Nutritional Variation among Different Camel Browse Vegetations
Nutritional Variation among Different Camel Browse Vegetations
Asad Ali Khaskheli1*, Gulfam Ali Mughal1, Muhammad Ibrahim Khaskheli2, Gul Bahar Khaskheli3, Allah Jurio Khaskheli2 and Arshad Ali Khaskheli1
1Department of Animal Nutrition, Sindh Agriculture University, Tando Jam, Pakistan
2Department of Plant Protection, Sindh Agriculture University, Tando Jam, Pakistan
3Department of Animal Products Technology, Sindh Agriculture University, Tando Jam, Pakistan
4Department of Biotechnology, Sindh Agriculture University, Tando Jam, Pakistan
ABSTRACT
Present study was conducted at the Department of Animal Nutrition, Sindh Agriculture University, Tando Jam during the year 2018. Study was subjected to screen and assess different vegetations though browse by camels surrounding the area of Mithi (sandy desert zone), Tando Allahyar (irrigated zone) and Thatta (coastal mangroves zone) of Sindh province. The attributes like major nutrients (moisture, dry matter, total organic matter, inorganic/mineral matter (ash), ether extract, crude protein, crude fiber, nitrogen free extract and total carbohydrate contents) were included. Comprehensive survey indicated year round availability of 19 different vegetations at study areas whereby dry matter contents in Calligonum polygonoides (93.63%) recorded significantly high, and in Trifolium alexandrinum it was low, while moisture content appeared vice versa to dry matter. Organic matter contents in Senegalia senegal (94.05%) followed by Calligonum polygonoides (93.80%) and Acacia jacquemontii (92.45%) appeared considerably high. Capparis deciduas (22.41%) and Suaeda fruticosa (21.21%) both possessed relatively similar crude protein contents and found considerably high and Cordia sinensis Linn. at bottom level. Zea mays (5.75%) revealed prominently high and Salvadora oleiodes and Cyamopsis tetragonoloba found considerably low in ether extract contents. Carbohydrate contents in Capparis deciduas (66.76%), Trifolium alexandrinum (62.10%), Haloxylon salicornicum (61.19%), Cordia sinensis Linn. (56.66%) and Suaeda fruticosa (54.84%) did not vary to each other and found significantly high from that of Salvadora oleiodes and comparatively low from that of Calligonum polygonoides. Nitrogen free extract in Calligonum polygonoides (62.04%) compared to Ziziphus nummularia, Acacia jacquemontii, Acacia nilotica, Zea mays, Trifolium alexandrinum, Capparis deciduas, Haloxylon salicornicum, Cordia sinensis Linn., Suaeda fruticosa and Salvadora oleiodes existed considerably high. Crude fiber in Zea mays (29.30%) noted remarkably high, and in Alhagi maurorum, Cyamopsis tetragonoloba and Calligonum polygonoides it was considerably low. Total inorganic matter in Cordia sinensis Linn. (33.63%) and Salvadora oleiodes (31.14%) recorded remarkably at abundant level. Present sudy concludes that the Trifolium alexandrinum noted to be high moistured vegetation, Senegalia senegal appeared considerably rich in organic matter and Cordia sinensis (Linn.) in total inorganic matter. Capparis deciduas pertained considerable concentration of crude protein, Zea mays high ether extract, Calligonum polygonoides beared significantly high level of carobohydrate.
Article Information
Received 28 May 2019
Revised 30 July 2019
Accepted 11 September 2019
Available online 02 April 2020
Authors’ Contribution
GAM and GK designed the experiments. AAK performed the experiments and prepared the manuscript. MIK contributed reagents, materials and analysis tools. AJK performed statistically analysis of data. AAK collected samples of camel browse vegetations.
Key words
Camel browse vegetations, Browsing, Nutritional analysis
DOI: https://dx.doi.org/10.17582/journal.pjz/20190528110501
* Corresponding author: khaskhelias@gmail.com
0030-9923/2020/0004-1393 $ 9.00/0
Copyright 2020 Zoological Society of Pakistan
INTRODUCTION
The camel is one of the most typical and best adopted animals of the desert. This animal is capable of enduring thirst and hunger for several days. Due to drought-resistant ability camels (Camelus dromedarius) have become an important facet in the economy, ecology and culture of pastoralists, particularly under current climate and environmental changes (Krätli et al., 2013; Megersa et al., 2014). In desert nomads of Pakistan this animal act as a primary source of milk, meat and play an important role in the socioeconomic uplift of the local community by providing transport facility and acting as racing/dancing animal (Hesse and Cotula, 2006; Liaqat et al., 2017).
In Pakistan, camel generally depends on the natural vegetations for feeding. Compared to other livestock species, camels are typically adapted to hot and arid environments which are less suitable for the crop production, thus contributing considerably to the food security of pastoral households (Amin et al., 2011). They are reliable milk producers during dry seasons when other livestock species like cattle, goat and sheep have lower milk production levels. Keeping in view the role played by camels in pastoral livelihoods, it seems very necessary not to ignore their dietary requirements. In fact, the nutrients requirement of camels has significant influence on their milk, and meat production and their performance as resilient animals (Dokata, 2014). Camels mostly prefer to browse naturally grown forage plants of desert and semi desert areas including trees, shrubs and hard-thorny, bitter and halophytic (salty) plants. Further, camel diets also include herbs, forbs and grasses (Dorges and Heucke, 2003; Iqbal and Khan, 2001). More often than not, camels tend to be selective in their diet during the wet season when forages are plentiful but become indiscriminate in their forage preference during the dry seasons due to forage shortage (McLeod and Pople, 2008).
