Inoculation of Sunflower with Endophytic Fungi Alters Soil Physio-Chemical Properties to Nullify Drought Stress
Inoculation of Sunflower with Endophytic Fungi Alters Soil Physio-Chemical Properties to Nullify Drought Stress
Nighat Seema1*, Muhammad Hamayun1, Husan Ara1 and Raham Sheer Khan2
1Department of Botany, Abdul Wali Khan University Mardan, Khyber Pakhtunkhwa, Pakistan; 2Department of Biotechnology, Abdul Wali Khan University Mardan, Khyber Pakhtunkhwa, Pakistan.
Abstract | Endophytic fungi cooperate more meticulously with their host plants and may be more capable to solubilize nutrients from soil and provide it to their hosts. In current study, endophytic fungi inoculation significantly affected the soil properties such as soil texture, organic matters, EC and pH with or without 8% PEG induced drought stress. Upon inoculation of different endophytic fungi with or without drought stress, the trend in pH values of soil remain variable (7.61~7.96) compared with the pH value before experiment. The 8% PEG induced drought stress elevated the EC value, however, the inoculation of all types of endophytic fungi lowered the EC to provide smooth medium for plant growth. Interestingly, the inoculation did enhance the extent of usage of organic compounds by the plants available in the soil and hence lowered the contents. During drought stress, ammonium ion concentration was increased in all endophytic treated soil except that of A. niger. Similarly, inoculation of endophytic fungi did also increased the value of soil nitrate ions in the range of 26.86±1.34~39.39±1.97 mg kg-1 with or without drought stress. Inoculation of endophytic fungi increased the amount of extractable P and K in growth media compared to soil samples before experiment and after experiment in control samples. In case of micronutrients, only copper was decreased in endophytic treated soil, whereas iron, zinc and manganese did not follow any specific trend with or without drought stress. Hence, it is suggested that all these endophytic fungi should be evaluated under open field drought stress conditions before final recommendation for agronomic purpose.
Received | December 03, 2018; Accepted | January 13, 2019; Published | January 25, 2019
*Correspondence | Nighat Seema, Department of Botany, Abdul Wali Khan University Mardan, Khyber Pakhtunkhwa, Pakistan; Email: seemazakaria@gmail.com
Citation | Seema, N., M. Hamayun, H. Ara and R.S. Khan. 2019. Inoculation of sunflower with endophytic fungi alters soil physico-chemical properties to nullify drought stress. Sarhad Journal of Agriculture, 35(1): 109-115.
DOI | http://dx.doi.org/10.17582/journal.sja/2019/35.1.109.115
Keywords | Macronutrients, Micronutrients, Polyethylene glycol, Abiotic stress, Soil medium
Introduction
The fertility of soil is affected by various physical, chemical and biological properties of soil. The soil physical properties mainly soil texture and chemical properties such as pH, lime, electrical conductivity (EC) and organic matter content and concentrations of macro- micronutrients mainly determine the soil fertility and its productivity.
The nutrient solution with proper pH provided to the plants is ensured to have contained the basic 16 mineral elements, 5 out of which occur in the soil and are readily available to be dissolved in soil by moisture and up taken by the roots. These elements are necessary for plant growth and if any one or more than one are lacking in soil, they can affect the plant growth. The pH has significant influence on solubility and bio-availability of nutrients. NO3 and NH4 -N are available in a relatively wider range of pH (6.0-8.5). In calcareous soils with high pH the availability of P to plants is decreased. The solubility of P is optimum over a narrow pH range (6.5-7.5). The micronutrients, Fe, Cu, Zn and Mn are more soluble in the pH range 5.0 to 6.0, and their availability in soils varies considerably with the seasonal changes in temperature, moisture and microbial activity (Hodgson, 1963). Copper, Fe, Mn and Zn are required by plants in minute quantities. Cu is slightly less abundant in soils than Zn (Lindsay, 1979). Elements reduce the uptake of one or more micronutrients by plants such as reduction in Fe and Mn uptake by plants as a result of P fertilization or Fe deficiencies caused by excess Ca and Mn (Olsen and Sommers, 1982).
