Non-Crop Vegetation as Habitat for Rodents in the Agricultural Landscape of Pothwar Plateau, Pakistan
Non-Crop Vegetation as Habitat for Rodents in the Agricultural Landscape of Pothwar Plateau, Pakistan
Nadeem Munawar1,2*, Tariq Mahmood2, Paula Rivadeneira1, Ali Akhter2 and Saqib Mehmood2
1Department of Soil, Water and Environmental Sciences, College of Agriculture and Life Sciences, The University of Arizona, Tucson, USA
2Department of Wildlife Management, Pir Mehr Ali Shah Arid Agriculture University, Rawalpindi, Pakistan
ABSTRACT
The community structure and richness of rodent populations in agricultural areas are related to variables such as habitat structure and complexity, temperature, rainfall, crop productivity, predation, trampling and grazing, surrounding landscape, and succession of the natural wild vegetation. Livestock grazing, cutting, harvesting (for fuel wood and animal feed) and burning of field boundary vegetation are common practices that affect rodents and their habitat. During crop season, local farmers in the Pothwar agro-ecosystem in Pakistan do not manage wild vegetation on the field edges, and that may impact rodent populations near their fields. This study was conducted to examine the effect of adjacent non-crop vegetation on rodent populations in the Pothwar agro-ecosystem in Pakistan. Over 14 months, vegetation analysis was conducted using the quadrate method to record the vegetation around rodents’ active burrows at field boundaries. The dominant wild vegetation included Cynodon dactylon, Saccharum griffithii, Dactyloctenium aegyptium, Dichanthium annulatum, Desmostachya bipinnata, Imperata cylindrical, Ziziphus nummularia, Achyranthes aspera, Calotropis procera, Sorghum halepense and Capparis deciduas. This vegetation supported the year-round population of rodents in the Pothwar agro-ecosystem by providing shelter, cover, and food. The data suggest that further research is needed to test various ecologically-based rodent management strategies e.g. management of non-crop habitats, cleaning of crop cache in post harvested fields, management of wild natural vegetation providing food and cover during non-crop seasons that would seem essential to maintenance of habitats for rodent species in agricultural landscapes
Article Information
Received 06 February 2019
Revised 22 Jule 2019
Accepted 06 July 2019
Available online 05 March 2020
Authors’ Contribution
NM conceived and designed the study. NM and TM analysed the data and wrote the manuscript. PR improved the manuscript through valuable discussion and constructive comments. AA and SM participated in the field-work.
Key words
Non-crop, Wild vegetation, Field boundaries, Analysis, Rodent
DOI: https://dx.doi.org/10.17582/journal.pjz/20190206230250
* Corresponding author: nadeemmunawer@gmail.com
0030-9923/2020/0003-1015 $ 9.00/0
Copyright 2020 Zoological Society of Pakistan
INTRODUCTION
Agricultural landscapes consist of mosaics of farmland and remnant natural habitats of woodlots, hedgerows, shelterbelts, and riparian zones that may offer an opportunity to conserve biodiversity while maintaining food production (Paoletti et al., 1992). Hedgerow and riparian habitats are particularly valuable for conservation of plant diversity in agricultural landscapes (Bunce and Hallam, 1993; Boutin et al., 2002). In addition, the varieties of habitats within agricultural landscapes help beneficial invertebrates (Altieri and Nicholls, 1999) and provide habitats for birds (Best, 1983). Rodent species are also common inhabitants of agricultural landscapes where they are an important prey source for a wide variety of avian, reptilian, and mammalian predators (Martin, 1994).
Buffer zones that employ tall vegetation can prevent erosion and runoff of nutrients, and may provide refuge and ecological corridors for wildlife
(Mauritzen et al., 1999), thereby helping to maintain local biodiversity in agro-ecosystems (Giusti et al., 1996; Marshall and Arnold, 1999; Tattersall et al., 2002). Vegetation is often cut back or removed entirely for rodent or other pest management or as a result of standard farming procedures, but there can be disadvantages to habitat reduction for beneficial species, including birds, arthropods and reptiles (Gorman and Reynolds, 1993; Atkinson et al., 2004). For the protection of these and other species in agro-ecosystems, high vegetation and well-developed vegetative cover are often advantageous, resulting in a conflict between the interests of pest management and the conservation of biodiversity in agro-ecosystems. Changes in vegetation height and vegetative cover can affect two important aspects of a small mammal’s life: food and shelter. In addition, vegetation can be important for building nests above or below ground and it provides thermal protection for rodents from heat and cold.