Normally, daily nutrients need of camel varies with the physiological functions of the body therefore, growth, fattening, gestation, lactation can be used as determining factors in order to estimate the daily nutrients demand. The deficiency of nutrients may leads to under nourishment, low productivity and predisposes the camels to parasitism, epidemics and breeding problems. The significance of forages for camel feeding is determined by their availability, palatability and nutrients composition (Khan, 2009). The most important objective in range management is animal production which totally depends on the nutritive value of accessible forages. There is very little information on the nutritive potential of forages in different arid and semiarid regions of the Pakistan. To preserve the optimum production and justifiable use of range resources in future the information regarding the availability, nutritive properties to maintain growth of animals and to assure the reasonable use of grazing lands are of utmost importance (Ganskopp and Bohnert, 2003).
Although some studies have been reported elsewhere on nutritional value of forage in natural rangelands and in cultivated fodder species (Nasrullah et al., 2003); no study exists for Sindh Province of Pakistan yet. This study was therefore focused to monitor the camel preferred forage species for their nutritive composition in desert, irrigated, coastal zone of Sindh Province of Pakistan.
MATERIALS AND METHODS
Location of study
The main location for conducting research on camel browse vegetations was Laboratory of Animal Nutrition, Faculty of Animal Husbandry and Veterinary Science, Sindh Agriculture University, Tando Jam, Sindh, Pakistan. Further, three different districts viz Mithi, Tando Allahyar and Thatta, respectively from ecological camel habitat zones like sandy desert, irrigated and coastal mangroves zones of Sindh province were included to monitor and collect samples of camel browse vegetations for current study.
Experimental procedure
Present study was conducted during the year 2018 whereby investigation was subjected into two phases. Firstly, comprehensive survey was performed at three different districts like Mithi, Tando Allahyar and Thatta of Sindh province in order to gather the data regarding availability of different camel browse vegetations. However, to assess the major nutrients of camel browse vegetations grown over three districts of different camel habitat zones of Sindh province representative samples were analyzed in the Laboratory of Animal Nutrition. A total of 19 different vegetations viz: Acacia nilotica, Ziziphus nummularia, Acacia jacquemontii, Prosopis juliflora, Cyamopsis tetragonoloba, Cordia sinensis Linn., Prosopis cineraria, Salvadora oleiodes, Capparis deciduas, Senegalia senegal, Sesamum indicum, Simmondsia chinensis, Calligonum polygonoides, Trifolium alexandrinum, Alhagi maurorum, Suaeda fruticosa, Haloxylon salicornicum, Tamarix passerinoides and Zea mays were sampled and brought to the laboratory of Animal Nutrition Department for assessing major nutrients, however composite sampling was performed in order to have replicated data.
Analysis of major nutrients of camel browse vegetations
Sample preparation
Fresh samples of different camel browse vegetations from respective location viz Mithi, Tando Allahyar and Thatta districts were dried in air-circulation oven at 60°C for overnight. Samples so obtained were ground to 1mm particle size using Wiley mill. Dried and ground samples were stored in air tight containers till further analysis (AOAC, 2000).
Moisture content
Moisture content was determined by evaporation method (Association of Official Analytical Chemists; AOAC, 2000). The washed aluminum dishes encoded with appropriate codes were moisture off in a hot air oven at 100±1°C for one hour. Sample of each camel browse vegetation (2g) was measured in pre-weighed empty dried aluminum dish and kept in hot air oven at 105±1°C for approximately 24hrs. It was then desiccated, weighed and re-dried in the hot air oven for further 30 min. It was again desiccated and weighed as before. The process of drying, desiccating and weighing were repeated till constant weight. The observed weight of sample with dish, dried sample with dish and empty dish were placed in the following formula was used to compute the percent of moisture content in camel browse vegetations:

Dry matter content
Dry matter of sample was analyzed using similar method as mentioned moisture. However, observations noted at each step were placed in the following formula to compute the percent of dry matter content in camel browse vegetations:

Inorganic/mineral (ash) matter
Inorganic/mineral (Ash) matter content was examined using Gravimetric method (AOAC, 2000). Sample (2g) in pre-weighed crucible was ignited in muffle furnace (600°C) for 6hrs, and thereafter, it was desiccated for one hour and the weight was taken. The concentration of ash was calculated by applying the following formula on observations recorded in each step.

Total organic matter
Total organic matter among camel browse vegetations was computed by difference method. Percent of inorganic/mineral (ash) matter was subtracted from hundred to calculate the percent of total organic matter (i.e. %Total organic matter = 100 – %inorganic matter).
