphagous nature and ability to develop resistance against a number of insecticides makes it a major pest of many field crops (Du et al., 2016). More than 172 host plant species from 68 different families serve as its host (Cunningham and Zalucki, 2014) including okra, tomato, gram, cotton and pigeonpea and some other crops like pearl millet, maize, sorghum, groundnut and tobacco (Rajapakse and Walter, 2007). Its attack in Pakistan leads to 32-53% losses in many crops annually (Abbas et al., 2015). Gram, being one of the major crops is also attacked by this pest, leading to 6-20% losses each year in spite of many insecticidal applications (Ahmed et al., 2004).
Larvae of H. armigera prefer to feed and develop on reproductive plant parts containing rich nutritional components (Fitt, 1989) thereby cause direct damage to flowering and fruiting parts. Before reaching the pupal stage, the larvae can consume several fruits (Nadeem et al., 2010). Host plant diversity is an important tool to regulate insect pest populations in an ecosystem. Amassed plant species richness is helpful in reducing insect herbivory risks because specific host plant resistance plays significant role in minimizing of crop losses (Castagneyrol et al., 2014).
The resistance offered by host plants can significantly contribute towards management of insect pests by altering their preference, lifecycle and biology (Sarwar et al., 2011). In particular, the host plants exhibiting the phenomenon of antixenosis and antibiosis usually influence the physicomorphic traits of insects in a number of ways e.g. reduction in body size, weight, longevity, reproduction, survival, ingestion and food digestion and fitness besides prolonged developmental time (Sharmad et al., 2005, Marchioro and Foerster, 2014).
How physiological and morphological (physicomorphic) characters of insects are influenced by host plats at species level may give a better insight of utilizing most suitable crops and cultivars in a pest management program. Since consumption and utilization of different host plants affect the nutritional and chemical responses of insect pests, their nutritional physiology and growth responses are important to be addressed in a good pest management program. Current study was conducted in the same context, aiming to evaluate the effects of different natural host plants; Okra (Abelmoschus esculentus L.), tomato (Lycopersicon esculentum L.) and gram (Cicer arietinum L.) -including an artificial diet- on the physicomorphic characters of H. armigera.
Materials and methods
The experiments were conducted at the laboratory of the Department of Entomology, University College of Agriculture and Environmental Sciences, The Islamia University of Bahawalpur (IUB), Pakistan.
Insect culture
The adults of H. armigera were collected by using light traps from the infested okra (A. esculentus) grown at the research farm of IUB campus within the coordinates of 29.3544°N, 71.6911°E. The adults were allowed to mate, and eggs were collected for rearing. The newly hatched larvae were shifted into 100 ml plastic containers kept under laboratory conditions (25±2oC and 75±5% RH) and fed on artificial diet until they reached up to the pupal stage. The artificial diet was prepared in laboratory with the following ingredients: ascorbic acid (4.7g), methyl-4-hydroxe benzoate (3g), sorbic acid (1.5g), streptomycin (1g), yeast (48g), agar (7.5g), linseed (12ml) and vitamins mixture (Vanderzants NBC) (5ml) mixed in 500ml distilled water. The adults were transferred to glass cages (40 x 30 cm); coated internally with white standard paper for oviposition; fed with sugar solution (3:1); kept under room conditions (25±2oC and 75±5% RH). The eggs were collected and placed in Petri plates in laboratory conditions until the hatching of caterpillars; thus, keeping up the complete cycle for constant supply of individuals at the same age in the further studies.
Host plants
Okra, tomato, and gram plants were grown in earthen pots under greenhouse condition. The average minimum and maximum temperatures of greenhouse ranged from 20 to 32oC. Sowing was done in February with mineral fertilization for okra (150-100-150 N-P-K kg ha-1), tomato (100-90-60 N-P-K kg ha-) and for gram (20-60-60 N-P-K kg ha-) (Akhtar et al., 2017; Siddiq et al., 2009; Moniruzzaman et al., 2007). Plants were irrigated twice a week to avoid drought stress. No insecticide was applied during experimentation. Healthy leaves from three host plants were collected and brought into laboratory in wet polythene bags.