The Pothwar plateau has sub-tropical dry scrub vegetation and rich floral diversity (Nawaz et al., 2010). Trees and shrubs bearing specific characteristics of scrub forest are abundant in this area (Nawaz et al., 2012). Acacia modesta, Olea ferruginaea and Tecomella undulata are important tree species while several shrubs and grasses are native to the Pothwar region, including the most abundant species of Dodonaea viscosa, Justicia adhatoda, Maytenus royleanus Ziziphus nummularia, Achyranthes aspera Chrysopogon serrulatus, Heteropogon contortus, Dichanthium foveolatum, Cynodon dactylon and Aristida mutabilis (Ahmad et al., 2008). On the Pothwar plateau, agricultural fields have invariably thick undisturbed field boundaries that are maintained to conserve water. Along the field boundaries, apart from wild vegetation of shrubs, fast growing trees are planted for browse and fodder purposes (Hussain et al., 2003).
This study was designed to determine the relationship of native plant abundance around rodent burrows at the field edge and structural diversity of adjacent non-crop vegetation in an agricultural landscape.
MATERIALS AND METHODS
Study area
The current study was conducted in the Pothwar Plateau (33o 30ʹ 0ʹʹ N and 73o 0ʹ 0ʹʹ W) which is a dissected region with undulating topography, gullies, low fertility and erratic rainfall mainly in July and August. The total area of Pothwar Plateau is 13,000 km2 with elevation varying between 305-610 m. Climate is semi-arid to humid and average annual rainfall ranging from 630-708 mm (Sarwar et al., 2017). The summer temperature ranges between 15o C and 40o C while the range of winter temperature is generally between 4o C and 25o C but it can occasionally drop below freezing (Hussain et al., 2003). Agriculture consists of two types of cropping systems: wheat-maize/millet and wheat-groundnut. The Pothwar ecosystem consists of non-cultivated (scrub forest and range land) and cultivated croplands. The cropland tracks also bear some wild vegetation on the thick field boundaries, kept undisturbed and intact for conservation of rain water (Munawar et al., 2018). The Pothwar Plateau is a habitat of seven rodent species (Roberts, 1997); the lesser bandicoot rat (Bandicota bengalensis), the short-tailed mole rat (Nesokia indica), the Indian gerbil (Tatera indica), the soft-furred field rat (Millardia meltada), the desert jird (Meriones hurrianae), the bush rat (Golunda ellioti) and Mus species.
We selected 20 study sites (five in each) in the four districts i.e. Attock, Chakwal, Jhelum and Rawalpindi. The sites were selected after consultation with local agriculture functionaries, taking farmers into confidence, considering logistic (road) approach to the site and appropriate level of rodent infestation. Approximately, each experimental site was of 5.0 ha area, with contiguous cropland habitat. Each site was located about 4-5 km apart.
Study design
The vegetation around the active burrow entrance was recorded and the burrows of rodent species were distinguished on the basis of fresh digging, size of soil particles, burrow openings, foot tracks, damage pattern to the surrounding crop plants and most importantly the fecal droppings. In the study area three rodent species were observed, the lesser bandicoot rat (Bandicota bengalensis), the short-tailed mole rat (Nesokia indica), and the Indian gerbil (Tatera indica).
Vegetation analyses were conducted seasonally from April 2016 to May 2017 using the quadrate method. A 1×1 m quadrate containing 100 squares of 10 cm2 each was used to determine density, frequency, and cover of each plant species around active rodent burrow. The quadrate was placed on steel pegs 30 cm above the ground at 10 randomly selected active burrows at each site on a single day.