Ether extract content
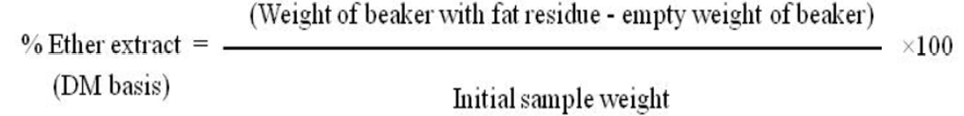
Ether extract content of camel browse vegetations was examined through Soxhlet method (AOAC, 2000). Ground sample (2 g) in thimble was extracted with diethyl ether (150 ml) into pre-weighed clean and dry fat beaker for six hrs. Fat Beaker was transferred to the Hot air oven to evaporate the residual ether. It was placed in desiccators and finally weighed. The percent of ether extract was calculated by using formula given below:
Crude protein content
Crude protein content was computed from nitrogen content present in sample, whereby it was firstly processed using Kjeldhal method (BSI, 1990). Sample (1g) was measured in Kjeldhal flask to which copper sulfate (0.2g) and sodium sulfate (2g) as catalyst were added. Further, sulfuric acid (25ml) as an oxidizing agent was delivered into similar flask and digested in Micro-Kjeldhal digester till solution became transparent. It was transferred into volumetric flask (250ml) and made up to mark with distilled water. Diluted sample (5ml) was distilled with 40% sodium hydroxide (5ml) using Micro-Kjeldhal distillation unit, where steam was distilled over 2 percent boric acid (5ml) containing an indicator for 3 minutes. The ammonia trapped in boric acid was titrated with 0.01N HCl, and the volume of HCl used was recorded. Parallel to this, a blank sample though containing only distilled water instead of sample was processed in similar manner as mentioned above. In the last, percent of nitrogen content present in camel browse vegetations was computed using formula given below:

Moreover, the percent of crude protein content in sample was calculated by multiplying the Nitrogen content obtained in the above procedure with conversion factor of 6.25.
% Crude protein = Nitrogen percent × 6.25
Crude fiber content
Crude fiber was determined using VanSoest method (AOAC, 2000). Ether extracted sample (2g) was transferred to the 600 ml beaker. Pre-heated H2SO4 having normality 0.2N was delivered into the beaker (200ml) and solution was gently boiled for about 30min. Contents of beaker were filtered through buchner funnel, beaker was rinsed with 50ml of boiling water. Residues were transferred back into the beaker containining boiled NaOH having normality 0.3N (200ml) and boiled exactly for 30min. Contents were filtered as above and washed with 25ml of boiling H2SO4 (0.2N) and then with 50ml H2O. The residues were dried at 65oC for about 24hrs and weighed. The residues were transferred into a clean, labeled and pre-weighed crucible and placed under muffle furnace (600oC) for 4hrs. Crucible containing sample was cooled in the desiccator and weighed using analytical weight balance. The observations recorded during this procedure were put in the following formula to compute the crude fiber percent.

Nitrogen free extract content
Nitrogen free extract in different camel browse vegetations was analyzed by difference method whereby sum of ether extract; crude protein; crude fiber and ash content was subtracted from Hundred i.e. Nitrogen free extract (%) = 100 – (%crude protein + % ether extract + % crude fiber + % mineral/ash content)
Total carbohydrate content
Percent of Nitrogen free extract and crude fiber was summed together to calculate the total carbohydrate content in different camel browse vegetations (i.e. % Total carbohydrate = % Nitrogen free extract + % crude fiber).
Statistical analysis
A computerized statistical package i.e. Student Edition of Statistix (SXW), Version 8.1 (Copyright 2005, Analytical Software, USA) was applied to assess the data. Statistical procedure of completely randomized analysis of variance (ANOVA) under linear models was used to observe the significant variations among the variables within district as well as between vegetations, and in case of the significant differences found among the means, the least significant difference (LSD) test was applied (Gomez and Gomez, 1984).
RESULTS AND DISCUSSION
Current study was conducted in order to monitor and analyze different camel browse vegetations at Mithi (Desert), Tando Allahyar (Irrigated) and Thatta (Costal) for nutrient composition. Survey of above said districts was made, meanwhile, sampling of available vegetations were performed, and examined at the Laboratory of Animal Nutrition Department for assessing their major nutrients. Dry matter content in camel browse vegetations differ plant to plant. However, in some occassions no statistical variation occurred among them. For instace, Senegalia senegal (75.95%) and Cyamopsis tetragonoloba (74.55%) found statistically similar to each other in dry matter contents, did not show any signifcant variation against Acacia nilotica and Acacia jacquemontii, and Cyamopsis tetragonoloba and Sesamum indicum (Fig. 1). Moreover, Concentration of dry matter contents in both of Senegalia senegal and Morai observed comparatively (p<0.05) low from that of noted in Calligonum polygonoides (93.63%). Similarly, the percent of dry matter in Trifolium alexandrinum (18.20%) did not vary (P>0.05) from that of recorded in Suaeda fruticosa, Haloxylon salicornicum, Zea mays, Salvadora oleiodes and Cordia sinensis Linn., but found comparatively (p<0.05) low from that of noted in other vegetations under current investigation. Further, dry matter content of Ziziphus nummularia (52.02%), Prosopis cineraria (49.25%), Prosopis juliflora (45.54%), Tamarix passerinoides (44.00%), Capparis deciduas (40.53%) and Alhagi maurorum (35.69%) although vary to each other, but differences among them existed statistically non-significant (p>0.05). Nevertheless, the percent of dry matter content in Calligonum polygonoides (93.63%) observed considerably high (p<0.05), while in Trifolium alexandrinum it was low (18.20%). It is noteworthy that in current study results indicate that species of plant in some extent had influence on dry matter contents. Although there were no significant variation among dry matter contents of some vegetations, but this variation had no close relationship to each other. The dry matter contents in different vegetations under present study found either in agreement or comparable with other reported studies. For instance, result regarding the Calligonum polygonoides (93.63) in the present sudy is very much related with the findings of El-Amier and Abdullah (2015) who reported 91% dry matter contents in Calligonum polygonoides. On the other hand, findings of dry matter contents of Cyamopsis tetragonoloba and Sesamum indicum revealed strong negative correlation with the results reported for degummed guar seeds from Mediterranean area (Chiofalo et al., 2018). However, it was not supported by Chiofalo et al., (2018) who otherwise observed somewhat lower concentration from the current study.