Insect growth and feeding indices parameters
The same sized second instar larvae were isolated from the rearing culture. One larva was released into single Petri dish containing leaves of either host plant or artificial diet. Leaves of tomato and additionally the pods in case of gram were weighed before offering to larvae. All the treatments were randomized with a completely randomized design (CRD) and replicated four times with 12 larvae in each replicate. The natural and artificial diets were changed on daily basis and the physicomorphic response of larvae was studied.
The data for larval weight (g), length (cm), diet weight (g), faeces weight (g) were recorded before and after the feeding at an interval of 24 h to calculate feeding indices parameters by using high precision balance (Shimadzu Scientific Instruments, ATX/ATY Unibloc Analytical Balance, Japan) with a range of 200g/0.0001g. Caterpillar length (cm) was measured using a measuring scale. The pupal weight (g) was also recorded on the day of pupae formation. Equations to calculate growth indices were derived from suggestions of Waldbauer (1968) as follows:
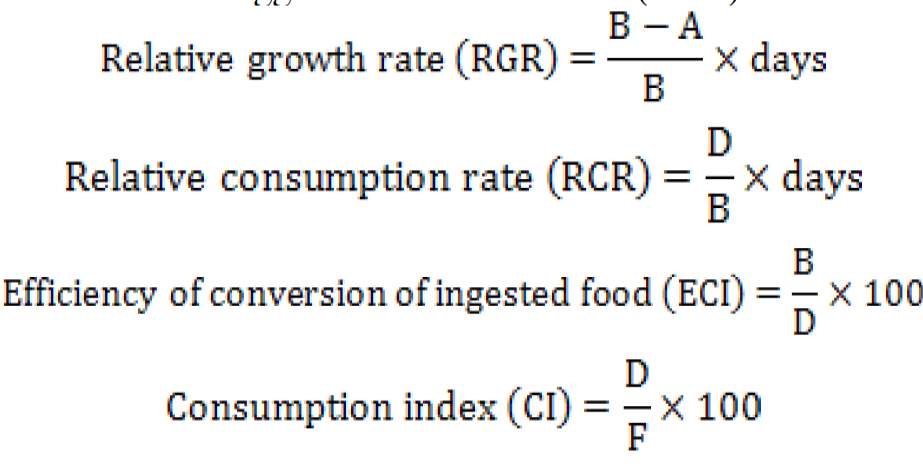
Where, A is the mean weight (g) of the insects on last day, B is the original mean weight of insects (g), D is food biomass ingested (g) per insect and F is faeces biomass produced (g) per insect.
Morphological parameters
After emergence, 15 adults were isolated from each treatment; killed by potassium cyanide to observe their morphometric variations. The wings, head and legs of each selected adult were separated carefully and mounted onto glass slides. Then, slides mounted specimen/body parts were digitally photographed using ultra-small high-performance zoom lens at 100 X magnification of stereo microscope (Micros, Austria). Measurements of different morphological parameters (fore and hind wing areas, head width, tibia and femur lengtsh) were made on a computer-using image J (2.0 UNIX) software. The desired portions of images were selected and colored. The lengths and areas were measured in centimeter in spreadsheet for further analysis.
Data analyses
Data were analyzed using one-way analysis of variance (ANOVA) to compare feeding indices parameters and morphometric variation of H. armigera between host plants. All linear measurements were log-transformed for analysis. For morphometric variation the measurements were analyzed with the null hypothesis that there was no significant difference among measurement of H. armigera on different diets Means were compared with Tukey’s HSD (honestly significant difference) test using MINITAB 16.1 software.