Plants are sometime exposed to extreme environmental conditions. Drought stress is one of the major abiotic constraint limiting crop growth and productivity worldwide. In general, drought stress occurs when available soil moisture decreases and atmospheric conditions cause transpiration or evaporation to cause loss of water (Khajeh Hosseini et al., 2003). Endophytic fungi play an important role in protecting plants and making plants more suitable for coping with biotic and abiotic stresses, reducing water consumption and increasing biomass. The plant growth regulator produced by endophytic fungi residing in the plant tissues play major roles in enhancement of growth and development of plants in general and crops in particular (Kang et al., 2014). Application of polyethylene glycol induced drought stress in combination with host plants and endophytic fungi significantly increased plant biomass and related growth parameters as compared to control plants. By influencing plant morphology, development, and physiological and biochemical responses to stress, fungal endophytes can induce mechanisms of drought tolerance in their hosts (Malinowski and Belesky, 2000).
The objective of this study was to evaluate the physico- chemical properties (texture, pH, EC, lime and organic matter) of soils and macro (N, P and K) and micronutrients (Zn, Cu, Fe and Mn) in soils, with and without endophytic fungi under drought stress.
Materials and Methods
The present experiment was conducted at the department of Botany, AWKUM during sunflower growing season 2013-2017. Pots were filled with autoclaved sand (300 g). The endophytic fungi were isolated by using standard protocols and were identified through ITS-1 and ITS-4 primers. The experiment was carried out as CRBD with three replicates. The experiment consists of the following treatments.
Experimental work plan.
| S.No. | Treatment |
| 1 | Control |
| 2 | Control + 8% PEG |
| 3 |
Plants treated with Phoma spp. |
| 4 |
Plants treated with Phoma spp. + 8% PEG |
| 5 |
Plants treated with Aspergillus niger |
| 6 |
Plants treated with Aspergillus niger + 8% PEG |
| 7 |
Plants treated with C. gracile |
| 8 |
Plants treated with C. gracile + 8% PEG |
| 9 |
Plants treated with F. proliferatum |
| 10 |
Plants treated with F. proliferatum + 8% PEG |
| 11 |
Plants treated A. terreus |
| 12 |
Plants treated with A. terreus + 8% PEG |
Fungal DNA isolation, identification and phylogenetic analysis
Genomic DNA was extracted from fungal isolates using standard method of Khan et al. (2008). Fungal isolate was identified by sequencing the internal transcribed region (ITS) of rDNA using universal primers: ITS-1; 5’-TCC GTA GGT GAA CCT GCG G-3’ and ITS-4; 5’-TCC TCC GCT TAT TGA TAT GC-3’. The BLAST search program (http:// blast.ncbi.nlm.nih.gov) was used to compare the nucleotide sequence similarity of ITS region of related fungi. The closely related sequences obtained were aligned through CLUSTAL W using MEGA version 7.0 software and a neighbor joining tree was constructed using the same software. The bootstrap replications (550) were used as a statistical support for the nodes in the phylogenetic tree.
Soil characterization and macro/micronutrients analysis
The analytical procedures for each physicochemical parameter are as follows.
Soil texture
Soil texture was calculated by textural triangle (Koehler et al., 1984). 10 ml of 1 N Na2CO3 was added to 50 g air dried sand sample and distilled water (50 mL) in a dispersion cup and stirred for 10 min and was then transferred to a 1000 mL cylinder. Reading was noted with hydrometer after 40 seconds and 2 h for silt + clay and clay respectively.
Soil pH and soil electric conductivity (EC)
The pH of soil samples was measured by preparing soil water suspension (1:5). Ten gram of air dried soil along with 50 mL distilled water was taken in flask and shaken for twenty-five minutes on magnetic stirrer for homogenous mixing, and then filtered with What man No. 42 filter paper. The pH of the suspension was determined with pH meter, while EC of the suspension (1:5) was determine in µS/cm using the EC meter (Richard, 1954).
Soil organic matter content
One-gram soil was mixed with 10 mL (1 N) potassium dichromate solution and 20 mL conc. H2SO4 into 250 mL volumetric flask. Upon cooling, distilled water (200 mL) was added into the mixture followed by filtration and addition of few drops ortho phenolphthalein. The filtrate was then titrated against 0.5 N FeSO4.7H2O solution until the appearance of maroon color (Nelson and Sommers, 1982).