Regarding the dry matter content in Senegalia senegal under current study, results found inconsistent with that of Heuzé et al. (2016) who reported 40.8% dry matter in Senegalia senegal (Acacia senegal). In case of Ziziphus nummularia, dry matter content (53.67%) resulted in current investigation appeared in agreement with different studies (Chandra and Mali, 2014; Khanum et al., 2007). Moreover, percent of dry matter in Capparis deciduas (40.53%) recorded in the present study found dissimilar with the reported results of Gull et al. (2015) who reported ~ 1.7 fold higher dry matter in Capparis deciduas. Further, Ullah et al. (2013) disagreed with present findings of Alhagi maurorum (35.69) who reported ~ 2.5 fold higher concentration of dry matter in Alhagi maurorum at Tank and South Waziristan areas of Pakistan. Nevertheless, findings of dry matter in Salvadora oleiodes (31.35) found comparble with the study of Samreen et al. (2016) who reported 61.6% dry matter in Salvadora oleiodes at Darazinda FRDI Khan, Pakistan. Dry matter content in Haloxylon salicornicum (23.04) and Suaeda fruticosa (18.58) disagreed with the findings of Towhidi and Zhandi (2007) and Ashraf et al. (2013) who reported 93.9 and 70-80% dry matter, respectively, while findings of Khanum et al. (2007) regarding the dry matter of Haloxylon salicornicum was somewhat relevant to present findings. Result regarding the dry matter contents in Zea mays held strong correlation with the findings of Khanum et al. (2007) who reported 25.5 ± 0.9% dry matter in Zea mays. Mohsen et al. (2011) reported 11 to 12 % dry matter in Trifolium alexandrinum which seems little bit close to dry matter of current study. Percent of dry matter content of Acacia nilotica (40.08%) did not match with that of reported by Khanum et al. (2007) i.e 60.4 ± 1.9%.
Moisture content although varied plant to plant, but most of them did not statistically differ with each other. For example, concentration of moisture content in Senegalia senegal (24.05%) and Cyamopsis tetragonoloba (25.45%) against Acacia nilotica (40.08%) and Acacia jacquemontii (38.50%), and versus Cyamopsis tetragonoloba (12.15%) and Sesamum indicum (8.95%) did not vary statistically (p>0.05), but against Calligonum polygonoides (6.37%) it occurred comparatively abundent (p<0.05). Similarly, the percent of moisture content in Trifolium alexandrinum (81.80%) versus Suaeda fruticosa (81.43%), Haloxylon salicornicum (23.04%), Zea mays (74.48%), Salvadora
oleiodes (68.65%) and Cordia sinensis Linn. (67.75%) existed staitistically similar (p>0.05) but it appeared significantly high (p<0.05) against other vegetations. Further, the level of moisture content in Ziziphus nummularia, Prosopis cineraria, Prosopis juliflora, Tamarix passerinoides, Capparis deciduas and Alhagi maurorum although varied plant to plant, but it was not statistically cosiderable (p>0.05) (Fig. 2). Moreover, moisture content examined from Calligonum polygonoides found comparatively low, while from Trifolium alexandrinum it was significantly high. It could be incurred from current results that plant specaie had some impact on moisture content of different vegetations, although majority of them did not vary to each other. Moisture content of Acacia nilotica, Ziziphus nummularia, Suaeda fruticosa, Haloxylon salicornicum, Capparis deciduas, Alhagi maurorum, Senegalia senegal (Fig. 2) in the current study did not appear in line with that of reported studies of different authors (Towhidi and Zhandi et al., 2007; Ashraf et al., 2013; Ullah et al., 2013; Abdullah et al., 2017) and found quit different, while in Prosopis juliflora, Cordia sinensis Linn., Salvadora oleiodes and Calligonum polygonoides it was in accordance with different reported studies (Murray et al., 2001; Mabrouk et al., 2008; El-Amier and Abdullah, 2015; Samreen et al., 2016).
Concentration of organic matter in Senegalia senegal, Calligonum polygonoides, Acacia jacquemontii, Cyamopsis tetragonoloba, Zea mays, Alhagi maurorum, Capparis deciduas, Prosopis juliflora, Sesamum indicum, Prosopis cineraria, Ziziphus nummularia and Cyamopsis tetragonoloba did not vary statistically (p>0.05) to each other but appeared significantly high from that of noted in Trifolium alexandrinum, Tamarix passerinoides, Acacia nilotica, Haloxylon salicornicum and Suaeda fruticosa though existed statistically similar (Fig. 3). Further, the level of organic matter in Salvadora oleiodes and Cordia sinensis Linn. found noticeably low (p<0.05) contrast to that of noted in all above said vegetations. The level of organic matters recorded in the present study for Senegalia senegal, Calligonum polygonoides, Acacia jacquemontii, Alhagi maurorum, Capparis deciduas, Prosopis juliflora, Prosopis cineraria, Ziziphus nummularia and Trifolium alexandrinum found relatively in accordance with that of reported in different studies (Mohsen et al., 2011; Ullah et al., 2013; Chandra and Mali, 2014; El-Amier and Abdullah, 2015; Heuzé et al., 2016; Heuzé et al., 2016; Rasool et al., 2017; Kathirvel et al., 2011). Nevertheless, slight variation occurred among them. This minor difference may be concerned with the environmental changes and/or variety distinction. However, the level of organic matter in Acacia nilotica, Haloxylon salicornicum, Suaeda fruticosa, Salvadora oleiodes and Cordia sinensis Linn. in current study totally disagreed with that of stated by different authers (Murray et al., 2001; Towhidi and Zhandi, 2007; Ashraf et al., 2013; Chandra and Mali, 2014; Bwai et al., 2015; Samreen et al., 2016).