Results
Feeding indices parameters
The results of present study showed that diets had significant differences in relative consumption rate (RCR) (F= 66.9, P<0.05) and relative growth rate (RGR) (F= 116, P<0.001) of H. armigera. However, no significant difference of efficiency of conversion of ingested food (ECI) (F= 2.47, P>0.05), and consumption index (CI) (F= 2.80, P>0.05), of H. armigera was found for different diets.
The highest RGR (2.09 mg/mg/day) of H. armigera was observed when feeding on gram host plant while the lowest (0.44 mg/mg/day) was on okra host. Similarly, the RCR and ECI were also indicated the similar trend and the values for RCR (10.14 mg/mg/day) and ECI (70.65 %) were found maximum when larvae were fed on gram. The minimum RCR and ECI values were recorded on okra host as 3.96 mg/mg/day and 66.05 % respectively. The consumption index (CI) mainly refers to the dry mass of food consumed per insect. The highest CI (1.49 %) was observed for gram plants and the lowest CI (0.95 %) was recorded during feeding on tomato plants (Table I).
Table I.- Feeding indices parameters (Means±SE) for H. armigera larvae in response to feeding on different host plants and artificial diet.
|
Diet |
RGR (mg/mg/day) |
RCR (mg/mg/day) |
ECI (%) |
CI (%) |
|
Tomato |
0.96±0.15c |
5.91±0.71c |
67.74±1.21a |
0.95±0.114b |
|
Okra |
0.44±0.09d |
3.96±0.65d |
66.05±1.14a |
0.99±0.082b |
|
Gram |
2.09±0.32a |
10.14±0.99a |
70.65±1.65a |
1.49±0.152a |
|
Artificial |
1.22±0.18b |
7.25±0.82b |
69.32±1.43a |
1.31±0.134ab |
Means in columns sharing similar letters are not significantly different according to Tukey HSD at P>0.05.
Growth parameters
The results showed that larval weight was significantly (F= 9.63, P<0.001) different according to the diet. However, no significant difference of larval length (F= 1.93, P>0.05), and pupal weight (F= 1.80, P>0.05) of H. armigera were found feeding on different diets. Both natural host plants and artificial diet affected the larval growth and pupal stage differently. The larval weight was found highest (0.38g) by feeding on gram leaves followed by artificial diet (0.32g) and okra (0.26g). The larval weight was found lower (0.21g) in case of tomato leaves. While, there was no significant difference (P>0.05) in the larval length as a response to feeding on the same food sources. Despite that there were no significant differences on pupal weight, it was observed that the maximum pupal weight (0.22 g) was gained by feeding on gram and the minimum pupal weight (0.14 g) was recorded on tomato plant feeding. The artificial diet and okra plant had almost similar effects on pupal weights (0.19 g, 0.18 g), respectively (Fig. 1).
Larval survivorship (%)
The results for larval survivorship feeding on different diets also showed significance (F= 60.3, P<0.001). Highest larval survivorship (97.23 %) was recorded when larvae fed on gram host plant followed by 88.6% on artificial diet. The lowest survival rate (62.39 %) was observed during feeding on okra plants (Fig. 2).