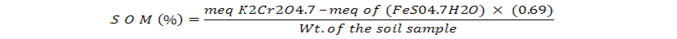
Total mineral nitrogen in soil
Total mineral N in soil was determined by the steam distillation method of (Nelson and Sommers, 1982). In this method soil sample (25 g) was shaken with 100 ml of 1 M KCl for 1 hour and filtered. 20 mL of filtrate was distilled with MgO to recover NH4-N and was collected in 5 ml boric acid mixed indicator solution and then titrated against 0.0005 moles HCl. The NO3-N was determined by subtracting the NH4-N from mineral N.
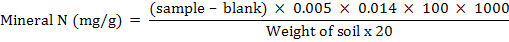
AB-DTPA extractable phosphorus and potassium
The soil extractable P and K was determined by mixing 10 g sample with 20 ml ammonium bicarbonate-diethylene triamine pentaacetic acid (AB-DTPA) solution in flask, then shaken on mechanical shaker for 20 minutes followed by filtration with the help of Wattman no 42 filter paper. For P and K determination ascorbic acid mixed indicator (5mL) was added into 1 ml filtrate and 3 ml distilled water in volumetric flask (25ml) and made a volume up to mark. After color development (blue) in dark, P and K concentrations was calculated at 410 nm using standard P and K by spectrophotometer (Soltanpour, 1985).
AB-DTPA extractable Fe, Mn, Zn and Cu
Micronutrients like iron, manganese zinc and copper were extracted through method describe by Soltanpour (1985). Then they were analyzed with help of AAS (atomic absorption spectrometry) (Perkin Elmer 2138) using acetylene gas and particular element cathode lamps.
Data analysis
The data were analyzed by Duncan Multiple Range Test (DMRT) using IBM SPSS software version 21.0 (SPSS Inc, Chicago, USA).
Results and Discussion
In the present study, the major challenge is being posed by the shortage of water leading to the conditions of drought. For this purpose, the endophytic fungi were first isolated from xerophytes and after initial screening the best growth promoting fungi were identified by sequencing the internal transcribed region (ITS) of rDNA using universal primers ITS-1 and ITS-4. These endophytic fungi were then inoculated on the Helianthus annuus L. (Sunflower Hysun-33) and their effect was recorded on various physical parameters with and without the application of drought stress (8% PEG). During current study, an effort was made to investigate the role of endophytic fungi in mobilization of important nutrients to plants and their possible role in alleviation of drought stress. The isolated fungal strains were found stimulatory to the nutrient accumulation in the rhizosphere. Fungal inoculation has the ability to restore the fertility of degraded land through several manners. These microorganisms surge the nutrient bioavailability and remediate soil structure by refining its accretion and stability.
Sand analysis
The physical parameters like pH, electrical conductivity and organic matter content of the sand samples were assessed before and after the experiments.
Soil pH and soil EC
It was depicted in the findings that there was varying change in the pH values of soil before and after experiments in different samples. The pH of soil in the control sample increased to 7.95 after conducting the experiment which was 7.59 before the experiment, while in other treatments there were differences. The pH was almost the same after experiments in the samples exposed to drought stress alone (7.55) and the sample without stress with inoculation of Phoma sp. and with stress and respective inoculation of C. gracile, A. niger and A. terreus having the values 7.61, 7.68 and 7.65 (Table 1). In the other samples with or without inoculations, the values of pH were higher almost equal to control values. The samples inoculated with F. proliferatum with and without stress had comparatively higher values than other samples which were 7.75 and 7.96 respectively, the latter being even higher than that of control. The EC of soil before conducting the experiment was recorded to be 0.28 dS m-1 and it was quite lower in all the other samples including control. However, in the samples only exposed to drought stress the value of EC was much higher which was 0.30 dS m-1. All the remaining treatments had the EC values in between 0.11 and 0.19 dS m-1, except for the sample of F. proliferatum inoculation which showed extremely low value of EC i.e., 0.05 dS m-1(Table 1).