No significant variation (P>0.05) occurred among crude protein contents of Capparis deciduas and Suaeda fruticosa, but in both of these vegetations it appeared comparatively high from that of recorded in Salvadora oleiodes, Ziziphus nummularia, Alhagi maurorum, Trifolium alexandrinum, Acacia jacquemontii, Haloxylon salicornicum, Sesamum indicum, Zea mays, Prosopis juliflora, Prosopis cineraria, Senegalia senegal, Calligonum polygonoides, Cyamopsis tetragonoloba, Cyamopsis tetragonoloba, Tamarix passerinoides and Acacia nilotica under which its level existed statistically non-significant to each other (Fig. 4). Nevertheless, the concentration of crude protein content in Cordia sinensis Linn. recorded significantly low compared to that of observed in all other vegetations under present investigation. In general, crude protein content in Capparis deciduas recorded in the present study found statistically similar to that of reported by Gull et al. (2015), while Abdullah et al. (2017) did not support it, their findings looks quite dissimilar from the present results. The level of crude protein content in Suaeda fruticosa and Khabar appeared dissimilar with that of observed by Samreen et al. (2016) but their concentration seems to be somewhat close to reported findings of Abdullah et al. (2017). The level of crude protein contents in Ziziphus nummularia, Acacia nilotica, Prosopis cineraria, Trifolium alexandrinum and Zea mays in present findings existed in agreement with that of reported results of different authors (Chandra and Mali, 2014). Further, the level of crude protein content in Prosopis juliflora, Prosopis cineraria and Acacia jacquemontii are very much different compared to that of reported in different studies (Mabrouk et al., 2008; Ullah et al., 2013; Rasool et al., 2017).
Ether extract in Zea mays followed by Trifolium alexandrinum revealed significantly high concentration, and in Salvadora oleiodes and Cyamopsis tetragonoloba it was comparatively low from that of other vegetations. Further, ether extract content in Sesamum indicum noted relatively similar to that of recorded in Trifolium alexandrinum, Senegalia senegal, Alhagi maurorum, Acacia nilotica, Acacia jacquemontii, but found signifcantly (p<0.05) high from that of Prosopis juliflora, Ziziphus nummularia, Cordia sinensis Linn., Haloxylon salicornicum, Tamarix passerinoides, Capparis deciduas, Prosopis cineraria, Calligonum polygonoides, Cyamopsis tetragonoloba and Suaeda fruticosa (Fig. 5). Variation in ether extract content of camel browse vegetations might be attributed with plant species and/or with environment of location whose effect has been noted in the present study. Ether extract content of Ziziphus nummularia, Prosopis juliflora, Salvadora oleiodes and Capparis deciduas at Tando Allahyar (irrigated) district noted comparatively (p<0.05) high followed by at Thatta (costal) and Mithi (desert). Nevertheless, the effect of environment of Mithi (desert) and Thatta (costal) on ether extract content of Ziziphus nummularia and Salvadora oleiodes was not prominent (p>0.05), where their concentration varied at both places but differences existed non-significant (p>0.05).
Further, the environment of Mithi (desert) supported the ether extract content of Acacia nilotica, where its concentration appeared comparatively high. However, environment of Tando Allahyar (irrigated) did not favor it and became at the bottom, while at Thatta (costal), the ether extract contents of Acacia nilotica was at intermediate level. It is noteworthy that percent of ether extract in most of the vegetations observed in the present study was in accordance with the findings of different authors. The concentration of ether extract content in Senegalia senegal, Cordia sinensis Linn., Suaeda fruticosa, Prosopis juliflora, Trifolium alexandrinum, Haloxylon salicornicum, Acacia nilotica, Capparis deciduas, Prosopis cineraria, Calligonum polygonoides and Ziziphus nummularia observed in the current study were in line with that of reported in different studies (Towhidi and Zhandi, 2007; Mabrouk et al., 2008; Mohsen et al., 2011; Ashraf et al., 2013; Chandra and Mali, 2014; El-Amier and Abdullah, 2015; Abdullah et al., 2017), while percent of ether extract in Alhagi maurorum, Salvadora oleiodes, Acacia jacquemontii, which recorded in current study found somewhat different from reported studies (Ullah et al., 2013; Samreen et al., 2016; Rasool et al., 2017).
Morover plants did not differ in total carbohydrate content from one another. For instance, differences in the concentration of carbohydrate contents of Calligonum polygonoides versus Cyamopsis tetragonoloba, Senegalia senegal, Cyamopsis tetragonoloba, Prosopis juliflora, Prosopis cineraria, Acacia jacquemontii, Alhagi maurorum, Sesamum indicum, Zea mays, Ziziphus nummularia, Acacia nilotica and Tamarix passerinoides existed statistically non-significant (p>0.05). Further, concentration of carbohydrate contents in Capparis deciduas, Trifolium alexandrinum, Haloxylon salicornicum, Cordia sinensis Linn. and Suaeda fruticosa did not differ (p>0.05) from one another but became significantly abundent from that of Salvadora oleiodes where its concentration recorded comparatively low (Fig. 6). Nevetheless, the concentration of carbohydrate contents recorded in the present study for different vegetations found either relavant or distinct to reported studies. For instance, Mabrouk et al. (2008) reported quite relevant results regarding the total carbohydrate level in Prosopis juliflora, while Rifat et al. (2018) reported little bit different concentration of carbohydrate content in Prosopis cineraria compared to current study. This difference among the results might be related with the variety, environmental distinction and soil composition. Differences in the results could also be related with the sample part of plant as in current study homogenous sample of leaves, seeds, pods were used, while in reported study of Rifat et al. (2018)only pods were focused. Total carobhydrate level in Alhagi maurorum disagreed with the results of Ullah et al. (2013) who reported lower percent of total carbohydrate content in Alhagi maurorum contrast to current findings. Findings of Malik et al. (1970) regarding total carbohydrate content in Ziziphus nummularia suported the results of current study, while Nazar et al. (2018) and Ashraf et al. (2013) disagreed with current results of total
carbohydrate of Acacia nilotica, Capparis deciduas and Haloxylon salicornicum, respectively though appeared totally distinct. Where ever, Murray et al. (2001), Rathore (2009) and Mohsen et al. (2011) reported relatively similar total carbohydrate in Cordia sinensis Linn., Salvadora oleiodes and Trifolium alexandrinum, respectively as recorded in current study.