Formulae used for quantification of shrubs and herbs species were as follows:
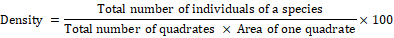
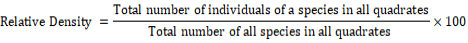

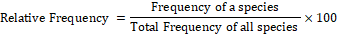

The plants of each study area were given a code number in the field during plant collections. Two representative specimens of each plant species were collected. One was pressed in newspapers for keeping as reference collection. The plants were identified using field guides or a herbarium reference collection.
Importance value index
This index is used to determine the overall importance of each species and measures of how dominant a species is in the given community structure. In calculating this index, the percentage values of the relative frequency, relative density and relative cover are summed up together and this value is designated as the Importance Value Index or IVI of the species (Curtis, 1959).
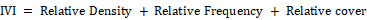
RESULTS
The fields of the Pothwar Plateau were variable in size with thick and wide undisturbed boundaries in order to conserve water. Wild vegetation provides shelter/cover and food to rodents when there is no cultivation or at early stage of crop growth. Results of vegetation analysis conducted in fallow and uncultivated fields during the course of this study period are detailed below:
Vegetation analysis during summer
The number of shrubs and herb/grass species recorded at the field boundaries on the burrow entrances of three rodent species; the lesser bandicoot rat (Bandicota bengalensis), the short-tailed mole rat (Nesokia indica), and the Indian gerbil (Tatera indica) during summer season corresponding to various agricultural practices of the study area are presented in Table I. A total of 30 plant species (included eight shrubs and 22 herbs/grass species) were recorded over the 54 burrows of bandicoot rat which provided shelter/cover and food during summer season. The shrubs given with IVI were comprised of Achyranthes aspera (26.4), followed by Capparis decidua (16.6), Artemisia dubia (15.7), Carthamus oxycantha (15.2), Aerva javanica (12.6), Calotropis procera (12.0), Ziziphus nummularia (6.12) and Cannabis sativa (4.30). The three herbs/grass species scoring highest Importance Value Index (IVI) were Saccharum griffithii (44.2) followed by Cynodon dactylon (42.2) and Dichanthium annulatum (38.2), while the lowest IVI (2.94) was recorded for Chenopodium album. A total of 42 burrows of Nesokia indica were selected to record vegetation coverage during summer season at field edges. The flora was comprised of seven shrubs and 13 herbs/grass species. The shrubs included the highest value Achyranthes aspera (30.1). A detail of 13 herbs/grasses species is given in Table I. Three species having the highest Importance Value Index (IVI) were Saccharum griffithii (IVI = 50.0) followed by Desmostachya bipinnata and Cynodon dactylon while the lowest IVI (2.01) was recorded for Chenopodium album.
There were 26 burrows of Tatera indica that came across during the vegetation analysis. The herbaceous flora occurring over the burrows of this rat was comprised of 17 plant species including five shrub species and 12 herbs/grasses. The highest Importance Value Index (IVI) estimated for shrubs was Achyranthes aspera (18.1). The IVIs estimated for the three dominant herbs/grasses were; 40.1 for Saccharum griffithii, 37.2 for Desmostachya bipinnata and 27.5 for Cynodon dactylon. Chrozophora tinctoria was the least occurring grass species with IVI value of 3.62 only (Table I).
Vegetation analysis during autumn
A total of 26 plant species were recorded around 46 burrow entrances of B. bengalensis on field edges during autumn season. These included eight shrubs and 18 herbs/grass species. A detail of 18 herbs/grasses species is given in Table I. Three species having the highest IVI were Saccharum griffithii (IVI = 58.4) followed by Saccharm spontaneum (IVI = 55.0) and Cynodon dactylon (IVI = 45.0) while the lowest IVI (3.20) was recorded for Rumex dentatus. The 20 plant species recorded around 38 burrows of N. indica included six shrubs and 14 herbs/grass species. Three grasses/herbs having the highest IVI were Dichanthium annulatum, (IVI = 49.0) followed by Saccharm griffithii (IVI = 38.1) and Desmostachya bipinnata (IVI = 35.2) while the lowest IVI (2.61) was recorded for Rumex dentatus. The number of burrows recorded at field edges were 16 for T. indica. The herbaceous flora which provides shelter and food to this rat during autumn season was comprised of 15 plant species including four shrub species and eleven herbs/grass species (Table I).