Table 1: pH, EC and Organic matter content (0-30 cm) analysis of sand used for sunflower seedlings before and after experiment.
| S.No | pH (1:5) | EC (dS m-1) | Organic Matter (%) |
| Before experiment | 7.59±0.38a | 0.28±0.01h | 0.23±0.01d |
| Control | 7.95±0.40a | 0.19±0.01g | 0.13±0.01ab |
| 8%PEG | 7.55±0.38a | 0.30±0.01i | 0.53±0.03h |
|
Phoma sp. |
7.61±0.38a | 0.13±0.01de | 0.10±0.00a |
|
Phoma sp.+8%PEG |
7.94±0.40a | 0.18±0.01fg | 0.34±0.02f |
| A.niger | 7.91±0.40a | 0.11±0.01b | 0.13±0.01ab |
|
A. niger+8%PEG |
7.68±0.37a | 0.14±0.01de | 0.30±0.01e |
| C. gracile | 7.94±0.40a | 0.17±0.01fg | 0.18±0.01c |
|
C. gracile+8%PEG |
7.64±0.38a | 0.15±0.01f | 0.21±0.01d |
| F. proliferatum | 7.75±0.39a | 0.05±0.00a | 0.14±0.01c |
|
F. proliferatum+8%PEG |
7.96±0.40a | 0.12±0.01bc | 0.77±0.04i |
| A. terreus | 7.76±0.39a | 0.14±0.01e | 0.11±0.01ab |
|
A. terreus+8%PEG |
7.65±0.38a | 0.18±0.01fg | 0.50±0.00g |
Organic matter content
The decrease or increase in the organic matter content was according to the extent of usage of organic compounds by the plants in soil. The percentage of organic matter in soil before conducting the experiment was 0.23% while it went on decreasing in all the treatment samples with respect to the extent of usage of organic matter content. The least recorded organic matter was in the soil sample inoculated with A. niger and exposed to stress, which was 0.3% while the highest value was recorded in the sample inoculated with F. proliferatum along with exposure of drought stress, which was 0.77%. The organic matter content in control sample was 0.13% while in the sample exposed with drought stress alone, it was 0.53% (Table 1).
Ionic concentration of NH4+ and NO3-1 in soil samples
It is depicted that the values of ammonium ion concentration were not uniform in the treatments as compared to the values in control and the sample before experiments. Before experiment, it was recorded to be 9.5 mgkg-1 while after experiment it was 4.5 mgkg-1 in control sample. The drought stress sample of soil had 11.5 mgkg-1 of ions and all the remaining treatments had values between 6.5 and 21.7 mgkg-1. The trend of nitrate ions indication also did not follow any specific trend. Its value in the soil sample before experiment was 26.43 mgkg-1 while in the control sample after experiment it was 19.42 mgkg-1. The remaining treatments including the drought stress treatment had the values in between 23.21 and 39.39 mgkg-1 (Table 2).
Concentration of AB-DTPA extractable P and K
The extractable phosphorus was noted down in the soil sample before experiment and its value was 0.29 mgkg-1 while after experiment in control sample, its value was mgkg-1. The drought stress sample showed its value to be 0.63 mgkg-1 while all the other treatment samples had values higher than these in inoculated samples. The highest value was noted in the samples inoculated with A. terreus and Phoma sp. both exposed to drought stress and the values read to be 5.97 and 6.46 mgkg-1 respectively. The trend of potassium ion detection was also the same as that for phosphorus in the soil samples before and after conducting the experiments. Its value before experiment was 20.43 mgkg-1 while after experiment in control it decreased only a little bit to 19.42 mgkg-1. The sample exposed drought stress was 40.21 mgkg-1 and the one exposed to stress and inoculated by Phoma sp. was 39.93 mgkg-1. (Table 2) All the remaining inoculations both with and without stress exposure showed high values of potassium between 27.32 for A. terreus and 39.39 for sample inoculated with C. gracile exposed to stress.