The percent of nitrogen free extract in Calligonum polygonoides existed non-significant with that of recorded in Senegalia senegal, Cyamopsis tetragonoloba, Cyamopsis tetragonoloba, Prosopis cineraria, Prosopis juliflora and Alhagi maurorum, while it appeared comparable (p<0.05) with that of Ziziphus nummularia, Acacia jacquemontii, Acacia nilotica, Zea mays, Trifolium alexandrinum, Capparis deciduas, Haloxylon salicornicum, Cordia sinensis Linn., Suaeda fruticosa and Salvadora oleiodes. Further, the concentration of nitrogen free extract in Suaeda fruticosa and Salvadora oleiodes did not differ (p>0.05) from that of recorded in Trifolium alexandrinum, Capparis deciduas, Haloxylon salicornicum and Cordia sinensis Linn., but found remarkably (p<0.05) low compared to that of other vegetations (Fig. 7). It is of interest to note that majority of vegetations in the present investigation resulted non-significant variation in nitogen free extract contents, but its concentration level found distinct against reported studies. In contrast to current study, the findings of nitrogen free extract contents in Calligonum polygonoides, Acacia nilotica, Ziziphus nummularia, Haloxylon salicornicum and Suaeda fruticosa found dissimilar with that of reported studies (Towhidi and Zhandi, 2007; Abdullah et al., 2017). However, Nitrogen free extract of Prosopis cineraria, Trifolium alexandrinum and Suaeda fruticosa existed in agreement with that of reported studies of different authors (Mohsen et al. (2011); Chandra and Mali, 2014; Abdullah et al., 2017).
The level of crude fiber content in Zea mays noted significantly high, while in Alhagi maurorum, Cyamopsis tetragonoloba and Calligonum polygonoides it was considerably (p<0.05) low. The difference in percent of crude fiber content in Acacia jacquemontii contrast to Capparis deciduas and Suaeda fruticosa; Haloxylon salicornicum versus Capparis deciduas, Suaeda fruticosa, Sesamum indicum and Cyamopsis tetragonoloba, and Prosopis cineraria against Cordia sinensis Linn., Khabar, Trifolium alexandrinum, Senegalia senegal, Tamarix passerinoides, Calligonum polygonoides, Alhagi maurorum and Cyamopsis tetragonoloba noted statistically non-significant (P>0.05). These above said results indicate that most of the vegetations had non-significant variation in crude fiber contents among them (Fig. 8). However, their concentration level might be either comparable or distinct against reported studies. It was noted that the findings of reported studies regarding crude fiber in Senegalia senegal, Zea mays, Capparis deciduas, Suaeda fruticosa, Prosopis juliflora, Acacia nilotica and Trifolium alexandrinum (Ahmed et al., 2009; Mohsen et al., 2011; Abdullah et al., 2017) are quite supportive to the current study. Moreover, percent of crude fiber content in Cordia sinensis Linn., Calligonum polygonoides, Senegalia senegal, Kandero, Acacia jacquemontii, Ziziphus nummularia, Khabar, Acacia nilotica, recorded under present investigation appeared distinct compared to that of reported findings (Murray et al., 2001; Ullah et al., 2013; El-Amier and Abdullah, 2015; Heuzé et al., 2016; Samreen et al., 2016; Rasool et al., 2017). It was further observed that regardless, the level of inorganic/mineral matters differ plant to plant, but in
most of the cases, differences existed non-significant (p>0.05) among them. Moreover, total inorganic/mineral matter in Cordia sinensis Linn. and Salvadora oleiodes observed remarkably (p<0.05) high, while it was significantly low in Cyamopsis tetragonoloba, Ziziphus nummularia, Prosopis cineraria, Sesamum indicum, Prosopis juliflora, Capparis deciduas, Alhagi maurorum, Zea mays, Cyamopsis tetragonoloba, Acacia jacquemontii, Calligonum polygonoides and Senegalia senegal though did not vary significantly.
Further, no countable differences occurred in inorganic/mineral matter of Suaeda fruticosa, Haloxylon salicornicum, Acacia nilotica, Tamarix passerinoides and Trifolium alexandrinum but varied significantly (p<0.05) from that of other vegetations in the present investigation, neverthless their percent reached at intermediate level (Fig. 9). However, the level of inorganic/mineral matter in majority of vegetations disagreed with reported findings of different authors except some. Present results of inorganic/mineral matter in Cordia sinensis Linn., Senegalia senegal, Salvadora oleiodes, Suaeda fruticosa, Haloxylon salicornicum, Alhagi maurorum and Acacia nilotica did not appear in accordance with that of reported in different studies (Murray et al., 2001; Ullah et al., 2013; Samreen et al., 2016; Abdullah et al., 2017). While findings regarding inorganic matter in Suaeda fruticosa, Prosopis cineraria, Prosopis juliflora, Capparis deciduas, Calligonum polygonoides, Acacia jacquemontii, Trifolium alexandrinum and Ziziphus nummularia in the current study found in line with that of reported by different authors (Mohsen et al., 2011; Chandra and Mali et al., 2014; El-Amier and Abdullah, 2015; Abdullah et al., 2017; Chandra and Mali, 2016).