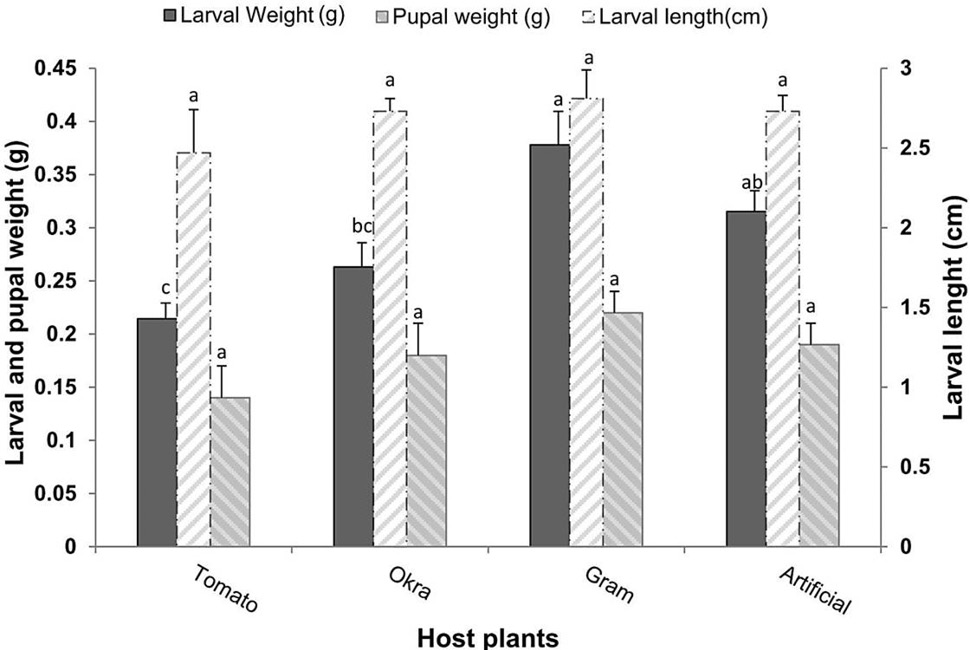
Fig. 1. Larval and Pupal weights and larval length (Means±SE) of H. armigera in response to feeding on different host plants and artificial diet, Pupal weight and larval length are not significant at P > 0.05.
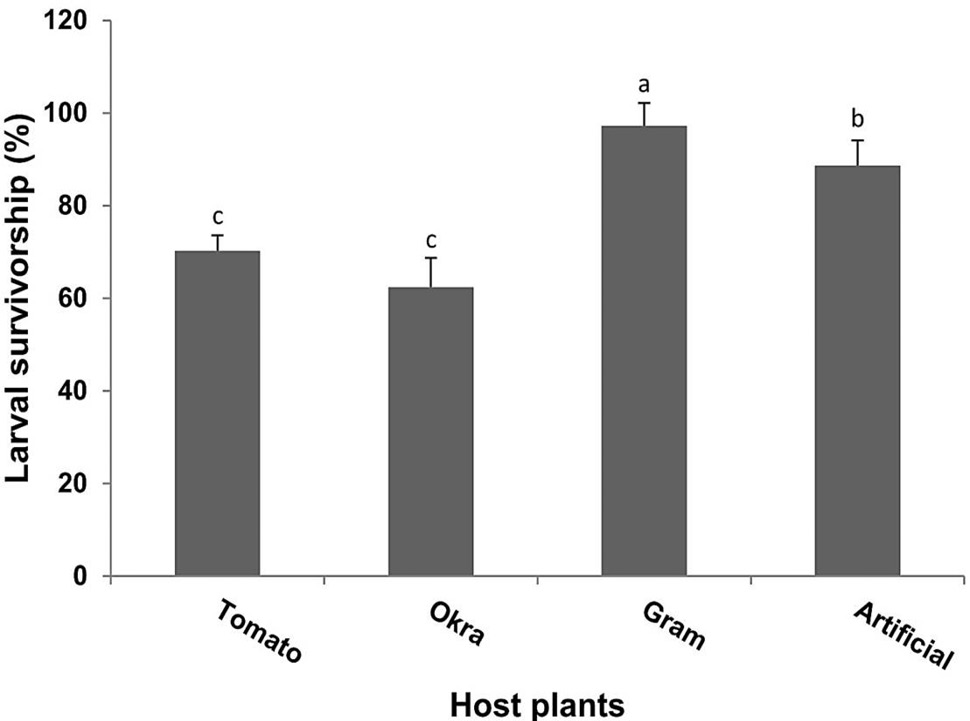
Fig. 2. Percent larval survivorship (Means±SE) of H. armigera in response to feeding on different host plants and artificial diet same letters representing the bars are not significant according to Tukey (HSD) test at P > 0.05.