Vegetation analysis during winter
The herbaceous flora occurring on crop field boundaries during winter season covering 34 burrow openings of bandicoot rat was comprised of 26 plant species including eight shrubs and 18 grasses/herbs. The IVIs estimated for the three dominant herbs/grasses were: 80.3 for Saccharum griffithii, 75.4 for Cynodon dactylon and 62.0 for Desmostachya bipinnata. Aristidia Cyanatha was the least occurring grass with IVI value of 2.80 only. A total of 25 plant species were recorded on field edges during winter season around 28 burrows of N. indica. These included eight shrubs and 17 herbs/grass species (Table I). No. of T. indica burrows examined for floral occurrence at field edges were 18. The wild vegetation was comprised of 21 plant species including five shrub and 16 herbs/grass species (Table I). The highest IVI recorded for shrubs were: Achyranthes aspera (23.5), while the lowest IVI recorded for Aristidia cyanatha was 1.32.
Vegetation analysis during spring
During spring season, we observed 46 burrows of bandicoot rat for vegetation analysis (Table I). A total of 27 floral species were recorded consisting of seven shrub species. A total of 23 species of wild flora were recorded from field edges during spring season around 24 burrows of Nesokia. The three top scoring IVIs herbs/grass were Cynodon dactylon (52.0), Saccharum griffithii (50.9) and Dichanthium annulatum (34.5). A member of this having lowest IVI (2.10) was Chrozophora tinctoria. A total of 19 plant species were recorded around 15 burrows of T. indica which included six shrub species and 13 herbs/grass species (Table I).
Table I. Importance Value Index (IVI) of different floral species recorded on crop field boundaries on rodent burrows during summer, autumn, winter and spring season in agro-ecosystem of Pothwar Plateau, Pakistan.
A.j, Aerva javanica; A.d, Artemisia dubia; A.a, Achyranthes aspera; C.p, Calotropis procera; C.d, Capparis deciduas; C.o, Carthamus oxycantha; C.s, Cannabis sativa; A.f, Avena fatua; Z.n, Ziziphus nummularia; S.g, Saccharum griffithii; D.b, Desmostachya bipinnata; C.d, Cynodon dactylon; D.a, Dichanthium annulatum; M.p, Medicago polymorpha; D.a, Dactyloctenium aegyptium; C.b, Conyza bonariensis; S.h, Sorghum halepense; E.p, Eclipta prostrate; I.c, Imperata cylindrical; D.m, Digera muricata; C.c, Cenchrus ciliaris; A.c, Aristidia Cyanatha; S.s, Saccharm spontaneum; O.c, Oxalis corniculata; R.d, Rumex dentatus; S.s, Solanum surattense; S.n, Solanum nigrum; T.t, Tribulus terrestris; C.a, Chenopodium album; E.c, Eragrostis cilianensis; C.t, Chrozophora tinctoria.
DISCUSSION
During this study, it has been observed that during the non-crop season, rodents depend on shrubs and herbs/grasses of field boundaries which are the chief source of their diet. Herbs/grass roots played a very important role in the diet of rodents. In the absence of cropland plants, they preferred the roots of wild flora at field edges, the highest preference of root was observed in the summer season. The summer floras recorded were relatively more diversified than those of other seasons. During summer season in the hot and dry period, when there is less vegetation cover in croplands, most of the rats shift toward field boundaries and construct burrows under wild vegetation. During this season the rainfall is an important factor, which accelerate the growth of weeds and grasses, therefore the number of vegetation observed were significantly higher than the rest of the seasons. Stenseth (1977) reported that habitat use by animals can change seasonally when rodents experience natural changes in resources and reduced survival rates during unfavourable times of the year in certain habitats.