Concentration of AB-DTPA extractable Cu, Fe, Zn and Mn
Extractable copper: The copper ion concentration in the samples before experiment was 1.23 mgkg-1 which
Table 2: Ionic Analysis of soil samples: Concentration of NH4+ and NO3- analysis of sand used for sunflower seedlings before and after experiment.
| S. No. |
mgkg-1 |
|||
|
NH4+ |
NO3- |
Extractable- P | Extractable- K | |
| Before exp. | 9.51±0.48d | 26.43±1.32c | 0.35±0.02a | 20.43±1.02a |
| Control | 4.51±0.23a | 19.42±0.97a | 0.29±0.01a | 19.42±0.97a |
| 8%PEG | 11.51±0.58e | 23.21±1.16b | 0.63±0.03b | 40.21±2.01e |
|
Phoma sp. |
7.50±0.38bc | 35.66±1.78e | 1.34±0.07e | 35.66±1.78c |
|
Phoma sp.+8%PEG |
12.51±0.63e | 31.93±1.60d | 6.46±0.32h | 39.93±2.00e |
| A. niger | 8.50±0.42cd | 26.86±1.34c | 1.22±0.06de | 36.86±1.84cd |
|
A. niger+8%PEG |
6.51±0.33b | 29.01±1.45c | 1.62±0.09f | 29.01±1.45b |
| C. gracile | 14.51±0.73f | 28.22±1.41c | 0.90±0.05c | 28.22±1.41b |
|
C. gracile+8%PEG |
19.51±0.98g | 39.39±1.97f | 1.61±0.08f | 39.39±1.97de |
| F. proliferatum | 12.51±0.63e | 23.43±1.18b | 0.46±0.02ab | 37.43±1.87cde |
|
F.proliferatum +8%PEG |
21.51±1.08h | 37.45±1.87ef | 0.97±0.05c | 21.45±1.91a |
| A. terreus | 11.80±0.59e | 27.32±1.37c | 1.08±0.05cd | 27.32±1.37b |
|
A. terreus+8%PEG |
21.71±1.09h | 35.64±1.78e | 5.97±0.3g | 35.64±1.79c |
Table 3: Concentration of AB-DTPA extractable Cu, Fe, Zn and Mn of sand used for sunflower seedlings before and after experiment.
| S. No. |
mgkg-1 |
|||
| Cu | Fe | Zn | Mn | |
| Before exp. | 1.23±0.06i | 2.23±0.11d | 0.04±0.00a | 1.28±0.06b |
| Control | 0.95±0.07h | 1.98±0.10bc | 0.34±0.02f | 0.90±0.00a |
| 8%PEG | 0.77±0.04f | 3.98±0.20g | 0.28±0.01e | 1.92±0.10c |
|
Phoma sp. |
0.16±0.01d | 2.98±0.15f | 0.13±0.01b | 0.91±0.05a |
|
Phoma sp.+8%PEG |
0.34±0.02e | 1.29±0.06a | 0.23±0.01d | 0.86±0.04a |
| A. niger | 0.11±0.01bcd | 1.91±0.10b | 0.14±0.01b | 1.98±0.10c |
|
A. niger+8%PEG |
0.12±0.01cd | 2.28±0.11d | 0.28±0.01e | 2.68±0.13e |
| C. gracile | 0.10±0.00bcd | 2.17±0.11cd | 0.26±0.01e | 2.18±0.11d |
|
C. gracile+8%PEG |
0.06±0.00ab | 2.32±0.12d | 0.16±0.01c | 2.98±0.15e |
| F. proliferatum | 0.03±0.00a | 1.87±0.09b | 0.25±0.01e | 2.18±0.11d |
|
F. proliferatum +8%PEG |
0.02±0.00a | 2.65±0.13e | 0.17±0.01c | 2.86±0.14e |
| A. terreus | 0.09±0.00bc | 2.33±0.12d | 0.29±0.01e | 2.29±0.11d |
|
A. terreus+8%PEG |
1.09±0.05h | 1.86±0.09b | 0.56±0.03g | 0.87±0.04a |
was quite higher than the findings in other samples. After conduction of experiments, it fell considerably to values below 1.0 mgkg-1, in subsequent samples. In control sample, it was recorded to be 0.95 mgkg-1 while in the sample exposed to stress, its value was 0.77 mgkg-1. In all the inoculated samples, the values of copper ion concentrations were founded to be between 0.02 and 0.34 mgkg-1 (Table 3).