Morphological characters
Significant variations of fore and hind wing area (F=8.55, P<0.05; F=13.0, P<0.001, respectively) and tibia length (F=47.1, P<0.001) of H. armigera adults were observed. However, no significant variations in the length of femur (F=0.83, P>0.05) and head (F=0.37, P>0.05) were observed among the treatments (Fig. 3). The values for all the morphological characters (areas of fore and hind wings, lengths of head, femur and tibia of hind legs) were observed higher in the individuals that emerged from the pupae of those larvae, which fed on gram plants followed by artificial diet and okra plants. The areas of fore and hind wings (0.98 cm2 and 0.79 cm2) and lengths of tibia (0.82 cm) were higher in the adults feeding on gram plant leaves. The lowest morphological developments were observed during feeding on tomato plants. This has indicated that tomato is the least suitable host plant for H. armigera developmental physiology among the provided food sources (Fig. 3).
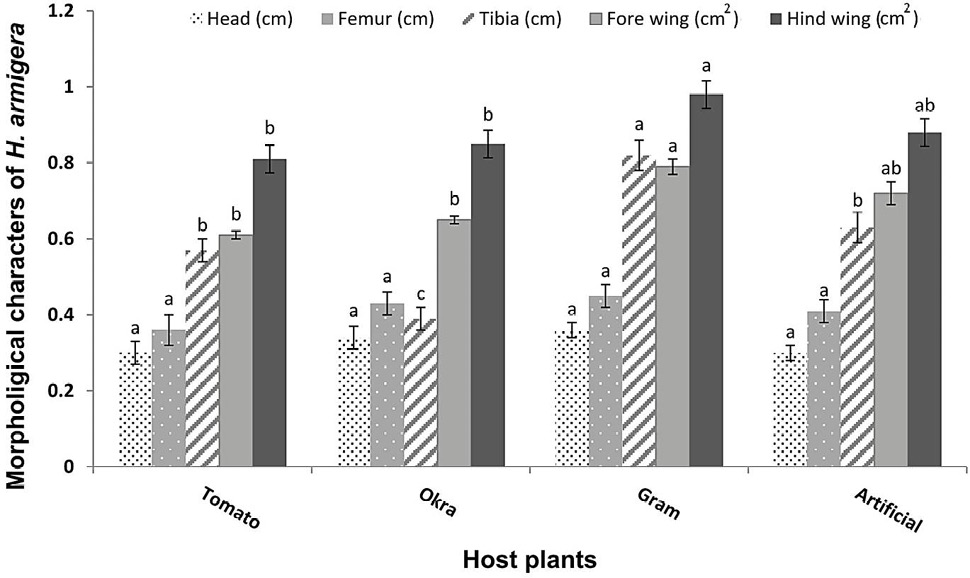
Fig. 3. Variations among morphological character measurements (cm2/cm) (Means±SE) of H. armigera adults in response to feeding on different host plants and artificial diet (Treatments showed significance only for tibia length and areas of fore wing and hind wing at P < 0.001).
Discussion
The variations of host plants might exert selection pressure on the insect populations, coming up with differential changes in their physiology and morphology as induced by the new food preferences (Barros et al., 2010). As H. armigera is highly polyphagous, it has ability to modify certain aspects of its life history, which may affect its ability to utilize different host plants. The differences in larval survivorship and development in H. armigera -on account of different host plants and artificial diet- is the function of difference in nutritional quality, chemical stress, antibiotic effects and secondary plant biochemicals of different host plants (Satpute et al., 2005). The best larval and pupal performance was also depicted in terms of their weights on gram leaves. This indicates the preference of H. armigera towards gram plants for oviposition and most of its population development in different crop combinations (Sequeira et al., 2001), which can be a significant constraint in gram production.