CONCLUSION
It could be concluded from present sudy that the Trifolium alexandrinum, Suaeda fruticosa, Haloxylon salicornicum, Zea mays, Salvadora oleiodes and Cordia sinensis (Linn.) noted to be high moistured vegetations, Senegalia Senegal followed by Calligonum polygonoides and Acacia jacquemontii appeared considerably rich in organic matter contents, and Cordia sinensis (Linn.) and Salvadora oleiodes in total inorganic/mineral matter. Capparis deciduas and Suaeda fruticosa both pertained considerable concentration of crude protein contents, Zea mays and Salvadora oleiodes high ether extract, Calligonum polygonoides beared significantly high level of carobohydrate contents and Zea mays revealed remarkably maximum percentage of crude fiber.
Statement of conflict of interest
The author declares there is no conflict of interest.
REFERENCES
Abdullah, M., Rafay, M., Sial, N., Rasheed, F., Nawaz, M.f., Nouman, W., Ahmad, I., Ruby, T. and Khalil, S., 2017. Determination of forage productivity, carrying capacity and palatability of browse vegetation in arid rangelands of cholistan desert (pakistan). Appl. Ecol. environ. Res., 15: 623-637. https://doi.org/10.15666/aeer/1504_623637
Abdulrazak, S.A., Orden, E.A., Ichinohe, T. and Fujihara, T., 2000. Chemical composition, phenolic concentration and in vitro gas production characteristics of selected Acacia fruits and leaves. Asian Australasian J. Anim. Sci., 13: 935-940. https://doi.org/10.5713/ajas.2000.934
Ahmad, S., Yaqoob, M., Hashmi, N., Zaman, M. and Tariq, M., 2009. Economic importance of camel: Unique alternative under crisis. Pak. Vet. J., 30: 191-197.
Amin, A.S., Abdoun, K.A. and Abdelatif, A.M., 2011. Observations on the seasonal browsing and grazing behaviour of camels (Camelus dromedarius) in southern Darfur-Sudan. Res. Opin. Anim. Vet. Sci., 1: 213-216.
A.O.A.C., 2000. Official methods of analysis. Association of Official Analytical Chemists International. Maryland, USA.
Ashraf, M.A., Karamat, M., Wajid, A., Qureshi, A.K. and Gharibreza, M., 2013. Chemical constituents of Haloxylon salicornicum plant from Cholistan desert, Bahawalpur, Pakistan. J. Fd. Agric. Environ., 11: 1176-1182.
BSI, 1990. Methods of testing the food. British Standards Institution: London, UK.
Bwai, M.D., Uzama, D., Abubakar, S., Olajide, O.O., Ikokoh, P.P. and Magu, J., 2015. Proximate, elemental, phytochemical and anti-fungal analysis of Acacia nilotica fruit. Pharmaceut. Biol. Eval., 2: 52-59.
Chandra, J. and Mali, M.C., 2014. Nutritional evaluation of top five fodder tree leaves of mimosaceae family of arid region of Rajasthan. Int. J. Inn. Res. Rev., 2: 14-16.
Chiofalo, B., Presti, V.L., Agata, A.D., Rao, R., Ceravolo, G. and Gresta, F., 2018. Qualitative profile of degummed guar (Cyamopsis tetragonoloba L.) seeds grown in a Mediterranean area for use as animal feed. J. Anim. Physiol. Anim. Nutri., 102: 260-267. https://doi.org/10.1111/jpn.12687
Dokata, M.D., 2014. Factors influencing camel milk production in central division of Isiolo District: A case of three camel milk women self help groups in Isiolo County, Kenya, MA dissertation. University of Nairobi. Kenya.
Dorges, B. and Heucke, J., 2003. Demonstration of ecologically sustainable management of camels on aboriginal and pastoral land. Alice Springs, N.T. pp.123-150.
El-Amier, Y.A. and Abdullah, T.J., 2015. Evaluation of nutritional value for four kinds of wild plants in Northern sector of Nile Delta, Egypt. Open J. appl. Sci., 5: 393-402. https://doi.org/10.4236/ojapps.2015.57039
Ganskopp, D. and Bohnert, D., 2003. Mineral concentration dynamics among 7 northern Great Basin grasses. J. Range Manage., 56:174-184. https://doi.org/10.2307/4003902
Gomez, K. and Gomez, A., 1984. Statistical procedures for agricultural research institute. Second edition, Los Banos Philipines: Jhon Wiley Sons Inc.
Gull, T., Mahmood, Z., Anwar, F., Sultana, B., Nouman, W., Shahid, S.A. and Iqbal, M.Z., 2015. Variation of proximate composition and minerals within different parts of Capparis decidua (Forssk.) Edgew. as a function of harvesting seasons. Pak. J. Bot., 47: 1743-1748.
Hesse, C. and Cotula, L., 2006. Climate change and pastoralists: Investing in people to respond to adversity. Sustainable development opinion, IIED. http://pubs. iied. org/pdfs/11059IIED. pdf. Retrieved on July 5, 2018, 8.00.