Extractable iron: The trend of iron concentration detection did not follow the pattern as that for copper as there were marked changed in the concentrations of iron in different samples. In the soil sample before experiment, the level of iron was detected to be 2.23 mgkg-1 while after experiment, it was noted to be 1.98 in control sample and 3.98 in the sample exposed to drought stress. In the inoculated samples, the values of iron did not follow any anomaly and fell in the ranges between 2.98 and 1.29 mgkg-1.
Zinc concentration in soil samples: The value of zinc was noted in the sample before experiment and it came out to be 0.04 mgkg-1 while in control sample it was recorded to be 0.34 mgkg-1. On the other hand, the sample exposed to drought stress showed its value to be 0.28 mgkg-1. The samples inoculated with C. gracile and with F. proliferatum exposed to drought stress showed the values to be 0.16 and 0.17 mgkg-1 respectively (Table 3).
Manganese concentration in soil samples: The value of manganese ions in soil samples before conducting experiment was 1.28 mgkg-1 while after experiment its value in control was recorded to be 0.9 mgkg-1. On the other hand, the concentration of Mn in sample exposed to drought stress was 1.92 mgkg-1. All the other values for inoculations fell in the range between 0.91 and 2.98 mgkg-1 (Table 3).
Soil is an important part of the whole system in which plants grow and it is the major source of moisture, different ions and organic content and houses a lot of microorganisms for the benefit of plants. That is why it is very important to maintain the integrity and composition of soil so that it may not outbalance the concentration of ions, molecules and organic content in the plants as well. The isolated fungal strains were found stimulatory to the nutrient accumulation in the rhizosphere. Bacterial and fungal inoculation have the ability to restore the fertility of degraded land through several manners. These microorganisms surge the nutrient bioavailability by nitrogen fixation and mobilization of important nutrients to plants but remediate soil structure by refining its accretion and stability (Rashid et al., 2016).
Phosphorus is not only an important part of the nucleic acids but also of different enzymes and proteins found in plant and is vital for their proper functioning. Also it must be available in such a form which is easily taken up by the plant root (Nannipieri et al., 2011). Therefore, its availability in soluble form is very important. The presence of microorganisms in soil enhances the ability of root cells to absorb phosphorus and accumulate it in plants. The extractable phosphorus was noted down in the soil sample before experiment and its value was 0.35 mgkg-1 while after experiment in control sample, its value was 0.29mgkg-1. Richardson (2001) reported increase in soil phosphorus content through inoculation with microorganisms. Similarly, potassium is important in the plants undergoing drought stress because it is the major factor involved in the opening and closing of stomata. Therefore, its optimum concentration is necessary for maintaining and regulating the opening and closing of stomata. Studies have shown that the fungal inoculants considerably increased the absorption of potassium by roots from soil which is an important benchmark in managing the drought stress (Al-Karaki, 2006; Evelin et al., 2009).
There is a direct relationship between the heavy metal ions absorption and drought stress management and it was seen evidently in the copper ions absorption pattern. The absorption of heavy metal ions has a direct link with oxidative stress management. Copper ions are reported to decrease the SOD activity in plants (Gallego et al., 1996). It was obvious in the findings of the experiment that copper ions concentration is much higher in the soil before conducting experiments and it has decreased considerably which shows that it was absorbed by the plant roots. In all the samples inoculated with fungal strains, the absorption was much better and it was shown to be far less in soil after experiment. Water is considered as the major limiting factor in different physiological reactions which also reduces the nutrient uptake in plants however, fungal inoculation increases the absorption of different types of nutrients even if the plant is under severe stress condition. These results were in agreement with those reported by Singh and Kapoor (1999) and Singh and Reddy (2011)
Author’s Contribution
Nighat Seema: Presented and designed the idea of the work.
Muhammad Hamayun: Supervision of the idea and research.
Hussan Ara: Literature review and graphs setting.
Raham Sheer Khan: Manuscript writing and graphs setting.