The body growth is an important biological indicator among insect population as determined by food consumption and utilization (Hosseininejad et al., 2015). Feeding indices contributes to the determination of relative growth rate, relative consumption rate and efficiency of conversion of ingested food. Feeding indices contribute to identify the resistant cultivars to implement insect pest control strategies including cultural and insecticidal control. The feeding indices for H. armigera were maximum in case of larval feeding on gram plants as compared to other diets. It is inferred that gram plant provided more nutritious diet and most suitable ingredients to be digested for improved body growth to the larvae of H. armigera for better biological performance (Liu et al., 2004). Gram is considered as one of the most suitable hosts for H. armigera as growth of H. armigera was enhanced when fed on it. So, prior to using gram as a trap crop for H. armigera management, its preference and performance should be tested on gram in field conditions. Reduced feeding indices parameters may lead to delayed larval growth, longer larval span and smaller sized pupae, which can directly influence the fecundity and longevity subsequently in adults (Sogbesan and Ugwumba, 2008). But, H. armigera has enough phenotypic plasticity to adapt and develop on all the plants studied in these experiments. All studied parameters were enough to support the highest consumption index in case of gram as a host plant in comparison to other diets. The larvae, feeding on gram as highly nutritious diet, develop more rapidly and complete their life span quickly as compared to the other diets (Hwang et al., 2008).
Physiological responses have great impact on the morphological characters as well and always depicted in terms of morphological changes (Ho and Pennings, 2013) and morphometric analysis of insect wings, head, femur and hind tibia can be used to predict the suitability of host plants as a substitute indicator for growth and development into an adult (Musundire et al., 2012). The morphological characters like wing areas, lengths of head, femur and tibia were also in accordance with the physiological outcome of the larvae who fed on gram plants with the highest recorded values while the lowest morphological developments were observed on tomato host plant (Ballabeni et al., 2003).
When the quantity of ingested food decreases, the duration of development is extended, and the insect becomes smaller and lighter as we had observed in case of tomato host. Kotkar et al. (2009) reported that legumes such as gram and pigeon pea had the highest protein contents while tomato had very low protein content, which may lead to lower consumption of tomato plants by H. armigera. Further, Liu et al. (2004) reported that tomato is a less suitable host plant for H. armigera larvae as compared to cotton, corn, hot pepper, tobacco, and common bean plants. These findings suggest that physicomorphic compensatory responses towards food quality is determined by host plant nutritional composition and its defense chemicals, which ultimately enhance or reduce the larval performance whilst adult characters are mainly depended upon larval performance (Couture et al., 2016).
Conclusion
In conclusion, the most suitable host plant can improve insect physiological and morphological fitness by providing balanced nutrition. Analysis of nutritional indices helps understand the behavioral and physiological basis of insect response towards host plants. Our results suggest that the gram plant is more suitable for increasing H. armigera population. Future studies should focus on demographic and nutritional parameters of this pest under laboratory and field conditions on different host plants.
Acknowledgements
The authors are thankful to Dr. Muhammad Afzal, Dean Faculty of Agriculture, University of Sargodha, for his support and facilitation to this study.
Statement of conflict of interest
The authors declare that there is no conflict of interests regarding the publication of this article.
References
Abbas, G., Hassan, N., Farhan, M., Haq, I. and Karar, H., 2015. Effect of selected insecticides on Helicoverpa armigera Hubner (Lepidoptera: Noctuidae) on tomato (Lycopersicon esculentum Miller) and their successful management. Adv. Ent., 3: 16-23. https://doi.org/10.4236/ae.2015.31003
Ahmed, S., Zia, K. and Shah, N.U.R., 2004. Validation of chemical control of gram pod borer, Helicoverpa armigera (Hub.) with new insecticides. Int. J. agric. Biol., 6: 978-980.
Akhtar, M.F.Z., Jamil, M., Ahamd, M. and Abbasi, G.H., 2017. Evaluation of biofertilizer in combination with organic amendments and rock phosphate enriched compost for improving productivity of chickpea and maize. Soil Environ., 36: 59-69.