Heuzé, V., Thiollet, H., Tran, G., Hassoun, P., Bastianelli, D. and Lebas, F., 2016. Gum arabic tree (Acacia senegal). Feedipedia, a programme by INRA, CIRAD, AFZ and FAO. http://www.feedipedia.org/node/342. Retrived on July 4, 2018, 17:00.
Iqbal, A. and Khan, B.B., 2001. Feeding behaviour of camel., Pak. J. agric. Sci., 38: 58-63.
Kathirvel, P. and Kumudha, P., 2011. Chemical composition of Prosopis juliflora (SW.) DC (mosquito bean). Int. J. appl. Biol. Pharmaceut. Technol., 2: 5-14.
Khan, F.M., 2009. Ethno-veterinary medicinal usage of flora of Greater Cholistan desert (Pakistan). Pak. Vet. J., 29: 75-80.
Khanum, S.A., Yaqoob, T., Sadaf, S., Hussain, M., Jabbar, M.A., Hussain, H.N., Kausar, R. and Rehman, S., 2007. Nutritional evaluation of various feedstuffs for livestock production using in vitro gas method. Pak. Vet. J., 27: 129-133.
Krätli, S., Huelsebusch, C., Brooks, S. and Kaufmann, B., 2013. Pastoralism: A critical asset for food security under global climate change. Anim. front., 3: 42-50. https://doi.org/10.2527/af.2013-0007
Liaqat, I., Arshad, N., Arshad, M., Mirza, S.A., Ali, N.M. and Shoukat, A., 2017. Antimicrobial activity of some medicinal plants extracts against food industry isolates. Pakistan J. Zool., 49: 523-530.
Mabrouk, H., Hilmi, E. and Abdullah, M., 2008. Nutritional value of Prosopis juliflora pods in feeding nile tilapia (Oreochromis niloticus) fries. Arab Gulf J. scient. Res., 26: 49-62.
Malik, N.S. and Nath, K., 1970. Chemical composition and nutritive value of green pala (Ziziphus nummularia) leaves. Ind. J. Anim. Sci., 40: 41-45.
McLeod, S.R. and Pople, A.R., 2008. Modelling management options for management of feral camels in central Australia. Technical Report. No 48. Desert Knowledge CRC, Alice Springs, NT.
Megersa, B., Markemann, A., Angassa, A. and Zárate, A.V., 2014. The role of livestock diversification in ensuring household food security under a changing climate in Borana, Ethiopia. Fd. Secur., 6: 15-28. https://doi.org/10.1007/s12571-013-0314-4
Mohsen, M.K., El-Santiel, G.S., Gaafar, H.M.A., El-Gendy, H.M. and El-Beltagi, E.A., 2011. Nutritional evaluation of berseem. 2. Effect of nitrogen fertilizer on berseem fed as silage to goats. Arch. Zootech., 14: 21-31.
Murray, S.S., Schoeninger, M.J., Bunn, H.T, Pickering, T.R. and Marlett, J.A., 2001. Nutritional composition of some wild plant foods and honey used by Hadza foragers of Tanzania. J. Fd. comp. Anal., 14: 3-13. https://doi.org/10.1006/jfca.2000.0960
Nazar, H., Mohammed, A.K., Ibrahim, O.A., Ishiyaku, Y.M., Ahmed, S.A. and Abdullah, M., 2018. Nutritional composition of some forage species consumed by one-humped camels (Camelus dromedarius) in Zaria sub-humid region of Nigeria. J. Anim. Prod. Res., 29: 365-370.
Nasrullah, M.N., Akashi, R. and Kawamura, O., 2003. Nutritive evaluation of forage plants grown in South Sulawesi, Indonesia. Asian-Aust J. Anim. Sci., 16: 693-701. https://doi.org/10.5713/ajas.2003.693
Rasool, F., Ishaque, I., Yaqoob, S. and Tanveer, S., 2017. Chemical composition and ethnobotanical uses of Acacia jacquemontii Benth. in the Thal desert in Pakistan. Bois Forêts Trop., 331: 1-10.
Rathore, M., 2009. Nutrient content of important fruit trees from arid zone of Rajasthan. J. Hort. Forest., 1:103-108.
Rifat, U.K.M., Farooq, M.U., Qamar, I.A., Ahmad, S., Razaq, A. and Tiwana, U.A., 2018. Seasonal variation in nutritional characteristics of forage species in Rakh Choti Dalana in District Dera Ghazi Khan Pakistan. Basic Res. J. agric. Sci. Rev., 6: 21-26.
Samreen, U., Ibrar, M., Badshah, L. and Ullah, B., 2016. Nutritional and elemental analysis of some selected fodder plants of Darazinda FRDI Khan. Pak. Adv. Pl. agric. Res., 4: 117-127.
Schawn, H.J., 2001. The biology of the camel. The one-humped camel in Eastern Africa, Weikersheim, Verlag Josef Margraf:10-29.
Towhidi, A. and Zhandi, M., 2007. Chemical composition, in vitro digestibility and palatability of nine plant species for dromedary camels in the province of Semnan, Iran. Egypt. J. Biol., 9: 47-52. https://doi.org/10.1017/S1752756200021360
Ullah, Z., Baloch, M.K, Khader, J.A., Baloch, I.B., Ullah, R., AbdEIslam, M.N. and Noor, S., 2013. Proximate and nutrient analysis of selected medicinal plants of Tank and South Waziristan area of Pakistan. Afri J. Pharm. Pharmacol., 7: 179-184. https://doi.org/10.5897/AJPP12.766
To share on other social networks, click on any share button. What are these?