Food is vital for the maintenance of body function and reproduction. Food availability impacts individual growth rates, reproductive success, population density and recruitment (Desy and Batzli, 1989). The breeding activity of summer season is correlated with the environmental factors (rainfall, day-length and temperature). It provides moist soil and good stands of wild vegetation for shelter and cover. While in autumn season there was less vegetative cover inside the fields due to early growth stage of wheat crop, the rodents preferred the naturally occurring food available in the shape of seeds of herbs/grasses or shrubs. The climatic conditions in this season were favorable but the duration is short, so, therefore, the wild vegetation didn’t give the rodents prolonged support. The number of burrows observed under wild vegetation in this season was relatively low. The seasonal changes in the population could be correlated with the type of cover, food and climatic factors.
During winter season fewer number of wild vegetation was observed and number of rodent burrows was also less due to unfavorable and extreme weather conditions. The rodents found reproductively inactive in this season. The drastic reduction in the population of rodents in winter was due to the natural cycle of fluctuations in population size with growth, peak and decline depending on several biotic and abiotic factors. In winter season groundnut crop was at maturity stage, with the formation of nuts, the rodents started feeding on them and made extensive burrow systems. The crop field had moist soil with good stand of groundnut plants provided cover to the rodent burrows, so, therefore, their preference toward shrubs and grasses was less. In spring season the breeding activity of the rodents reaches at peak, which corresponds with the maturity of wheat crop and moderate temperature and photoperiod (Hussain et al., 2003). Likewise, the growth of wild vegetation (weeds/shrubs and herbs/grasses) also started in spring that corresponds with the favorable weather conditions, moderate temperature and photoperiod. This season provided more diversified habitat to rodents both inside the croplands and at field edges, because their breeding activity started in this season. De Cauver et al. (2006) reported that vegetation regrowth after mowing grass or harvesting crops can in fact be more nutritious than the vegetation removed. It is argued that this could lead to a higher carrying capacity of mown habitats for small mammal populations; however, most studies contradict this view (Slade and Crain, 2006).
For rodents in agro-ecosystems, habitat use may also be linked to crop cycles and can involve movements between refuge habitats and crop habitats according to seasonal changes in food supply or intra-specific competition (Hansson, 1977; Redhead and Singleton, 1988; Chambers et al., 1996). The composition and structure of vegetation fluctuate naturally depending on season. These changes are usually enhanced by agriculture. Seasonal changes in vegetation height and vegetative cover are, therefore, inherent to agricultural land use. Their intensity depends on the cropping system and on the cultivation system, such as rotation and choice of crops.
Jacob and Halle (2001) suggested that seasonal reduction in vegetation height is drastic in grain growing systems (e.g. wheat, rice), but much less pronounced for pastures and other grasslands. Short-term effects can be caused by farming practices, such as mowing and harvesting. These effects may range from behavioural responses of rodents regarding movements and feeding to local extinction of rodent populations (Jacob, 2003; Cavia et al., 2005).
It is concluded from this study that the burrows of rodents were found mostly on the undisturbed field boundaries during non-crop season as the year-around vegetation is dominated by Saccharum griffithii, Cynodon dactylon, Saccharum spontaneum, Desmostachya bipinnata, Dichanthium annulatum, Ziziphus nummularia, Achyranthes aspera, Calotropis procera, Capparis deciduas and Rumex dentatus. This mixed type of wild natural flora were consumed round the year and provides the ideal conditions (shelter and cover) for the rodents to maintain their population and to avoid predators during non-cropping seasons.
CONCLUSION
Few studies have been carried out regarding the population effects of vegetation on rodent communities in agro-ecosystems of Pothwar Plateau at a management scale. Use of agrochemicals, livestock grazing and cutting should be rationalized to the possible extent for reducing their negative impact on flora and fauna (including rodents). Therefore, work is needed for environment education and awareness of local communities and to gather information about balancing the potential benefits of adjacent non-crop vegetation on the field margins. With such information it would be possible to weigh up the needs of rodent control and the benefits for conservation and ecosystem services provided by wild vegetation in marginal habitats of agro-ecosystems.