Ballabeni, P., Gotthard, K., Kayumba, A. and Rahier, M., 2003. Local adaptation and ecological genetics of host-plant specialization in a leaf beetle. Oikos, 101: 70–78. https://doi.org/10.1034/j.1600-0706.2003.12569.x
Barros, E.M., Torres, J.B., Ruberson, J.R. and Oliveira, M.D., 2010. Development of Spodoptera frugiperda on different hosts and damage to reproductive structures in cotton. Ent. exp. Appl., 137: 237–245. https://doi.org/10.1111/j.1570-7458.2010.01058.x
Castagneyrol, B., Jactel, H., Vacher, C., Brockerhoff, E.G. and Koricheva, J., 2014. Effects of plant phylogenetic diversity on herbivory depend on herbivore specialization. J. appl. Ecol., 51: 134–141. https://doi.org/10.1111/1365-2664.12175
Couture, J.J., Mason, C.J., Habeck, C.W. and Lindroth, R.L., 2016. Behavioral and morphological responses of an insect herbivore to low nutrient quality are inhibited by plant chemical defenses. Arthropod-Pl. Interac., 10: 341-349. https://doi.org/10.1007/s11829-016-9439-7
Cunningham, J.P. and Zalucki, M.P., 2014. Understanding Heliothine (Lepidoptera: Heliothinae) pests: What is a host plant? J. econ. Ent., 107: 881-896. https://doi.org/10.1603/EC14036
Du, Y., Zhang, J., Yan, Z., Ma, Y., Yang, M., Zhang, M., Zhang, Z., Qin, L. and Cao, Q., 2016. Host preference and performance of the yellow peach moth (Conogethes punctiferalis) on chestnut cultivars. PLoS One, 11: 1-17. https://doi.org/10.1371/journal.pone.0157609
Fitt, G.P., 1989. The ecology of Heliothis species in relation to Agroecosystems. Annu. Rev. Ent., 34: 17–53. https://doi.org/10.1146/annurev.en.34.010189.000313
Görür, G., 2000. The role of phenotypic plasticity in host race formation and sympatric speciation in phytophagous insects, particularly in aphids. Turk. J. Zool., 24: 63-68.
Ho, C.K. and Pennings, S.C., 2013. Preference and performance in plant-herbivore interactions across latitude-A study in U.S. Atlantic salt marshes. PLoS One, 8: 1–11. https://doi.org/10.1371/journal.pone.0059829
Hosseininejad, A.S., Naseri, B. and Razmjou, J., 2015. Comparative feeding performance and digestive physiology of Helicoverpa armigera (Lepidoptera: Noctuidae) larvae-fed 11 corn hybrids. J. Insect Sci., 15: 1–6. https://doi.org/10.1093/jisesa/ieu179
Hwang, S.Y., Liu, C.H. and Shen, T.C., 2008. Effects of plant nutrient availability and host plant species on the performance of two Pieris butterflies (Lepidoptera: Pieridae). Biochem. System. Ecol., 36: 505–513. https://doi.org/10.1016/j.bse.2008.03.001
Kotkar, H.M., Sarate, P.J., Tamhane, V.A., Gupta, V.S. and Giri, A.P., 2009. Responses of midgut amylases of Helicoverpa armigera to feeding on various host plants. J. Insect. Physiol., 55: 663–670. https://doi.org/10.1016/j.jinsphys.2009.05.004
Liu, Z., Li, D., Gong, P. and Wu, K., 2004. Life table studies of the cotton bollworm, Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae), on different host plants. Environ. Ent., 33: 1570–1576. https://doi.org/10.1603/0046-225X-33.6.1570
Marchioro, M. and Foerster, L.A., 2014. Preference‒performance linkage in the diamondback moth, Plutella xylostella and implications for its management. J. Insect Sci., 14: 85. https://doi.org/10.1673/031.014.85
Moniruzzaman, M., Uddin, M.Z. and Choudhury, A.K., 2007. Response of okra seed crop to sowing time and plant spacing in south eastern hilly region of Bangladesh. Bangladesh J. agric. Res., 32: 393-402. https://doi.org/10.3329/bjar.v32i3.541
Musundire, R., Chabi-Olaye, A. and Krüger, K., 2012. Host plant effects on morphometric characteristics of Liriomyza huidobrensis, L. and L. trifolii (Diptera: Agromyzidae). J. appl. Ent., 136: 97-108. https://doi.org/10.1111/j.1439-0418.2010.01597.x
Nadeem, S., Shafique, M., Hamed, M., Atta, B.M. and Shah, T.M., 2010. Evaluation of advanced chickpea genotypes for resistance to pod borer, Helicoverpa armigera (Hubner) (Lepidoptera: Noctuidae). Pak. J. agric. Sci., 47: 132-135.