References
Al-Karaki, G.N. 2006. Nursery inoculation of tomato with arbuscular mycorrhizal fungi and subsequent performance under irrigation with saline water. Sci. Hortic. 109: 1-7. https://doi.org/10.1016/j.scienta.2006.02.019
Evelin, H., R. Kapoor and B. Giri. 2009. Arbuscular mycorrhizal fungi in alleviation of salt stress: Rev. Ann. Bot. 104: 1263-1280. https://doi.org/10.1093/aob/mcp251
Gallego, S.M., M.P. Benavides and M.L. Tomaro. 1996. Effect of heavy metal ion excess on sunflower leaves: evidence for involvement of oxidative stress. Plant Sci. 121: 151-159. https://doi.org/10.1016/S0168-9452(96)04528-1
Kang, S.M., M. Waqas, A.L. Khan and I.J. Lee. 2014. Plant-growth-promoting rhizobacteria: Potential candidates for gibberellins production and crop growth promotion. Use of Microbes for the Alleviation of Soil Stresses. Volume 1. Springer.
Khan, S.A., M. Hamayun, H. Yoon, H.Y. Kim, S.J. Suh, S.K. Hwang, J.M. Kim, I.J. Lee, Y.S. Choo and U.H. Yoon. 2008. Plant growth promotion and Penicillium citrinum. BMC Microbiol. 8: 231. https://doi.org/10.1186/1471-2180-8-231
Khajeh-Hosseini, M., A. Powell and I. Bingham. 2003. The interaction between salinity stress and seed vigour during germination of soyabean seeds. Seed Sci. Technol. 31: 715-725. https://doi.org/10.15258/sst.2003.31.3.20
Koehler, F., C. Moodie and B. Mcneal. 1984. Laboratory manual for soil fertility, Wash. State Univ. Pullman Wash.
Lindsay, W.L. 1979. Chemical equilibria in soils. John Willey and Sons, New York.
Hodgson, J.F. 1963. Micronutrients availability. J. Adv. Agron. 15: 119
Malinowski, D.P. and D.P. Belesky. 2000. Adaptations of endophyte-infected cool-season grasses to environmental stresses: mechanisms of drought and mineral stress tolerance. Crop Sci. 40: 923-940. https://doi.org/10.2135/cropsci2000.404923x
Nannipieri, P., L. Giagnoni, L. Landi and G. Renella. 2011. Role of phosphatase enzymes in soil. Phosphorus Action. Springer.
Nelson, D. and L.E. Sommers. 1982. Total carbon, organic carbon, and organic matter1. Methods soil Anal. Part 2. Chem. Microbiol. Prop. 539-579.
Olsen, S.R. and L.E. Sommers. 1982. Phosphorus. In: Methods of soil analysis. (Part 2, 2nd edition). A.L. Page, R.H. Miller and D.R. Keeney (eds.). American Society of Agronomy, USA.
Rashid, M.I., L.H. Mujawar, T. Shahzad, T. Almeelbi, I.M. Ismail and M. Oves. 2016. Bacteria and fungi can contribute to nutrients bioavailability and aggregate formation in degraded soils. Microbiol. Res. 183: 26-41. https://doi.org/10.1016/j.micres.2015.11.007
Richard, L. 1954. Diagnosis and improvement of saline and alkali soils. USDA hand book. No. 60. US Govt. Press, Wash. DC. 160.
Richardson, A.E. 2001. Prospects for using soil microorganisms to improve the acquisition of phosphorus by plants. Funct. Plant Biol. 28: 897-906. https://doi.org/10.1071/PP01093
Singh, S. and K. Kapoor. 1999. Inoculation with phosphate-solubilizing microorganisms and a vesicular-arbuscular mycorrhizal fungus improves dry matter yield and nutrient uptake by wheat grown in a sandy soil. Biol. Fert. Soils. 28: 139-144. https://doi.org/10.1007/s003740050475
Singh, H. and M.S. Reddy. 2011. Effect of inoculation with phosphate solubilizing fungus on growth and nutrient uptake of wheat and maize plants fertilized with rock phosphate in alkaline soils. Eur. J. Soil Biol. 47: 30-34. https://doi.org/10.1016/j.ejsobi.2010.10.005
Soltanpour, P. 1985. Use of ammonium bicarbonate DTPA soil test to evaluate elemental availability and toxicity. Commun. Soil Sci. Plant Anal. 16: 323-338. https://doi.org/10.1080/00103628509367607
To share on other social networks, click on any share button. What are these?