ACKNOWLEDGEMENTS
This research was made possible by the financial support of Higher Education Commission, Islamabad, Pakistan under a research project “Ecologically-based rodent management in croplands of Pothwar Plateau, Pakistan” with grant code No. 20-2547/NRPU/R & D/HEC/13/97. . Special thanks to Prof. Dr. Sarwat N. Mirza and Dr. Amir Saleem for helping in plant species identification.
Statement of conflicts of interest
The authors declare that there is no conflict of interests.
REFERENCES
Ahmad, I., Hussain M., Ahmad M.S.A. and Hameed, M., 2008. Spatio-temporal effects on association of plant species in Soon Valley of Pakistan. Pakistan J. Bot., 40: 1865-1876.
Altieri, M.A. and Nicholls, C.I., 1999. Biodiversity, ecosystem function, and insect pest management in agricultural systems. In: Biodiversity in agroecosystems (eds. W.W. Collins and C.O. Qualset). CRC Press, Boca Raton, FL, USA, pp. 69-84. https://doi.org/10.1201/9781420049244.ch5
Atkinson, P.W., Buckingham, D. and Morris, A.J., 2004. What factors determine where invertebrate-feeding birds forage in dry agricultural grasslands? Ibis, 146: 99-107. https://doi.org/10.1111/j.1474-919X.2004.00346.x
Best, L.B., 1983. Bird use of fence rows: Implications of contemporary fencerow management practices. Wildl. Soc. Bull., 11: 333-347.
Boutin, C., Jobin, B., Be´langer, L. and Choinie`re, L., 2002. Plant diversity in three types of hedgerows adjacent to cropfields. Biodiv. Conserv., 11: 1-25. https://doi.org/10.1023/A:1014023326658
Bunce, R.G.H. and Hallam, C.J., 1993. The ecological significance of linear features in agricultural landscapes in Britain. In: Landscape ecology and agroecosystems (eds. R.G.H. Bunce, L. Ryszkowski and M.G. Paoletti). Lewis Publishers, Boca Raton, FL, USA, pp. 11-19.
Cavia, R., Gomez Villafane, I.E., Cittadino, E.A., Bilenca, D.N., Mino, M.H. and Busch, M., 2005. Effects of cereal harvest on abundance and spatial distribution of the rodent Akodon azarae in central Argentina. Agric. Ecosyst. Environ., 107: 9-95. https://doi.org/10.1016/j.agee.2004.09.011
Chambers, L.K., Singleton, G.R. and van Wensveen, M., 1996. Spatial heterogeneity in wild populations of house mice (Mus domesticus) on the Darling Downs, South-eastern Queensland. Wildl. Res., 23: 23-38. https://doi.org/10.1071/WR9960023
Curtis, J.T., 1959. The vegetation of Wisconsin: An ordination of plant communities. University of Wisconsin Press, Madison, Wisconsin. pp. 657.
De Cauver, B., Reheul, D., Nijs, I. and Milbau, A., 2006. Dry matter yield and herbage quality of field margin vegetation as a function of vegetation development and management regime. Wageningen J. Life Sci., 54: 37-60. https://doi.org/10.1016/S1573-5214(06)80003-5
Desy, E.A. and Batzli, G.O., 1989. Effects of food availability and predation on prairie vole demography: A field experiment. Ecology, 70: 21-411. https://doi.org/10.2307/1937546
Giusti, G.A., Whisson, D.A. and Gorenzel, W.P., 1996. Rodents and cover crops – a review. 17th Vertebrate Pest Conference, University of California, Davis, pp. 59-61.
Gorman, M.L. and Reynolds, P., 1993. The impact of land-use change on voles and raptors. Mammal. Rev., 23: 6-121. https://doi.org/10.1111/j.1365-2907.1993.tb00423.x
Hansson, L., 1977. Spatial dynamics of field voles, Microtus agrestis, in heterogeneous landscapes. Oik, 29: 44-539. https://doi.org/10.2307/3543592
Hussain, I., Cheema A.M. and Khan, A.A., 2003. Small rodents in the crop ecosystem of Pothwar Plateau, Pakistan. Wildl. Res., 30: 269-274. https://doi.org/10.1071/WR01025
Jacob, J and Halle, S., 2001. The importance of land management for population parameters and spatial behaviour in common voles (Microtus arvalis). In: Advances in vertebrate pest management II (eds. H.J. Pelz , D.P. Cowan and C.J. Feare). Filander Verlag, Fürth, pp. 30-319.