Rajapakse, C.N.K. and Walter, G.H., 2007. Polyphagy and primary host plants: oviposition preference versus larval performance in the lepidopteran pest Helicoverpa armigera. Arthropod-Pl. Interac., 1: 17–26. https://doi.org/10.1007/s11829-007-9003-6
Sarwar, M., Ahmad, N. and Toufiq, M., 2011. Identification of susceptible and tolerant gram (Cicer arietinum L.) genotypes against gram pod borer (Helicoverpa armigera) (Hubner). Pak. J. Bot., 43: 1265-1270.
Satpute, N.S., Deshmukh, S.D., Rao, N.G.V., Nimbalkar, S.A., Birch, L.C., DeWitt, R.M., Howe, R.W., Laughlin, R., Nanthagopal, R., Uthamasamy, S., Wellik, M.J. and Pedigo, L.P., 2005. Life tables and the intrinsic rate of increase of Earias vittella (Lepidoptera: Noctuidae) reared on different hosts. Int. J. trop. Insect. Sci., 25: 73-79. https://doi.org/10.1079/IJT200561
Scriber, J.M., 2002. Latitudinal and local geographic mosaics in host plant preferences as shaped by thermal units and voltinism in Papilio spp. (Lepidoptera). Eur. J. Ent., 99: 225-239. https://doi.org/10.14411/eje.2002.032
Sequeira, R.V., Mcdonald, J.L., Moore, A.D., Wright, G.A. and Wright, L.C., 2001. Host plant selection by Helicoverpa spp. in chickpea-companion cropping systems. Ent. exp. Appl., 10: 1-7. https://doi.org/10.1046/j.1570-7458.2001.00884.x
Shanower, T.G., Romeis, J. and Minja, E.M., 1999. Insect pests of Pigeonpea and their management. Annu. Rev. Ent., 44: 77–96. https://doi.org/10.1146/annurev.ento.44.1.77
Sharmad, H.C., Pampapathy, G., Lanka, S.K. and Ridsdill-Smith, T.J., 2005. Antibiosis mechanism of resistance to pod borer, Helicoverpa armigera in wild relatives of chickpea. Euphytica, 142: 107–117. https://doi.org/10.1007/s10681-005-1041-5
Siddiq, S., Yaseen, M., Mehdi, S.A.R., Khalid, A. and Kashif, S.U.R., 2009. Growth and yield response of tomato (Lycopersicon esculentum mill.) to soil applied calcium carbide and l-methionine. Pak. J. Bot., 41: 2455-2464.
Sogbesan, A.O. and Ugwumba, A.A.A., 2008. Nutritional evaluation of Termite (Macrotermes subhyalinus) meal as animal protein supplements in the diets of Heterobranchus longifilis (Valenciennes, 1840) Fingerlings. Turk. J. Fish. aquat. Sci., 8: 149-157.
Waldbauer, G.P., 1968. The consumption and utilization of food by insects. Adv. Insect. Physiol., 5: 229-287. https://doi.org/10.1016/S0065-2806(08)60230-1
To share on other social networks, click on any share button. What are these?