Jacob, J., 2003. Short-term effects of farming practices on populations of common voles. Agric. Ecosyst. Environ., 95: 5-321. https://doi.org/10.1016/S0167-8809(02)00084-1
Marshall, E.J.P. and Arnold, G.M., 1999. Factors affecting field weed and field margin flora on a farm in Essex, UK. Landsc. Urban Plan., 31: 16-205. https://doi.org/10.1016/0169-2046(94)01047-C
Martin, S.K., 1994. Feeding ecology of American martens and fishers. In: Biology and conservation (eds. S.W. Buskirk, A.S. Harestad, M.G. Raphael and R.A. Powell). Comstock Publishing Association. Cornell University Press, Ithaca and London, pp. 297-315.
Mauritzen, M., Bergers, P.J.M., Andreassen, H.P., Bussink, H. and Barendse, R., 1999. Root vole movement patterns: Do ditches function as habitat corridors? J. appl. Ecol., 36: 21-409. https://doi.org/10.1046/j.1365-2664.1999.00414.x
Munawar, N., Hussain, I. and Mahmood, T., 2018. Occurrence of rodent species in agricultural lands during cropping and non-cropping seasons of Pothwar Plateau, Pakistan. Pakistan J. Zool., 50:1663-1669.
Nawaz, T., Hameed, M., Naz, N., Ahmad, M.S.A. and Chaudhry, A.A., 2010. Impact of fencing on vegetation structure in Lehri and Jindi sub-mountaineous open scrub forest. Int. J. Biol. Biotech., 7: 227-233.
Nawaz, T., Hameed, M., Ashraf, M., Ahmad, F., Ahmad, M.S.A., Hussain, M., Ahmad, I., Younis, A. and Ahmad, K.S., 2012. Diversity and conservation status of economically important flora of the Salt Range, Pakistan. Pak. J. Bot., 44: 203-211.
Paoletti, M.G., Pimentel, D., Stinner, B.R. and Stinner, D., 1992. Agroecosystem biodiversity: Matching production and conservation biology. Agric. Ecosyst. Environ., 40: 3-23. https://doi.org/10.1016/B978-0-444-89390-1.50004-4
Redhead. T.D. and Singleton, G.R., 1988. The PICA Strategy for the prevention of losses caused by plagues of Mus domesticus in rural Australia. EPPO Bull., 18: 48-237. https://doi.org/10.1111/j.1365-2338.1988.tb00371.x
Roberts, T. J., 1997. The mammals of Pakistan, revised edition. Oxford University Press, pp. 525.
Sarwar, M., Hussain, I., Anwar, M. and Mirza S.N., 2016. Baseline data on anthropogenic practices in the agro-ecosystem of Pothwar Plateau, Pakistan. J. Anim. Pl. Sci., 26: 850-857
Sarwar, M., Hussain, I., Ashraf, N., Anwar, M. and Mirza S. N., 2017. Baseline data on wild flora of crop field boundaries in the agroecosystem of Pothwar Plateau, Pakistan. Pak. J. Bot., 49: 249-258.
Slade, N.A. and Crain, S., 2006. Impact on rodents of mowing strips in old fields of Eastern Kansas. J. Mammal., 87: 97-101. https://doi.org/10.1644/05-MAMM-A-006R2.1
Stenseth, N.C., 1977. On the importance of spatio- temporal heterogeneity for the population dynamics of rodents: towards a theoretical foundation of rodent control. Oik, 29: 52-545. https://doi.org/10.2307/3543593
Tattersall, F.H., Macdonald, D.W., Hart, B.J., Johnson, P., Manley, W. and Feber, R., 2002. Is habitat linearity important for small mammal communities on farmland? J. appl. Ecol., 39: 52-643. https://doi.org/10.1046/j.1365-2664.2002.00741.x
To share on other social networks, click on any share button. What are these?










